Abstract
Bitterness-masking agents support the intake of functional food ingredients, which taste bitter. However, evidence-based methodology to find bitterness-masking agents remains to be established. In the present study, focusing on the fact that a bitter tastant-binding compound is expected to have a bitterness-masking effect, several epigallocatechin gallate (EGCg)-binding peptides were identified from the amino acid sequence of ribulose 1,5-bisphosphate carboxylase/oxygenase using peptide array technology. Deletion analysis and alanine scanning analysis of these peptides revealed that electrostatic interaction contribute strongly to the binding mechanism. EGCg-binding peptides identified in this study, namely, MHFRVLAKALR and FTGLKSTSAFPVTRK, successfully suppressed the activation of the bitter-taste receptor hTAS2R39 by administration of EGCg. These results suggest that peptide array technology can be used to screen bitterness-masking peptides.
Introduction
Functional food ingredients represented by green tea polyphenols have numerous health benefits, such as antiobesity, antihypertensive, and anticancer properties (Iwata et al., 2019, Terada et al., 2019, Legeay et al., 2015, Lan et al., 2015, Ito et al., 2013, Lesschaeve et al., 2005). In recent years, the market for functional foods with health benefits has expanded rapidly (Jędrusek-Golińska et al. 2020). However, while they are beneficial to health, several functional food ingredients represented by polyphenols and flavonoids taste unpleasantly bitter (Kuroda et al., 2016; Oladokun et al., 2016; Yamazaki et al., 2013; Narukawa et al., 2011).
Taste-masking technology reduces bitterness and supports the intake of functional food ingredients (Imai et al., 2019). The bitter taste, especially problematic to manufacturing functional foods, is caused by the activation of 25 types of bitter-taste receptors, hTAS2Rs, by bitter tastants (Chandrashekar et al., 2000). Each hTAS2R is activated by specific agonists, including epigallocatechin gallate (EGCg) as a main bitter tastant of green tea, which activates hTAS2R39 (Narukawa et al., 2011). Therefore, a compound that captures a bitter tastant can be expected to have a bitterness-masking effect by preventing hTAS2R activation. A typical bitterness-masking compound is cyclodextrin, which captures EGCg in a hydrophobic pocket composed of six to eight units of glucose, and is used frequently in functional foods, such as EGC-genriched green tea beverages (Lv et al., 2019; Hayashi et al., 2010). As another example, casein has also been reported to exhibit a bitterness-masking effect by binding to EGCg (Bohin et al., 2013). Ogi et al. reported that fatty acids contained in cheese show a bitterness-masking effect by forming a complex with quinine (Ogi et al., 2015; Homma et al., 2012). To date, while several bitterness-masking agents have been found empirically by mainly sensory evaluation, the evidence-based methodology to develop bitterness-masking agents has not been established.
This research aims to develop a novel methodology to screen bitterness-masking agents. We investigated the applicability of peptide array technology, which is used in epitope mapping of antibodies (Martínez-Botas and de la Hoz, 2016), to screen for bitterness-masking agents. In the present research, we focused on peptides in tea-leaf protein hydrolysates as bitterness-masking agent candidates. Tea leaves contain about 30% protein that has no effective use (Ozbayram et al., 2020; Zheng et al., 2017; Yang et al., 2015), and most of the large quantities of green tea leaves produced during the manufacturing process are discarded. In a preliminary experiment using the TS-5000Z taste-sensing system (Intelligent Sensor Technology, Inc., Kanagawa, Japan), we found that a protein hydrolysate of tea-leaf waste had a bitterness-masking effect on EGCg. This result indicated the possibility that EGCg-binding peptides are present in tealeaf hydrolysates. We screened the EGCg-binding peptides on the amino acid sequence of ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) using peptide array technology, since the main protein of green tea leaves (Camellia sinensis) is Rubisco. As a result, several EGCgbinding peptides were identified to successfully suppress the response of the bitter-taste receptor, hTAS2R39, by administering EGCg. The screening of food ingredient-binding peptides using peptide array technology, as presented in this study, can be applied to developing bitterness-masking agents (Fig. 1).

Materials and Methods
Materials and chemicals EGCg-rich green tea (PubChem CID: 65064) was purchased from Ito En, Ltd. (Tokyo, Japan). EGCg was obtained from Nacalai Tesque, Inc. (Kyoto, Japan). Reagents for peptide synthesis were purchased from Watanabe Chemical Industries, Ltd. (Hiroshima, Japan). Flp-In TREx 293T cells and Lipofectamine 2000 were purchased from Life Technologies (USA). Fluo8-AM was obtained from Probes Inc. (USA). hTAS2R39/pEAK10 plasmid was kindly provided by Dr. Misaka (The University of Tokyo) (Narukawa et al., 2011; Yamazaki et al., 2013). A DNA fragment encoding the chimeric G-protein subunit Gα16gust44 was kindly provided by Takashi Ueda (Nagoya City University) (Ueda et al., 2003). All other reagents were obtained from standard suppliers.
Physicochemical analysis of the bitterness-masking effect of green tea-leaf hydrolysates Green tea leaves were rinsed 10 times with hot water at 80 °C. The residual wet paste (tealeaf waste) was suspended in 10 mM of Na-phosphate buffer (pH 8.0) containing the food processing protease, PROTIN SD-NY10 (Amano Enzyme Inc., Nagoya, Japan), and the tea-leaf paste was stirred at 37 °C for 5 h. The reaction was stopped by heating at 95 °C for 3 min. Then, the insoluble residue was removed by centrifugation at 12 000 × g for 10 min, and the supernatant was freeze-dried.
The taste properties of the hydrolysates were analyzed preliminarily using the TS-5000Z taste-sensing system (Intelligent Sensor Technology, Inc.). The system quantitates physicochemical properties that are common to a specific taste substance in a test sample, according to its interaction with an artificial lipid-membrane sensor. The hydrolysates were added to a commercially available EGCg-enriched green tea beverage. The bitterness intensity of the supernatant was analyzed after centrifugation at 39 700 × g at 20 °C for 20 min.
Peptide array analysis The peptide array was synthesized according to the amino acid sequence of C. sinensis Rubisco (UniProt: Q8WIZ5, A0FIP7) using a ResPep SL peptide synthesizer (Intavis AG, Cologne, Germany) (Frank, 1992). After spotting an activated Fmoc amino acid on the cellulose membrane, the remaining amino acid groups were blocked with 5% acetic anhydride. At each elongation step, the N-terminal amino acids were deprotected by 20% piperidine, and then the peptide-chain extension was confirmed through bromophenol blue staining. After the final step, the side-chain protecting groups were removed with a mixture of 95% trifluoroacetic acid, 3% triisopropylsilane, and 2% ultrapure water. The membrane was washed consecutively with dichloromethane, N,N′-dimethylformamide, ethanol, and ultrapure water. In the synthesized peptide array, the C-terminal carboxyl group of peptides was bound to the cellulose membrane.
To analyze the EGCg-binding peptides, the peptide array membrane was soaked in 50 mM of Na-phosphate buffer (pH 6.0) containing 0.1 mM EGCg for 30 min at 25 °C. The membrane was rinsed thrice for 5 min with 50 mM Na-phosphate buffer (pH 6.0). The EGCg-binding peptides on the membrane were detected through redox-cycling staining using nitroblue tetrazolium/glycine (Mori et al., 2010). The signal intensity of each spot was measured using ImageJ software.
Cell-based assay for the bitter-taste receptor The human bitter-taste receptor for EGCg is hTAS2R39 (Narukawa et al., 2011). The functional expression of hTAS2R39 and other details were described previously (Narukawa et al., 2011). Cells that stably expressed chimeric G-protein subunit Gα16gust44 (Flp-In G cells) were constructed from Flp-In TREx 293T cells. The Peak10 vector encoding hTAS2R39 was transfected into Flp-In G cells using Lipofectamine 2000.
The cells were incubated for 60 min with 3 µM Fluo-8 AM, and the cellular response to ligand administration was measured using the Flexstation II microplate reader (Molecular Devices, USA). The fluorescence (excitation: 485 nm, emission: 525 nm) was monitored at 2-s intervals at 25 °C. The average fluorescence value before sample administration was regarded as the baseline value (F0). After applying the ligand solutions, changes in the fluorescence value were measured for 80 s, and the highest value (F) was employed to calculate the [Ca2+]i response using the equation [(F−F0)/F0]. As a negative control, the empty pEAK10 vector (mock) was transfected to Flp-In G cells. The response value was calculated by subtracting the response of mock cells from that of hTAS2R39-expressing cells. The dose–response curve parameters were determined by a four-parameter logistic model (Hill equation) using Prism 4.0a software (GraphPad Software, USA).
Statistical analysis Data obtained in the experiment were analyzed by the Tukey-Kramer test. Significance was accepted at p < 0.05.
Results and Discussion
Preliminary analysis of the bitterness-masking effect of tea-leaf hydrolysates The EGCg solution was physicochemically analyzed using the taste-sensing system TS-5000Z, and the results showed that the bitterness signal increased proportionally as a function of the EGCg concentration (data not shown). The tea-leaf hydrolysates reduced the bitterness signal of the EGCg-enriched green tea beverage in a concentration-dependent manner, although the change was not statistically significant (Fig. 2). This result suggests that tea-leaf hydrolysates contain bitterness-masking agents for EGCg, and the agents inhibit the interaction between EGCg and the artificial membrane of the taste-sensing system, as the taste-sensing system quantifies the physicochemical stimulus between taste substances and the artificial membrane. We hypothesized that the peptides generated from tea-leaves can form a complex with EGCg. On the other hand, the taste-sensing system is not a device that measures on the same principle as human taste sense. In order to demonstrate the bitterness-masking effect, verification by sensory evaluation or the analysis of taste receptor response is required.

Screening of EGCg-binding peptides by peptide array analysis The results of the taste sensor analysis suggested that the tea-leaf hydrolysates contain EGCg-binding peptides. In C3 plants, including tea plants, large amounts of nitrogen (15–35% of total nitrogen in green leaves) are present as Rubisco (Evans, 1989). Therefore, we assumed that those peptides were derived from C. sinensis Rubisco, the major protein present in tea leaves, and we attempted to determine each amino acid sequence through peptide array analysis. A total of 598 pentadecapeptides corresponding to the Rubisco amino acid sequence were synthesized on a cellulose membrane (peptide array). This peptide library comprises all the amino acid sequences of large and small Rubisco subunits. After redox-cycle staining for the peptide array membrane, EGCg-binding peptides were stained violet according to the strength of the bond (Fig. 3A). The results indicated that TKASVGFKAGVKDYK, TIKPKLGLSAKNYGR, KNHGM HFRVLAKALR, HGMHFRVLAKALRMS, PAQASMAAPFT GLKS, FTGLKSTSAFPVTRK, and VQCMKVWPPLGLKKF showed strong binding ability to EGCg (Fig. 3B).

Subsequently, to determine the EGCg-binding region on each peptide, we conducted deletion analysis from the N- or C-terminus of peptides (Fig. 3C–I). Similar to the original pentadecapeptides, several smaller peptides also showed binding to EGCg. Based on the significant decrease in binding activity due to 1 amino acid deletion, TIK, HGMHFR, MHFRVLAKALR, RMS, FTGLKSTSAFPVTRK, and FTGLK derived from TIKPKLGLSAKNYGR, KNHGMHFRVLAKALR, HGMHFRVLAKALRMS, HGMHFRVL AKALRMS, FTGLKSTSAFPVTRK, and FTGLKSTSAF PVTRK were identified as typical EGCg-binding peptides. Although peptide array is a semi-quantitative analysis tool that depends on the synthesis efficiency of each peptide, the experimental results were reproducible. The chain length of these peptides was not correlated with the binding strength, whereas those bound to EGCg with high affinity contained the basic amino acid residues (Arg or Lys).
Analysis of the binding mechanisms between peptides and EGCg Alanine scanning analysis was conducted on TIK, HGMHFR, MHFRVLAKALR, RMS, FTGLKSTSAFPVTRK, and FTGLK to determine the importance of each amino acid residue in the peptides to EGCg binding (Fig. 4). The results indicated that alanine substitution in the basic amino acids (Arg or Lys) significantly decreased the binding strength of most peptides. Typically, for TIK, alanine substitution at the K3 position reduced the signal intensity to 2% (Fig. 4A). In the other case, when the three basic amino acid residues (K5, R14, and K15) in FTGLKSTSAFPVTRK were replaced, the signal intensity was reduced to 35% (Fig. 4E). These results indicate that the basic amino acid residues in these peptides contributed strongly to their binding capacity to EGCg. However, several peptides that lacked the basic amino acid residues (e.g., MHFAVLAAALA in Fig. 4C or FTGLASTSAFPVTAA in Fig. 4E) were also capable of binding to EGCg. The EGCg-binding peptides discovered in the present study probably bound to EGCg mainly through electrostatic interactions, but with other minor supporting interactions such as hydrophobic interactions.

There are several reports of protein-polyphenol interactions (Feng et al., 2019; Cao et al., 2011; Ishii et al., 2011; Ishii et al., 2010). EGCg interacts directly with fibronectin, MMP-2, MMP-9, vimentin, HSP90, GPR78, IGF1R, Fyn, ZAP70, G3BP1, Pin1, and TRAF6 (Saeki et al., 2018). The interactions between EGCg and these proteins involve several mechanisms, such as hydrogen bonding, hydrophobic interaction, pi interaction, and electrostatic interaction. Among the proteins identified as molecules that EGCg interacts directly with, the 67-kDa laminin receptor (67LR) is an EGCg receptor with a higher affinity (Tachibana et al., 2004). Fujimura et al. identified the EGCg-binding peptides from the amino acid sequence of 67LR (Fujimura et al., 2012). The Lys166 in a peptide derived from 67LR contributed significantly to EGCg binding. It is a reasonable explanation that the Rubisco-derived peptides identified in the present study bound to EGCg through electromechanisms similar to that for the 67LR-derived peptides, since the deprotonation of the phenolic hydroxyl group of EGCg generated a negative charge (Xiang et al., 2016).
Evaluation of the bitterness-masking effect through taste receptor assay Two peptides, MHFRVLAKALR and FTGLKSTSAFPVTRK, were identified as peptides with strong binding ability to EGCg (Fig. 4C, E). The bitterness-masking effects of these peptides were evaluated through a cell-based taste receptor assay. It was found that hTAS2R39-expressing cells responded to EGCg in a concentration-dependent manner (Fig. 5A) (Narukawa et al., 2011). The EC50 value was 0.37 mM. As a negative control peptide, Gly-Gly did not suppress the activation of hTAS2R39 by EGCg (Fig. 5B). MHFRVLAKALR and FTGLKSTSAFPVTRK did not activate hTAS2R39 and did not give non-specific stimuli to the cells at concentrations below 0.5 mM. Both peptides suppressed hTAS2R39 activation caused by EGCg in a concentration-dependent manner (Fig. 5C, D). The IC50 values for MHFRVLAKALR and FTGLKSTSAFPVTRK for 0.30 mM EGCg were 0.36 and 0.39 mM, respectively. Thus, both peptides identified through peptide array analysis evidently masked the bitterness caused by EGCg. hTAS2R39 and hTAS2R14 have been reported as bitter taste receptors for EGCg (Narukawa et al., 2011; Yamazaki et al., 2013). In particular, the hTAS2R39 activation correlates with the values of sensory evaluation (Narukawa et al., 2011). Therefore, the peptides identified through peptide array analysis can be expected to have a bitterness-masking effect for EGCg by the binding property. Several EGCg-binding peptides were discovered in this study, which were synthesized according to the Rubisco amino acid sequence (Fig. 3), suggesting that those peptides also possessed a bitterness-masking property by capturing EGCg. These results indicated that bitterness-masking agents can be found rapidly and easily through peptide array analysis. This screening method will likely be applicable to developing bitterness-masking agents for a wide range of bitter components. Indeed, in the case of tangeretin (data not shown), three tangeretin-binding peptides (PIVMHD, AVFARE, and IIGFND) identified through peptide array analysis successfully suppressed the activation of hTAS2R14, which is a bitter-taste receptor for tangeretin (Kuroda et al., 2016). The IC50 values were 0.82 mM, 0.47 mM and 0.31 mM for PIVMHD, AVFARE, and IIGFND, respectively.
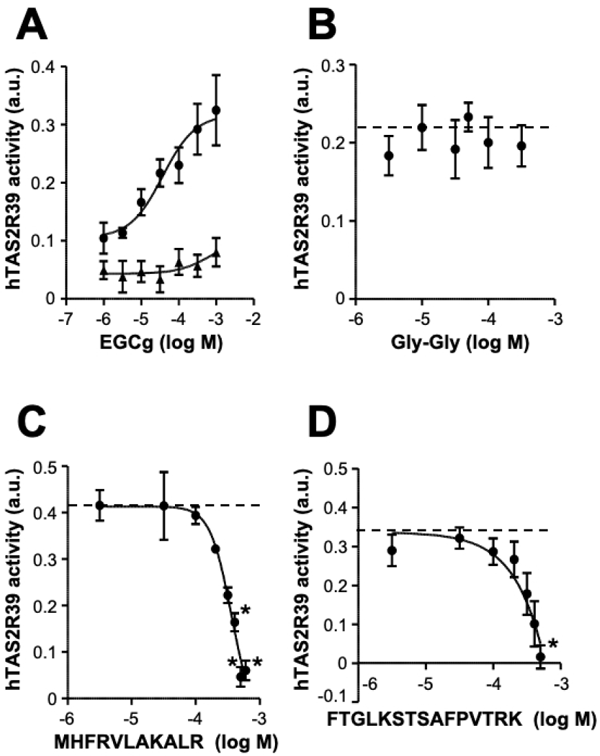
The effects of complex formation on the physiological activity of EGCg should be considered in evaluating bitterness-masking agents. Regarding this concern, Han et al. reported that conjugation with proteins improves the bioavailability of EGCg (Han et al. 2020). One of the reasons for this phenomenon is thought to be the improved stability of EGCg by binding to the protein (Ishii et al., 2011; Mori et al., 2010; Bae et al., 2009), although the mechanism has not been clarified. The EGCg-binding peptides found in the present study may also improve EGCg bioavailability.
Conclusion
In the present study, we found novel EGCg-binding peptides through peptide array analysis (Fig. 3). This analysis allowed us to not only identify target-binding peptides but also understand the binding mechanism (Figs. 3, 4). The EGCg-binding peptides evidently suppressed the activation of the bitter-taste receptor of EGCg (Fig. 5).
The demand for bitterness-masking agents is growing rapidly because of the increased consumption of functional foods (Jędrusek-Golińska et al., 2020). The development of bitterness-masking agents that bind to bitter tastants, such as the EGCg-binding peptides identified in the present study, requires screening for a binding molecule against each compound. Therefore, the screening method for a bitter tastant-binding agent should be rapid and simple. A peptide array is an effective technology used for the rapid epitope analysis of antibodies (Martínez-Botas et al., 2016; Frank, 1992), and also can be applicable for the purpose of screening for bitter tastant-binding (bitterness-masking) peptides.
Acknowledgements This work was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (grant numbers: 18K19753, 18H03195, 19K15792), and the Fuji Foundation for Protein Research. The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- Bae, M.J., Ishii, T., Minoda, K., Kawada, Y., Ichikawa, T., Mori, T., Kamihira, M., and Nakayama, T. (2009). Albumin stabilizes (-)-epigallocatechin gallate in human serum: binding capacity and antioxidant property. Mol. Nutr. Food Res., 53, 709-715.
- Bohin, M.C., Roland, W.S., Gruppen, H., Gouka, R.J., van der Hijden, H.T., Dekker, P., Smit, G., and Vincken, J.P. (2013). Evaluation of the bitter-masking potential of food proteins for EGCG by a cell-based human bitter taste receptor assay and binding studies. J. Agric. Food Chem., 61, 10010-10017.
- Cao, H., Chen, L., and Xiao, J. (2011). Binding citrus flavanones to human serum albumin: effect of structure on affinity. Mol. Biol. Rep., 38, 2257-2262.
- Chandrashekar, J., Mueller, K.L., Hoon, M.A., Adler, E., Feng, L., Guo, W., Zuker, C.S., and Ryba, N.J. (2000). T2Rs function as bitter taste receptors. Cell, 100, 703-711.
- Evans, J.R. (1989). Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia, 78, 9-11.
- Feng, H., Jin H., Gao, Y., Zhu X., Zhao, Q., Liu C., and Xu J. (2019). The Effect of (-)-Epigallocatechin-3-Gallate non-covalent interaction with the glycosylated protein on the emulsion property. Polymers (Basel), 11, 1688.
- Frank, R. (1992). Spot-synthesis: an easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron, 48, 9217-9232.
- Fujimura, Y., Sumida, M., Sugihara, K., Tsukamoto, S., Yamada, K., and Tachibana, H. (2012). Green tea polyphenol EGCG sensing motif on the 67-kDa laminin receptor. PLoS One, 7, e37942.
- Han, Y.C, Chiu, H.F., Ho, Y.T., Venkatakrishnan, K., and Wang, C.K. (2020). Improved bioavailability of EGCG after complexation with royal jelly protein. Food Biochem., 25, e13372.
- Hayashi, N., Chen, R., Hiraoka, M., Ujihara, T., and Ikezaki, H. (2010). Beta-cyclodextrin/surface plasmon resonance detection system for sensing bitter-astringent taste intensity of green tea catechins. J. Agric. Food Chem., 58, 8351-8356.
- Homma, R., Yamashita, H., Funaki, J., Ueda, R., Sakurai, T., Ishimaru, Y., Abe, K., and Asakura, T. (2012). Identification of bitterness-masking compounds from cheese. J. Agric. Food Chem., 60, 4492-4499.
- Imai, K., Ikeda, A., Shimizu, K., and Honda., H. (2019). Selective elimination of bitter peptides by adsorption to heat-treated porous silica gel. Food Sci. Technol. Res., 25, 179-186.
- Ishii, T., Ichikawa, T., Minoda, K., Kusaka, K., Ito, S., Suzuki, Y., Akagawa, M., Mochizuki, K., Goda, T., and Nakayama, T. (2011). Human serum albumin as an antioxidant in the oxidation of (-)-epigallocatechin gallate: participation of reversible covalent binding for interaction and stabilization. Biosci. Biotechnol. Biochem., 75, 100-106.
- Ishii, T., Mori, T., Ichikawa, T., Kaku, M., Kusaka, K., Uekusa, Y., Akagawa, M., Aihara, Y., Furuta, T., Wakimoto, T., Kan, T., and Nakayama, T. (2010). Structural characteristics of green tea catechins for formation of protein carbonyl in human serum albumin. Bioorg. Med. Chem., 18, 4892-4286.
- Ito, K., Hikida, A., Kawai, S., Lan, V. T., Motoyama, T., Kitagawa, S., Yoshikawa, Y., Kato, R., and Kawarasaki, Y. (2013). Analysing the substrate multispecificity of a proton-coupled oligopeptide transporter using a dipeptide library. Nat. Commun., 4, 2502.
- Iwata, S., Kato, T., Yokoyama, A., Hirota, J., and Ogihara, T. (2019). Specified kiwifruit extract blocks increase of body weight and visceral fat in high-fat-diet-fed mice by inhibiting intestinal lipase. Food Sci. Technol. Res., 25, 295-302.
- Jędrusek-Golińska, A., Górecka, D., Buchowski, M., Wieczorowska-Tobis, K., Gramza-Michałowska, A., and Szymandera-Buszka, K. (2020). Recent progress in the use of functional foods for older adults: a narrative review. Compr. Rev. Food Sci. Food Saf., 19, 835-856.
- Kuroda, Y., Ikeda, R., Yamazaki, T., Ito, K., Uda, K., Wakabayashi, K., and Watanabe, T. (2016). Activation of human bitter taste receptors by polymethoxylated flavonoids. Biosci. Biotechnol. Biochem., 80, 2014-2017.
- Lan, V.T., Ito, K., Ohno, M., Motoyama, T., Ito, S., and Kawarasaki, Y. (2015). Analyzing a dipeptide library to identify human dipeptidyl peptidase IV inhibitor. Food Chem., 175, 66-73.
- Legeay, S., Rodier, M., Fillon, L., Faure, S., and Clere, N. (2015). Epigallocatechin gallate: a review of its beneficial properties to prevent metabolic syndrome. Nutrients, 7, 5443-5468.
- Lesschaeve, I., and Noble, A.C. (2005). Polyphenols: factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr., 81, 330-335.
- Lv, S., Zhang, X., Liu, S., Lv, K., Yang, W., and Zhou, Z. (2019). Separation and purification of epigallocatechin gallate and epicatechin gallate by two-step chromatography involving β-cyclodextrin bonded agar. Food Sci. Technol. Res., 25, 187-195.
- Martínez-Botas, J., and de la Hoz, B. (2016). IgE and IgG4 epitope mapping of food allergens with a peptide microarray immunoassay. Meth. Mol. Biol., 1352, 235-249.
- Mori, T., Ishii, T., Akagawa, M., Nakamura, Y., and Nakayama, T. (2010). Covalent binding of tea catechins to protein thiols: the relationship between stability and electrophilic reactivity. Biosci. Biotechnol. Biochem., 74, 2451-2456.
- Narukawa, M., Noga, C., Ueno, Y., Sato, T., Misaka, T., and Watanabe, T. (2011). Evaluation of the bitterness of green tea catechins by a cell-based assay with the human bitter taste receptor hTAS2R39. Biochem. Biophys. Res. Commun., 405, 620-625.
- Ogi, K., Yamashita, H., Terada, T., Homma, R., Shimizu-Ibuka, A., Yoshimura, E., Ishimaru, Y., Abe, K., and Asakura, T. (2015). Long-chain fatty acids elicit a bitterness-masking effect on quinine and other nitrogenous bitter substances by formation of insoluble binary complexes. J. Agric. Food Chem., 63, 8493-8500.
- Oladokun, O., Tarrega, A., James, S., Smart, K., Hort, J., and Cook, D. (2016). The impact of hop bitter acid and polyphenol profiles on the perceived bitterness of beer. Food Chem., 15, 212-220.
- Ozbayram, E.G. (2020). Waste to energy: valorization of spent tea waste by anaerobic digestion. Environ. Technol., 23, 1-7.
- Saeki, K., Hayakawa, S., Nakano, S., Ito, S., Oishi, Y., Suzuki, Y., and Isemura, M. (2018) In vitro and in silico studies of the molecular interactions of epigallocatechin-3-O-gallate (EGCG) with proteins that explain the health benefits of green tea. Molecules, 23, 1295.
- Tachibana, H., Koga, K., Fujimura, Y., and Yamada, K. (2004). A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol., 11, 380-381.
- Terada, Y., Yamashita R., Ihara, N., Yamazaki-Ito, T., Takahashi, Y., Masuda, H., Sakuragawa, S., Ito, S., Ito, K., and Watanabe, T. (2019). Human TRPA1 activation by terpenes derived from the essential oil of daidai, Citrus aurantium L. var. daidai Makino. Biosci. Biotechnol. Biochem., 83, 1721-1728.
- Ueda, T., Ugawa, S., Yamamura, H., Imaizumi, Y., and Shimada, S. (2003). Functional interaction between T2R taste receptors and G-protein alpha subunits expressed in taste receptor cells. J. Neurosci., 23, 7376-7380.
- Xiang, D., Meng, Q., Liu, H., Lan, M., and Wei, G. (2016). The study of a curcumin-resembling molecular probe for the pH-responsive fluorometric assay and application in cell imaging. Talanta, 146, 851-856.
- Yamazaki, T., Narukawa, M., Mochizuki, M., Misaka, T., and Watanabe, T. (2013). Activation of the hTAS2R14 human bitter-taste receptor by (-)-epigallocatechin gallate and (-)-epicatechin gallate. Biosci. Biotechnol. Biochem., 77, 1981-1983.
- Yang, D., Liang, J., Wang, Y., Sun, F., Tao, H., Xu, Q., Zhang, L., Zhang, Z., Ho, C.T., and Wan, X. (2015). Tea waste: an effective and economic substrate for oyster mushroom cultivation. J. Sci. Food Agric., 96, 680-684.
- Zheng, Q., Han, C., Zhong, Y., Wen, R., and Zhong, M. (2017). Effects of dietary supplementation with green tea waste on growth, digestive enzyme and lipid metabolism of juvenile hybrid tilapia, Oreochromis niloticus × O. aureus. Fish Physiol. Biochem., 43, 361-371.





