2021 Volume 27 Issue 2 Pages 293-300
2021 Volume 27 Issue 2 Pages 293-300
In this study, we analyzed the molecular properties and thermal behavior of dried egg white (DEW) proteins to understand the mechanisms underlying DEW gel hardening via dry-heat treatment. Dry-heat-treated DEW proteins displayed a higher surface negative charge and more isopeptide bonds, such as those in lanthionine and lysinoalanine. In addition, secondary structure and surface hydrophobicity measurements suggested that structural changes that occur during heating in solution were suppressed in dry-heat-treated DEW. The size of the protein aggregates did not change during heating in the diluted solution, and was almost the same as that of the gel structure unit.
These results indicated that the structural changes of the protein in solution were restricted due to isopeptide bonds, and that the surface negative charge of the protein caused intermolecular repulsion. Consequently, protein interactions were limited, and the proteins formed a finer and more homogeneous network, which is believed to harden the gel.
Hen egg white from chickens (Gallus gallus domesticus) is capable of thermal gelling and foaming, and these distinctive functional properties make it an essential ingredient in the production of processed foods (Alleoni, 2006; Mine, 2015). Egg white mainly comprises 9.7%–12% (w/w) protein and water (Alleoni, 2006). Total egg white protein typically comprises ovalbumin (OVA, 54%), ovotransferrin (OVT, 12%), ovomucoid (11%), ovomucin (5.4%), ovoglobulin G2/G3 (4%), and lysozyme (LYZ, 3.4%) as well as small amounts of other proteins (Mine, 2015). Thermal gelling of egg white occurs when heat is applied, resulting in the denaturation of protein molecules and their subsequent coagulation and network formation (Handa et al., 1998; Ma and Holme, 1982; Nakamura et al., 1984).
Liquid egg white and dried egg white are products that are extensively used as ingredients in processed foods. Dried egg white is preferred in the processed food industry, because it has a longer shelf life and is more convenient to use than liquid egg white. Furthermore, the heat-induced gel of dried egg white is known to be harder and more elastic than that of liquid egg white prepared with the same protein concentration. These gelling properties have made dried egg white a vital material that contributes to the hardness and elasticity of processed foods, such as Chinese noodles, processed meat products, and surimi products (Han et al., 2019; Hunt et al., 2009; Tachi et al., 2004).
Dried egg white products are produced by first desugaring and then spray-drying liquid egg white; the powdered product is then pasteurized (Bergquist, 1995). Pasteurization is performed by heating the product at 65 °C–80 °C for 3–30 days in a dry state (dry-heat treatment) (Handa et al., 2001). Moreover, this process greatly influences the gel properties of the dried egg white. The dry-heat treatment conditions, i.e., the duration, temperature, and pH, are reported to impact the thermal gelling properties of dried egg white. The number of formed soluble protein aggregates with high molecular weights and the hardness of the thermal gel increase with increasing dry-heat treatment time or increasing pH (Kato et al., 1989; Mine, 1996). Observations of the thermal gel microstructure using scanning electron microscopy (SEM) have demonstrated that heated dried egg white (HDEW) exhibits a finer network than non-heated dried egg white (NDEW) (Ogawa et al., 2003). Furthermore, the average molecular weight of soluble protein aggregates and the physical properties of the gel are highly correlated for all types of dried egg white products (Handa et al., 2001).
As described above, research on the correlation between dried egg white protein characteristics and thermal gelling properties has been conducted. However, differences in conformational changes and interactions of proteins during heating in solution could also affect gel hardening. In this study, we evaluated the structural changes and intermolecular interactions of proteins during heating in solution. Our goal was to elucidate the mechanism underlying thermal gel hardening via dry-heat treatment in dried egg white.
Samples and reagents HDEW and NDEW were provided by Kewpie Egg Corporation (Tokyo, Japan). 8-Anilino-1-naphthalenesulfonic acid magnesium salt (ANS) and L-cysteine were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). 5,5′-Dithio-bis(2-nitrobenzoic acid) (DTNB) was purchased from Dojindo Laboratories, Inc. (Kumamoto, Japan). L-Cysteic acid was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). DL-Lanthionine was purchased from Sigma-Aldrich Co. LLC (St. Louis, USA). Lysinoalanine·2HCl was purchased from Bachem AG (Bubendorf, Switzerland). All other reagents were purchased from Fujifilm Wako Pure Chemical Co. (Osaka, Japan).
Sample preparation and heat treatment Dried egg white was dissolved in deionized water, and the pH was adjusted to 7.0 using 1 M HCl or 1 M NaOH. Next, insoluble proteins were removed via centrifugal separation, and the protein concentration was adjusted based on the values measured using a total nitrogen/total carbon analyzer (Sumigraph NC220-F; Sumika Chemical Analysis Service, Ltd., Osaka, Japan). The following protein concentrations were used: 10% (w/w) for gel hardness measurement, SEM, and electrophoresis samples; 0.02% (w/w) for surface hydrophobicity measurement samples; and 0.01% (w/w) for particle size distribution, zeta potential, and circular dichroic (CD) spectroscopy samples. The thermal gel and CD measurement samples were prepared by heating them at 80 °C for 30 min. For the heat treatment, each dried egg white solution was heated to 25 °C or between 40 °C and 90 °C for 30 min. The heating apparatus used for sample preparation was a water bath for surface hydrophobicity measurements and a thermal cycler (Veriti®; Applied Biosystems, Foster City, USA). All heated samples were cooled to room temperature before being used in their respective experiments. A 0.1 M sodium phosphate buffer solution (pH 7.0) was used for the stable CD measurement.
Gel hardness measurement Measurement of the physical properties of dried egg white gels was performed using a texture analyzer (Tensipresser My Boy II; Taketomo Electric, Tokyo, Japan) and a cylindrical plunger with a 2-mm diameter. The gel hardness was measured using the stress generated at 15% compression.
Polyacrylamide gel electrophoresis (PAGE) The heat-treated suspensions or gels from each temperature were pulverized using a Multi-beads Shocker MB1001C (Yasui Kikai, Osaka, Japan), and subsequently used as samples.
Blue-Native PAGE (BN-PAGE) was conducted according to the methods of Schägger and von Jagow (1991). A NativePAGE Bis-Tris Gel System 3%–12% (Invitrogen, Carlsbad, CA, USA) was used for electrophoresis. The pulverized samples were dispersed in a 50 mM sodium phosphate buffer solution (pH 7.0), and mixed with 200 mM Bis-Tris buffer (pH 7.0) containing 200 mM NaCl, 40% glycerol, and 0.004% Ponceau S. NativeMark Unstained Protein Standard (Invitrogen) was used as the molecular weight marker.
Sodium dodecyl sulfate (SDS)-PAGE was conducted according to the methods of Laemmli (1970). The pulverized samples were dispersed in 50 mM sodium phosphate buffer (pH 7.0) containing 2.0% (w/v) SDS, 3.0 M urea, and 1 mM ethylenedinitrilotetraacetic acid in the presence or absence of 50 mM dithiothreitol (DTT); these were subsequently used as samples for reducing and non-reducing SDS-PAGE, respectively. The sample solutions used for the reducing and non-reducing conditions were mixed with 40 mM Tris-HCl buffer solution (pH 6.8) containing 1.5 mM bromophenol blue, 3% SDS, and 61% glycerol with or without 50 mM DTT, respectively, and were then electrophoresed. XL-Ladder Broad (APRO Science, Tokushima, Japan) was used as the molecular weight marker on a 5%–20% gradient gel (e-PAGEL® E-T520L 5%–20%; Atto Co., Ltd., Tokyo, Japan). The gel dye used for PAGE was One Step CBB (Bio Craft, Tokyo, Japan).
SEM observations Heated sections of approximately 1 × 1 × 5 mm were cut from the center of the dried egg white gels that were prepared by heating at 80 °C for 30 min. After fixing in 2.5% glutaraldehyde, they were washed three times with 0.1 M phosphate buffer (pH 7.3), then sequentially dehydrated in 50%, 70%, 80%, 90%, 95%, 100% (I), and 100% (II) ethanol. The samples were cut in liquid nitrogen, then placed in t-butanol, followed by lyophilization in a lyophilizer (ES-2030; Hitachi, Tokyo, Japan). Next, the samples were coated with osmium using an osmium coater (HPC-1SW; Vacuum Device, Ibaraki, Japan), and observed using SEM (S-4800; Hitachi High Technologies, Tokyo, Japan). Image analysis software (Image-Pro Plus; Media Cybernetics, Rockville, USA) was used to measure the size of the aggregates that made up the gel network structure (gel structural units) from the pictures obtained during SEM observations. For these measurements, the radius of particles was measured at 50 randomly selected locations and averaged.
Particle size distribution and zeta potential measurements The zeta potential and particle size distribution for heat-treated samples were analyzed using a light scattering detector with a 532-nm laser beam (Mobius; Wyatt Technology Co., Santa Barbara, USA).
Surface hydrophobicity measurements The surface hydrophobicity of heat-treated samples was measured according to the methods of Hayakawa et al. (1985). Phosphate buffer (0.1 M, pH 7.0) containing 2 mM ANS was added to the samples, and the fluorescence intensity was measured using a spectrofluorophotometer (RF-5000; Shimadzu, Kyoto, Japan) with an excitation wavelength of 390 nm and an emission wavelength of 470 nm.
Circular dichroism (CD) spectroscopy The CD spectra of unheated samples and samples that were heated to 80 °C were measured using a CD spectrometer (J-720 circular dichroism spectrometer, JASCO, Tokyo, Japan) using 1-mm cells at 25 °C in the far ultraviolet region. The data are represented in terms of mean residue ellipticity.
Quantification of thiol groups and isopeptide bonds Dried egg whites were used for the quantification of thiol groups and isopeptide bonds. Free thiol groups were quantified according to the methods of Rombouts et al. (2016) using DTNB. Samples were suspended in 50 mM pH 7.0 sodium phosphate buffer containing 2.0% (w/v) SDS, 3.0 M urea, and 1 mM ethylenedinitrilotetraacetic acid, and then 1.0 mg/mL DTNB solution was added and allowed to react. The absorbance of the reaction mixture was measured at 412 nm.
The number of total thiol groups (the sum of free thiols and thiols forming disulfide bonds) was determined by oxidizing the samples in performic acid, then acid-hydrolyzing them in 6 M HCl. The total amount of thiol groups was measured as cysteic acid using a liquid chromatography/quadrupole-time-of-flight mass spectrometry (LC/Q-TOF) system (LC, 1260 Infinity; Q-TOF, 6530 Accurate-Mass Q-TOF LC/MS; Agilent Technologies, Santa Clara, USA). The analysis column was an Intrada Amino Acid column (100 × 2 mm, 3 µm; Imtakt, Kyoto, Japan). The column temperature was 35 °C, the mobile phases for the gradient method were acetonitrile containing 0.1% (v/v) formic acid (solution A) and 100 mM ammonium formate (solution B), and the flow rate was 0.4 mL/min. The detection of compounds was performed using electrospray ionization in negative-ion mode. The dry gas temperature was 300 °C, and the flow rate was 6 L/min. The fragmenter voltage was set to 175 V.
Isopeptide bond analysis was conducted according to the methods of Friedman (1999). Samples were acid-hydrolyzed in 6 M HCl at 110 °C for 24 h, and then used for analysis. LC/Q-TOF analysis was conducted in positive-ion mode using the ionization method, and all other conditions were the same as those used for the measurements of the total thiol groups.
Statistical analysis All experiments were performed at least in triplicate, and the measured values are expressed as means ± standard deviations. The statistical analysis software used to test for significant differences was Statcel 4 (OMS Publishing Inc., Tokyo, Japan). A student's t-test was used to test for differences between two groups.
Thermal gelling of dried egg white Gelation was found to begin at temperatures between 70 °C–75 °C in NDEW and 55 °C–60 °C in HDEW (Fig. 1A), indicating that dry-heat treatment lowered the temperature at which gelation started. The gel hardness increased in a temperature-dependent manner. The peak hardness value of HDEW was five times greater than that of NDEW, which is consistent with previous studies (Kato et al., 1989; Mine, 1996). Additionally, visual observation of the changes due to heating in solution (Fig. 1B) revealed the rapid development of opacity in NDEW at 60 °C; in contrast, opacity developed gradually at 55 °C–75 °C in HDEW, which remained more transparent than NDEW at all heating temperatures.
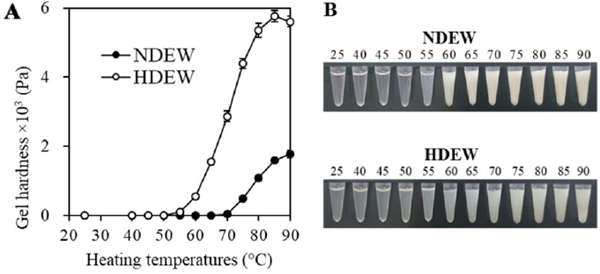
(A) Changes in the gel hardness of dried egg white solutions of NDEW (filled circles) and HDEW (open circles) at different temperatures. (B) Changes in the appearance of dried egg white solutions of NDEW (upper) and HDEW (lower). The numbers above the pictures indicate the heating temperatures (°C).
Gel microstructure The thermal gels observed via SEM revealed that NDEW was composed of large protein particles that formed a coarse, random network (Fig. 2A, C), while HDEW comprised small gel structural units that formed a dense and heterogeneous network (Fig. 2B, D). The average radius of the gel structural units in NDEW was 223.6 ± 36.7 nm. The average radius of the HDEW gel structural units (18.2 ± 2.6 nm) was significantly smaller (p < 0.01), suggesting the suppression of protein aggregation in HDEW.

Scanning electron microscope images of NDEW (A, C) and HDEW (B, D) gels. A and B are at 10 000 × magnification; C and D are at 50 000 × magnification. The white line represents the scale at different temperatures.
Thermal aggregation behavior First, the migration behavior of the unheated (25 °C) sample was observed (Fig. 3A–F). BN-PAGE showed OVA and OVT bands in the NDEW samples. In contrast, the HDEW samples had spread-out OVA and OVT bands, with putative protein polymers above the OVT band, smearing in the high molecular weight region, and bands at the top of the gel. This showed that dry-heat treatment caused the formation of various protein aggregates, which has been previously reported (Kato et al., 1989; Handa et al., 2001). The OVT band for HDEW in non-reducing SDS-PAGE was particularly thin, and there were aggregate bands in the polymer region and the top of the gel. However, the OVT band strength was recovered in reducing SDS-PAGE, and the bands at the top of the gel disappeared, but the smeared band in the polymer region remained. It suggests that, in HDEW, the aggregates are formed through disulfide bonds and other covalent bonds. Additionally, in both non-reducing and reducing SDS-PAGE, more bands with high mobility were seen in the OVA molecular weight range in HDEW samples than in NDEW samples. A possible reason for this increase in SDS-PAGE band mobility is that dry-heat treatment promotes the formation of covalent bonds other than disulfide bonds within OVA molecules, making the molecules more compact.
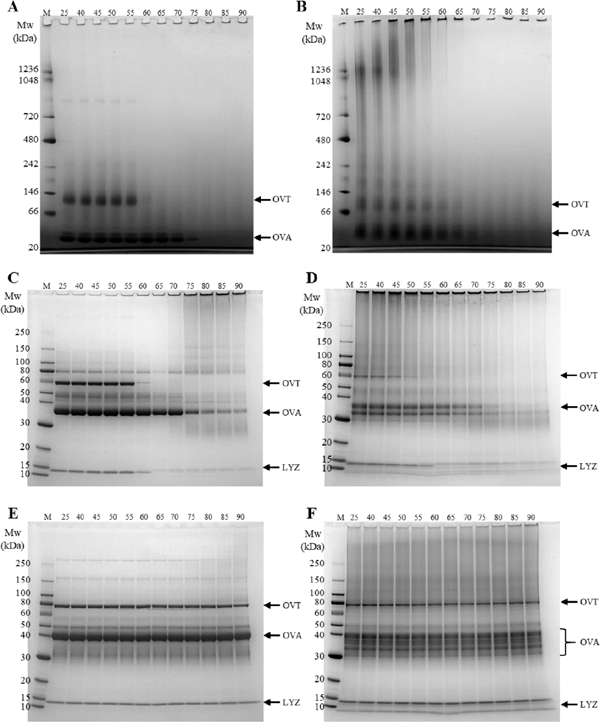
PAGE analysis of NDEW (A, C, E) and HDEW (B, D, F) solutions at different heating temperatures. A, B: Blue-Native PAGE; C, D: non-reducing SDS-PAGE; E, F: reducing SDS-PAGE. The numbers above the graph indicate the heating temperature (°C). M: molecular weight marker. OVT, OVA, and LYZ show ovotransferrin, ovalbumin, and lysozyme, respectively.
Next, the migration behavior of the samples heated at 40 °C–90 °C was observed. The BN-PAGE results showed that the NDEW OVT band suddenly disappeared at 60 °C, while most of the OVA bands disappeared at 75 °C (Fig. 3A, B). No increase in the polymer region bands was observed accompanying these changes, but this is thought to be because the generated aggregates were composed of molecules too large to penetrate the gel. In contrast, the HDEW OVT band gradually became fainter as the temperature increased from 40 °C, and the polymer region smear band showed increased polymerization.
Non-reducing SDS-PAGE showed similar trends to BN-PAGE for NDEW. In addition, most of the OVA band disappeared at 75 °C; this change was accompanied by the appearance of a polymer-region smear band, and the majority of the LYZ band suddenly disappeared at 65 °C (Fig. 3C, D).
Starting at 40 °C, the HDEW OVT band gradually grew fainter as the temperature increased, and it disappeared at 55 °C, which is lower than the temperature at which the band disappeared for NDEW. The LYZ band of HDEW also grew fainter at temperatures lower than 55 °C. We observed that the previously mentioned highly mobile OVA band did not fade as easily as ordinary OVA bands. A reason for this may be that the covalent bonds in the OVA molecule suppress its unfolding. Additionally, the smear bands in the polymer region gradually faded as the heating temperature increased, suggesting that covalent bonds formed between large molecules that were unable to penetrate the gel.
Reducing SDS-PAGE (Fig. 3E, F) revealed no difference between the band patterns of the heated samples and the unheated samples (25 °C) for NDEW and HDEW, indicating that disulfide bonds contribute to protein network formation for both NDEW and HDEW.
Mean particle size Unheated (25 °C) HDEW samples had a smaller particle size when compared to those of NDEW (Fig. 4A). This result is inconsistent with the observation that protein aggregates were found in only HDEW samples (Fig. 3B). This contradiction is thought to be due to weak interactions in the NDEW proteins, causing the formation of aggregates larger than those in the HDEW solution, and the pretreatment for BN-PAGE broke apart those weak interactions.
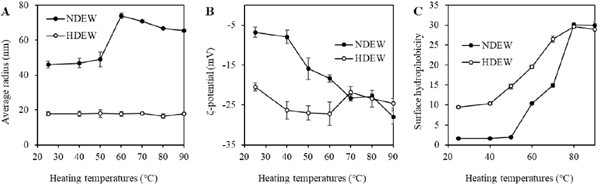
Thermal changes in mean particle size (A), zeta potential (B), and surface hydrophobicity (C) of NDEW (filled circles) and HDEW (open circles) proteins.
When NDEW was heated, the particle size drastically increased at 60 °C, and was slightly smaller at 70 °C and above. This is thought to be due to denatured OVA suppressing OVT aggregation at temperatures of 70 °C and above, causing the OVT aggregates to become smaller (Matsudomi et al., 2002). In contrast, for HDEW, no increase in the mean particle size was seen even with the heating of HDEW solutions. Consequently, HDEW proteins may be more resistant to aggregation than NDEW proteins. In the previously described PAGE experiments, protein aggregates were observed in the heated HDEW solutions (Fig. 3B, D), but this is thought to be due to the high protein concentration (10%).
The mean radius of NDEW particles that were heated to 80 °C for 30 min was 66.8 nm (Fig. 4A); the previously described SEM observations indicated that the mean radius of the structural units of the gel heated at 80 °C for 30 min was 234 nm, which is 3.5 times larger. The difference in protein concentration (0.1% versus. 10%) may account for this. Interestingly, however, the mean particle radius of HDEW heated at 80 °C for 30 min was 16.5 nm, and the SEM observations indicated that the mean radius of the gel structural units of the thermal gel was 18.2 nm, suggesting that the protein-soluble aggregates in HDEW become the gel structural units.
Surface charge In the unheated (25 °C) samples, HDEW proteins had a higher negative surface charge than the NDEW proteins (Fig. 4B). This suggested that dry-heat treatment caused protein molecules to unfold, exposing the buried acidic amino acid side chains to the surface. Even in purified OVA, the dry-heat treatment causes an increase in the surface negative charge (Ma et al., 2019).
When the solutions were heated, the surface charge of NDEW proteins increased at 40 °C and above. In contrast, no marked changes in the surface charge of heated solutions were observed for HDEW. These results suggested that strong intermolecular repulsive forces among protein molecules suppressed further aggregation when HDEW proteins were heated in solution (Fig. 4B).
Surface hydrophobicity In the unheated (25 °C) samples, HDEW had higher surface hydrophobicity than did NDEW (Fig. 4C), which is consistent with previous research (Kato et al., 1989; Mine, 1997). Furthermore, up to 80 °C, HDEW had higher surface hydrophobicity than did NDEW. The surface hydrophobicity of NDEW increased starting at 50 °C, and HDEW surface hydrophobicity began increasing from a lower temperature. The initial gelling temperature of the 10% protein solution was lower for HDEW than for NDEW (Fig. 1A). In non-reducing SDS-PAGE, the temperature at which OVT and LYZ began decreasing was also lower in HDEW than in NDEW (Fig. 3). This is thought to be caused by HDEW having more hydrophobic regions on the protein molecule surface, which facilitate the interaction of protein molecules. The increase in surface hydrophobicity due to rising temperature was gradual and small in HDEW when compared to that for NDEW. Furthermore, when heated above 60 °C, the NDEW proteins aggregated via surface hydrophobic interactions, consuming hydrophobic regions, which seemed to lower the surface hydrophobicity (Fig. 4C). These results suggest that the increase in surface hydrophobicity of the proteins during heating in solution is suppressed by dry-heat treatment.
Secondary structure The spectrum for the unheated NDEW sample showed negative peaks at 208 and 222 nm, whereas the spectrum for the unheated HDEW showed a negative peak at 214 nm (Fig. 5). Negative peaks at 208 and 222 nm have been reported to be derived from α-helix structures, and the negative peak at 214 nm has been reported to be derived from β-sheet structures (Zargorski, 1999). Thus, the secondary structure of HDEW has fewer α-helices and more β-sheets than that of NDEW.

Molar ellipticity of unheated (solid line) and heated at 80°C (dotted line) for NDEW (black line) and HDEW (gray line) solutions.
The spectrum of the heated NDEW solution showed a marked decrease in the characteristic α-helix peak intensity (Fig. 5), and the peak derived from β-sheets was not observed. This suggests that heating in solution causes the proteins to unfold, creating a structure with many random coils. In contrast, while heating the HDEW solution caused the absolute intensity of the spectrum to decrease when compared to that of the unheated solution, the peak at the characteristic β-sheet wavelength was maintained (Fig. 5). These results suggest that for sample solutions heated at 80 °C, the secondary structure of HDEW remained more intact than that of NDEW. These results are consistent with our findings that changes in the surface charge (Fig. 4B) and surface hydrophobicity (Fig. 4C) were small, suggesting that protein conformation changes due to heating in solution were suppressed. One factor behind this is believed to be the abundance of β-sheets in HDEW protein molecules. β-Sheets are more thermally stable than α-helices (Vijayakumar et al., 1993).
Quantification of thiol groups and isopeptide bonds When egg white and other proteins are heated, they form disulfide bonds and heat-irreversible isopeptide bonds, such as those in lanthionine (LAN) and lysinoalanine (LAL), as interand intra-molecular crosslinks (Friedman, 1999; Rombouts et al., 2016; Suda et al., 2010). Thus, we quantified the LAN, LAL, and thiol groups in dried egg white proteins (Table 1). HDEW had significantly (p < 0.01) fewer free thiol groups than did NDEW. The number of total thiols (free thiols and those forming disulfide bonds) was not significantly (p < 0.05) different between the HDEW and NDEW groups. HDEW had more LAN and LAL than did NDEW. LAN and LAL cannot be broken by reduction, suggesting that LAN and LAL contributed to the polymerization of protein molecules that was observed in the reductive SDS-PAGE of HDEW. Additionally, in general, LAN and LAL are generated due to β-elimination reactions of cysteine or serine residues followed by binding to cysteine and lysine, respectively (Friedman, 1999).
| NDEW | HDEW | ||
|---|---|---|---|
| Thiol groups | Free SH | 57.8 ± 1.0 | 51.1 ± 0.7 ** |
| Total SH | 190.0 ± 9.7 | 181.4 ± 5.7 | |
| Isopeptide bonds | LAN | 0.23 ± 0.02 | 1.11 ± 0.12 ** |
| LAL | 0.17 ± 0.04 | 3.90 ± 0.67 ** |
LAN, lanothionine; LAL, lysinoalanine; SH, thiol groups.
The LAL content was higher than the LAN content in HDEW. Recently, we found that more LAN was formed than LAL during heat-induced gelation of liquid egg white (Koyama et al., 2020). Thus, the formation rates for LAN and LAL seem to differ depending on the egg white status and the heating conditions.
Gel hardening mechanism Based on the results of this study, we propose a model for the thermal gel hardening of dried egg white due to dry-heat treatment. In NDEW, multiple hydrophobic regions are exposed at the protein surface during thermal gelling as the protein molecules unfold. Also, intermolecular repulsive forces are small. Thus, random aggregation of protein molecules occurs, resulting in the formation of large particles as the gel structural units, and the creation of a rough gel network, which is thought to make the gel softer. In HDEW, however, dry-heat treatment led to the formation of intra- and intermolecular heat-irreversible isopeptide bonds in the protein molecules. These bonds are thought to limit the unfolding of protein molecules, suppressing the exposure of hydrophobic regions and thiol groups at the protein surface. Furthermore, the surfaces of the protein molecules have a highly negative charge, which leads to a greater intermolecular repulsive force. Consequently, we propose that the bonding areas in the protein molecules are limited, creating a dense and homogenous gel network with small particles as the gel structural units that facilitate gel hardening.
Here, we elucidated one aspect of the hardening mechanism of the gel of dried egg white subjected to dry-heat treatment. Our results showed that the surface of dried egg white protein molecules had an increased negative charge due to the dry-heat treatment. Also, the formation of intra- and intermolecular heat-irreversible isopeptide bonds, i.e., those in LAN and LAL, was promoted by the dry-heat treatment.
Additionally, changes in surface hydrophobicity and the secondary structure of the protein molecules were suppressed when heating in solution. Also, the protein surfaces maintained a negative charge during heating in solution. As a result, intermolecular protein interactions were limited during thermal gelling, which caused the formation of a denser and more homogeneous network that is thought to have hardened the gel. In the future, we plan to elucidate the main proteins that contribute to the formation of soluble protein aggregates during dry-heat treatment of dried egg whites. Also, we will identify which amino acids from which proteins form isopeptide bonds. The mechanism responsible for the excellent gelation properties of dried egg white is expected to provide useful information for the development of egg white products with novel gelling properties.