2021 Volume 27 Issue 3 Pages 441-452
2021 Volume 27 Issue 3 Pages 441-452
A novel peptide PYPYEPYEPYPY from Yak bone hydrolysate (YBG) was purified by Sephadex G-25, RP-HPLC and identified by ESI-TOF-MS. The peptide exhibited some antioxidant activity in HepG2 cells and the dipeptidyl peptidase IV (DPP-IV) inhibition activities in vivo. In HepG2 cells, when the concentration of peptide was 20–160 µM, it could increase the half-lethal rate (LD50) of cells and showed cytoprotective effect, while at 20–40 µM reduced the reactive oxygen species (ROS) content. The peptide PYPYEPYEPYPY exhibited the highest DPP-IV inhibition activities with IC50 value of 189.4 µM among the nine peptides from YBG. Molecular docking predicted the interaction of peptide PYPYEPYEPYPY with DPP-IV residues TRP62, THR304 and SER462, and the binding between them mainly depends on van der Waals forces and hydrogen bonds. Glucose tolerance tests were performed on C57 mice orally administered synthesized PYPYEPYEPYPY. The maximal decrement in the blood glucose was 10.28 ± 0.47 mmol/L induced by one-time gavage of PYPYEPYEPYPY at 100 mg/kg. This is the first report that both DPP-IV inhibitory activity and antioxidant activity have been identified from yak bone hydrolysate.
Industrial processing of Yak produces with tons of its by-products which are normally utilized for production of low-value products, including fertilizers, animal feed, pet food, etc (Yang et al., 2010). Therefore, alternative utilization of Yak by-products for high-value products not only reduces resource waste but also provides the potential value-added products for the market. Yak bone, the main components of Yak by-products, can serve as promising sources of bioactive peptides (Toldra et al., 2016). Bioactive peptides are composed of 2–20 kinds of amino acids, which have a variety of physiological regulating functions (Gómez-Guillén et al., 2011). The past decade has witnessed a growing number of studies encompassing bioactive peptides with antihypertensive, antioxidant, antidiabetic, bone health-promoting effects (Fu et al., 2019). Li et al. (2009) founded the emulsification and foaming properties of Yak bone hydrolysate. Cao et al. (2020) isolated a hypotensive peptide from Yak bone. Ye et al. (2019) identified a new osteoblast-proliferating peptide from Yak bone, the sequence was GPSGPAGKDGRIGQPG.
Furthermore, more and more evidence shows bioactive peptides are unstable in vivo (Yu et al., 2017). Bioactive peptide sequences can be released from their precursors during in vivo through pepsin and trypsin (Fu et al., 2017). The whole process mainly occurs in the gastrointestinal tract of the animal. Therefore, it is also very important to evaluate the active peptides in vivo. There are many animal models for evaluating biologically active peptides. Among them, the mice model is widely used because of its fast, practical and stable characteristics (Hur et al., 2011; Maestri et al., 2019). However, there are few reports on the functional activity of Yak bone peptide in vivo. Therefore, the purpose of this study was to: (a) identify the peptides in Yak bone hydrolysate by using gel chromatography and reversed-phase high performance liquid chromatography (RP-HPLC) (b) determine the binding mechanism between peptide and DPP-IV through molecular docking simulation (c) verify the blood glucose effects in vivo using C57 mice. The present work contributes to the development of peptides from Yak by-products as functional food ingredients and provides some future directions and strategies to overcome these challenges.
Materials Yak bone was obtained from the Anhui Guotai Biotechnology CO., LTD. The papain, pepsin, pancreatin, 2′, 7′-dichlorofluorescein-diacetate (DCFH-DA) and DPP-IV enzyme were obtained from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS), Dulbecco's modified Eagle's medium (DMEM), penicillin/streptomycin (P/S) and trypsin solution were purchased from the Sangon Biotech Co., Ltd (Shanghai, China). The remainders of reagents were of analytical grade.
Preparation and molecular weight determination of Yak bone peptide The frozen bone was grinded into Yak bone powder, which was provided by the Anhui Guotai Biotechnology CO., LTD. Yak bone was dissolved in 30 volume distilled water, then mixed with papain (3500 U/g protein) and hydrolyzed at 60 °C for 8 h. Finally, the enzyme was boiled for 10 min to inactivate the enzyme, it was filtered by vacuum filter paper and lyophilized (Fan et al., 2006). The molecular weight distribution of Yak bone peptide was determined by gel permeation chromatography (Martinsen et al., 1991).
Purification of peptides from Yak bone
Ultrafiltration and Chromatography using Sephadex G-25 The hydrolysates were ultra-filtered through 5,000 Da molecular weight cut off according to the molecular weight test of Yak bone hydrolysate (Wang et al., 2016). The lyophilized samples were dissolved in distilled water to reach a concentration of 20 mg/mL, and 100 µL loaded on gel filtration column (1.0 × 60 cm; Solarbio Corp, Beijing, China) at room temperature. The distilled water was used as the eluent. Before loading, the samples were filtered through a 0.22 µm membrane. The UV absorbance of the effluent was set at 220 nm using an HD-6 spectrophotometer (Hu Xi, Shanghai, China). Four fractions (G1, G2, G3 and G4) were collected (Gu et al., 2015).
Purification of peptides by RP-HPLC The most active fractions were further purified using RP-HPLC (HPLC 1260, Agilent Technologies, Karlsruhe, Germany). The collection conditions of the fractions were: a CAPCELLPAK C18 AQ S-5 (10 × 150 mm, Shiseido, Tokyo, Japan); 100 µL injection volume; elution buffer A was water + 0.1% trifluoroacetic acid (TFA); elution buffer B was acetonitrile + 0.1% TFA. The content of solvent A was decreased from 100% to 95% in the first 10 min, decreased from 95% to 81% within 10–20 min, from 81% to 10% within 20–29 min and increased from 10% to 100% in the 29–40 min time period. 1 mL/min flow rate; and 218 nm detection wavelength (Pan et al., 2016).
Identification and synthesis of peptides Peptides from the RP-HPLC purification were identified by ESI-TOF-MS as described by Dang et al. (2019). These peptides were synthesized by the Sangon Biotech Co., Ltd (Shanghai, China) and identified by RP-HPLC and mass spectrometry (Dang et al., 2015). The purity of these peptides was above 99%.
Antioxidant activity assay of peptides
Determinations of antioxidant capacity by DPPH assay The DPPH radical scavenging activity was slightly modified according to the method of Hao et al. (2020). Let the reactants mix well and keep away from light at room temperature for 30 min.
Determination of ABTS free radical scavenging activity The antioxidant activity of the peptides was determined using ABTS, as previously described with slight modifications (Craciunescu et al., 2012). ABTS solution mixed with potassium persulfate in dark for 14 h. The OD was read at 734 nm using Microplate Reader.
Effect of peptide on HepG2 cell intracellular antioxidant
Cell culture HepG2 cells were purchased from the Ming Zhou Biotechnology Co., Ltd (Ningbo, China). The cells were cultured in DMEM medium (10% FBS; 1% P/S), and maintained in incubator containing 5% carbon dioxide at 37 °C.
Cytotoxicity experiments The cytotoxicity of the peptide was evaluated according to Zhang et al. (2016). The cells were cultured in DMEM medium at a density of 2 × 104 cells/well. The cells were cultured with different concentrations of peptide PYPYEPYEPYPY (10, 30, 100, 200, 300, and 600 µM) for 24 h, with each concentration was repeated five times. After 24 h of incubation, 10 µL CCK-8 was added to each hole, then returned to incubator and incubated for 2 h at 37 °C. In addition, the H2O2 concentration of the cell's half-lethal rate (LD50) was also determined within 4 h.
Cell protection of peptides The cells were cultured with peptides of different concentrations (20, 40, 80, 160 µM) for 24 h, these concentrations of peptides have been experimentally proven to be non-toxic to cells. Subsequently, the old medium was removed, and the new medium containing 800 µM H2O2 (LD50 concentration) was added for 4 h (Liang et al., 2019). A 40 µM GSH solution was used as the positive control.
Determination of intracellular ROS level The decrease of fluorescence from dichlorofluorescein (DCF) was used to study the cellular antioxidant activity (Liang et al., 2019). Cells were seeded in 96 well plates of DMEM at the density of 2 × 104 cells/well, and cultured for 6 h. Then, the old medium was removed and the new medium containing peptide was added for 24 h at cultured conditions. Afterwards, cells were incubated with 1% H2O2 in DMEM for 4 h. After incubation with 10 µL DCFH-DA for 30 min, the cells were washed twice with PBS, and fluorescence emission at 525 nm was determined with excitation at 488 nm using a microplate reader at 37 °C. Positive control (cells treated only with GSH) and negative control with untreated cells (only DMEM) were included.
Determination of DPP-IV inhibitory activity The DPP-IV inhibitory activity assay was performed as described by Lacroix and Li (2012) with slight modification. Briefly, the sample was diluted 4 times with buffer solution, 25 µL sample was mixed with 50 µL enzyme, incubated at 37 °C for 10 min, and then added 25 µL of 1 mM of Gly-Pro-p-nitroanilide and was measured with Microplate Reader.
The blood glucose Effect of DPP-IV Inhibitory Peptide in Animals Experiment The blood glucose changes on C57 mice were measured by oral glucose tolerance test (OGTT) to determine the hypoglycemic effect of PYPYEPYEPYPY in vivo. The mice were fasted for 18 h before OGTT. Twenty female mice (6 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (China). Each C57 mice were regularly received feed and tap water at a temperature of 25 ± 4 °C and a humidity of 40 ± 6%. C57 mice were randomly divided into four groups after a week of adaptation, with 6 mice in each group for experiment. Respectively, the synthetic peptide PYPYEPYEPYPY, sitaglipin and metformin were dissolved in distilled water as the sample group (100 mg/kg BW) and positive control group (100 mg/kg BW). 0.9% saline was used as the negative control group. Within 0, 15, 30, 60, and 120 min after treatment, the effect of DPP-IV inhibitors on C57 mice was measured by Glutest sensors (Sanwa Kagaku Kenkyusho Co., Ltd., Nagoya, Japan) at 37 °C (Hiroshi U et al., 2012).
In silico gastrointestinal digestion of peptide and digestive stability of simulated gastrointestinal tract The enzyme action module in bioactive peptides database (BIOPEP http://www.uwm.edu.pl/biochemia/biopep/start_biopep.php) was used to simulate the digestion of Yak bone peptides. After inputting the peptides sequence in the database and selecting the protease to simulate the digestion, the peptides could be obtained after the mimic enzyme digestion. These results were based on the specificity of the restriction sites. Some other databases, such as Peptide cutter (https://web.expasy.org/peptide _cutter/) can also be used to simulate the digestion of peptides.
In vitro gastrointestinal digestion was carried out according to the method of Minekus et al. (2014) with slight modifications. Peptides solution with a concentration of 1.0 mg/mL was mixed with simulated gastric fluid and incubated at 37 °C for 2 h, and then mixed with simulated intestinal fluid and incubated for 2 h at 37 °C. Finally, the peptides were quickly frozen in liquid nitrogen and then lyophilized. SIF (simulated intestinal fluid) and SGF (simulated gastric fluid) electrolyte stock solutions, an 800 U/mL pancreatin stock solution based on trypsin activity and a 25, 000 U/mL pepsin stock solution based on pepsin activity.
The method for evaluating the stability of peptide after simulated digestion was based on the Hao et al. (2020). Agilent 1260 HPLC system (Santa Clara, CA, USA) was used to evaluate the peptide stability after simulated digestion. The wavelength was monitored at 215 nm. The gradient elution solvent system consisted of mobile phase A (pure water with 1% TFA) and mobile phase B (ACN with 0.1% TFA). The content of solvent A was decreased from 100% to 95% in the first 10 min, decreased from 95% to 81% within 10–20 min, from 81% to 10% during the 20–29 min period and increased from 10% to 100% in the 29–40 min period. The flow rate was 1 mL/min, and the injection volume was 100 µL. The stability% = peak area after digestion/peak area before digestion.
Molecular Docking The functional peptide PYPYEPYEPYPY was molecularly docked by Discovery Studio 3.5 (DS, version 2.1, Neo Trident Technology LTD). According to the method of Sila et al. with some modifications (Sila et al., 2016). The crystal structure of the porcine dipeptidyl peptidase IV in complex (PDB code: 2AJBA) was optimized, water molecules and ligands were removed before molecular docking, and Zn2+ and Cl− were added to the DPP-IV model to optimize the structure of DPP-IV. The active site of molecular docking was carried out with coordinates X: 43.249, Y: 33.469, Z: 42.325 and the docking radius was 15 Å.
Statistical analysis One-way analysis of variance was used for data analysis, and then SPSS 13.0 was used to determine the least significant difference. A P value of less than 0.05 was considered statistically significant.
Molecular weight distribution of Yak bone peptides Molecular weight is an important parameter reflecting the degree of protein hydrolysis, and it is closely related to the biological activity after hydrolysis (Li et al., 2008). The molecular weight of Yak bone peptide was mainly less than 5 000 Da, 80.38% peptides was less than 2 000 Da (Table 1). It showed that Yak bone peptides had basically small molecular weight. They were further purified by Sephadex G-25.
| Molecular weight range (Da) | Peak area percentage (%) |
|---|---|
| > 5 000 | 3.19 |
| 5 000–3 000 | 7.21 |
| 3 000–2 000 | 9.22 |
| 2 000–1 000 | 19.80 |
| 1 000–500 | 27.07 |
| 500–180 | 24.36 |
| < 180 | 9.15 |
Purification of the peptides by Sephadex G-25 and RP-HPLC According to Fig. 1A, four fractions of Yak bone peptide were separated from Sephadex G-25 columns. The functions were determined after concentrating and freeze-drying. Based on our previous functional screening of Yak bone peptides before purification, the antioxidant and DPP-IV inhibitory activities were prominent. Results showed their activities of the fraction G2 was the highest, so it was chose for further purification, and then separated into eleven fractions (F1–F11) using RP-HPLC (Fig. 1B). The eleven fractions were separated by RP-HPLC system, and the antioxidant and DPP-IV inhibitory activities were determined (Table 2). Among them, fraction 4 has the highest antioxidant activity, with an inhibitory activity of 82.3 ± 2.30%. Fraction 10 has the highest hypoglycemic inhibitory activity, with an inhibitory activity of 32.2 ± 0.37%. So the two fractions were collected for the further identification.
| Fractions | DPPH (%) | DPP-IV (%) |
|---|---|---|
| F1 | 25.6 ± 0.29 | 24.8 ± 0.68 |
| F2 | 18.5 ± 0.16 | 23.0 ± 0.23 |
| F3 | 36.4 ± 1.61 | −3.8 ± 0.09 |
| F4 | 82.3 ± 2.30 | 20.0 ± 0.13 |
| F5 | 34.9 ± 1.12 | 1.7 ± 0.06 |
| F6 | 31.4 ± 0.61 | 23.1 ± 0.56 |
| F7 | 29.9 ± 0.39 | 14.1 ± 0.43 |
| F8 | 32.0 ± 1.23 | 2.1 ± 0.12 |
| F9 | 32.7 ± 1.32 | 13.8 ± 0.18 |
| F10 | 42.6 ± 1.03 | 32.2 ± 0.37 |
| F11 | 41.6 ± 1.93 | −0.3 ± 0.03 |

Purification of peptides with Antioxidant and DPP-IV inhibitory activity from YBG using multi-column chromatography.
Note: A: Sephadex G-25 column chromatography separation diagram of Yak bone hydrolysate. B: Separation of the peptides from the most active fraction G2 using the RP-HPLC system.
Identification of the peptide by ESI-TOF-MS The peptides from the RP-HPLC fractions F4 and F10 were identified by ESI-TOF-MS. Results showed the sequences of nine peptides were identified, peptide 7 and peptide 9 were from the fraction F4, and the rest were from the fraction F10. The amino acid sequences were as followings: peptide 1: Ile-Gly-Ile-Gly-Ile-Ser-Gly (IGIGISG); peptide 2: Gly-Leu-Leu-Gly-Leu-Gly-Ser (GLLGLGS); peptide 3: Ala-Gly-Pro-Ala-Gly-Pro-Lys (AGPAGPK); peptide 4: Asp-Phe-Pro-Phe- Pro-Leu-Pro-Lys (DFPFPLPK); peptide 5: Val-Gly-Pro-Ala-Gly-Pro-Asn-Gly-Phe (VGPAGPNGF); peptide 6: Gly-Pro-Ala-Gly-Pro-Ile-Gly-Pro-Val-Gly-Ala (GPAGPIGPVGA); peptide 7: Pro-Tyr-Pro-Tyr-Glu-Pro-Tyr-Glu-Pro-Tyr-Pro-Tyr (PYPYEPYEPYPY); peptide 8: Gly-Pro-Ala-Gly-Pro-Pro-Gly-Pro-Ile-Gly-Asn-Val (GPAGPPGPIGNV) and peptide 9: Thr-Gly-Pro-Ala-Gly-Pro-Ala-Gly-Pro-Ile-Gly-Pro-Val-Gly (TGPAGPAGPIGPVG). From above all, eight of the nine peptides were firstly identified except peptide 8, which was newly reported by Ye et al. (2020). The mass spectrogram of the peptide PYPYEPYEPYPY was listed as the representatives in Fig. 2. The injection concentration of our gel chromatography column was 200 mg/mL and the injection volume was 1 mL. According to the ratio of gel chromatographic peak G2 area to total peak area, and the ratio of RP-HPLC peak F4 area to total peak area, the peptide was roughly calculated the output of PYPYEPYEPYPY was 0.16%. Since the main purpose was to obtain a new functional peptide, we have roughly estimated the yield of peptide PYPYEPYEPYPY. In the future research, we will do it accurately.

Novel peptide PYPYEPYEPYPY from YBG identified by ESI-TOF-MS
Determination of ABTS and DPPH free radical scavenging activity ABTS and DPPH free radical scavenging ability is a common method to evaluate the antioxidant capacity of samples (Yoshiki et al., 2001). Among the nine identified peptides, PYPYEPYEPYPY showed the strongest activity that it was almost the same rate of ABTS free radical scavenging as GSH at the concentration of 1.0 mg/mL (Fig. 3). According to the research of Chen et al., peptides containing amino acid fragments such as Tyr, Lys, His, Pro and Met exhibited strong antioxidant activities. Generally, Phe, Trp and Tyr could provide hydrogen atoms for free radicals to achieve the elimination of free radicals effect (Chen et al., 1998). Therefore, PYPYEPYEPYPY had the highest antioxidant activity.

DPPH and ABTS free radical scavenging activity of the PYPYEPYEPYPY peptide. Different lowercase letters represent significant differences in free radical scavenging activities of peptide DPPH (p < 0.05), and different uppercase letters represent significant differences in free radical scavenging activities of peptide ABTS (p < 0.05)
Cytoprotective and cellular antioxidant effects The effect of sample on cell viability plays an important role in cytotoxicity experiments, which usually verifies whether the sample promotes cell growth (Wang et al., 2015). The cytotoxicity of different concentrations of peptide on HepG2 cells was shown in Fig. 4A. When the concentration added from 10 to 200 µM, this proliferation-promoting effect increased from 95.9% to 106.2%, the peptide PYPYEPYEPYPY could promoted the growth of HepG2 cells. This may be due to the peptide PYPYEPYEPYPY plays a role similar to growth factors (Ravallec-Plé et al., 2001). However, the concentration was 600 µM (p < 0.05), which showed slightly cytotoxic, and the proliferation rate was 92.9%. This may be due to the cells exceeding the optimal growth concentration and produced slight toxicity. Therefore, the optimal concentration was selected for further cell experiments (1–200 µM).

Effect of peptides on HepG2 cells.
Note: A: Cytotoxicity of peptide with concentration of 10–600 µM on cells. B: Protective effect of peptide on damaged cells. C: Effect of peptide on intracellular ROS content. D: Fluorescence images of HepG-2 cells. Different letters indicate significant differences in peptide activity (p < 0.05).
As shown in Figure 4B, the peptide has a protective effect on HepG2 cell oxidative damage induced by H2O2 in the range of 20–160 µM. GSH is a common antioxidant in cells, usually as a positive control. The results showed that the protective effect of 40 µM peptide was nearly half higher than that of 40 µM GSH. In addition, the peptide has protective effect on cells at the concentration of 20–160 µM (p < 0.05). Others have also studied the cytoprotective effects of peptides from different sources on damaged cells. For example, the peptides isolated from pine nuts and whey hydrolyzed protein have a cytoprotective effect, preventing H2O2 damage to HepG2 cells. Their peptides were Gln-Asp-His-Cys-His and Val-His-Leu-Lys-Pro (Kong et al., 2012; Liang and Zhang 2017).
The content of ROS in the cell is one of the important indicators for determining the antioxidant of the sample. The change of ROS content is usually due to the fact that DCFH-DA is digested by esterase on the cell membrane into DCFH. DCFH can freely pass through the cell membrane and specifically bind to cellular ROS, and after binding, it produces fluorescence. Determine the change of ROS content by measuring the change of fluorescence intensity (Fan et al., 2020). The ROS content produced by the peptide in the cell was shown in Fig. 4C and Fig. 4D. When the peptide concentration was 20–40 µM, the ROS content continuously decreases, and when the concentration was 160 µM, the ROS content was the highest, possibly because the high concentrations of peptide lead to the decrease of receptor binding. However, the content of ROS at all concentrations was lower than the H2O2 injury group. The experimental results showed that the peptide could effectively protect HepG2 cells from free radical damage.
Hypoglycemic Effects of PYPYEPYEPYPY The hypoglycemic effect of bioactive peptides in vivo is relatively more important than in vitro. Some chemically synthesized drugs have a strong effect of hypoglycemic in vitro, but their effects in vivo will be weakened (Fu et al., 2011). In order to measure the hypoglycemic effect of the peptide PYPYEPYEPYPY in vivo, the hypoglycemic effect was determined by the blood glucose changes on C57 mice within 2 h after oral administration. The blood glucose of the negative control group (0.9% saline solution) had no obvious change after oral administration for 2 h (Fig. 5). The final blood glucose of C57 mice was 6.5 ± 0.26 mmol/L. However, the sample group (peptide, 100 mg/kg) and the positive control group (sitaglipin and metformin, 100 mg/kg) significantly reduced the blood glucose of C57 mice (Fig. 5). The peptide had remained a significant hypoglycemic effect within 1 h of oral administration. The maximum hypoglycemic activity occurred at the 1th hour after oral administration, and the blood glucose was 10.28 ± 0.47 mmol/L. It was worth noting that PYPYEPYEPYPY at 100 mg/kg was amount to the maximum blood glucose lowering effect faster than sitaglipin at 100 mg/kg (1 h), which indicated that PYPYEPYEPYPY had better effect than sitaglipin in vivo. In addition, when oral administration dose was 100 mg/kg, the maximum decrease in blood glucose of the sample group and positive control group were 10.27 and 9.33 mmol/L, respectively, indicating that the hypoglycemic ability of peptide and sitaglipin in vitro and in vivo was not completely proportional. Remarkably, the hypoglycemic effect of PYPYEPYEPYPY 100 mg/kg was the same to the positive control (metformin, 100 mg/kg), suggesting that PYPYEPYEPYPY was a potential peptide for the prevention and treatment of hypoglycemic.

Effects of the saline solution, control group (sitaglipin and metformin, 100 mg/kg) and sample group (peptide, 100 mg/kg) on blood glucose of C57 mice
In silico gastrointestinal digestion of peptide and digestive stability of simulated gastrointestinal tract Active peptides can be digested and most peptides may change their functional activities after passing through the gastrointestinal tract. It is important to evaluate their stability or their bioaccessibility using the simulation of gastrointestinal digestion. In this sense, an in silico simulation of gastrointestinal digestion was performed, using the hydrolysis tool of the BIOPEP program. The results showed that the peptide PYPYEPYEPYPY was digested into PY-PY-EPY-EPY-PY. Therefore, if the peptide was not protected from the gastrointestinal environment, they would be hydrolyzed by digestive proteases, and their bioactivity modified. Studies have shown that the sequence, molecular weight and hydrophobicity of peptides will affect the gastrointestinal digestive stability (Bo and Ningning 2018). Peptides rich in Pro and Glu are generally more tolerant to the degradation of gastrointestinal digestive enzymes (Maestri et al., 2019).
The computer-simulated enzyme digestion results showed that the peptide was not stable, so we conducted a simulated digestion of the peptide in vitro to study its stability. The results showed that the stability of the peptide PYPYEPYEPYPY after digestion was 58.8%. This peptide PYPYEPYEPYPY was identified by ESI-TOF-MS, and the peptide was digested into peptides PYPYEPY, PYEPY, PY (Fig. 6A–F), it was mainly consistent with the in silico simulation. According to Lan et al. (2015) reported that the peptide PY has DPP-IV inhibitory activity. Furthermore, it has been demonstrated that DPP-IV could preferably cleave Pro, Ala and Ser in protein sequence as the second N-terminal residue (Davy et al., 2000). Peptides including Pro and Ala may have the potential to be DPP-IV inhibitors. Therefore, the high proportion of Pro in peptide PYPYEPYEPYPY may be the reason for DPP-IV inhibitory activities (Gomez-Guillen et al., 2011). However, the activities of peptides PYPYEPY, PYEPY, PY were not as good as peptide PYPYEPYEPYPY. The analysis may be due to the synergy between these three peptides. We will conduct further research later.
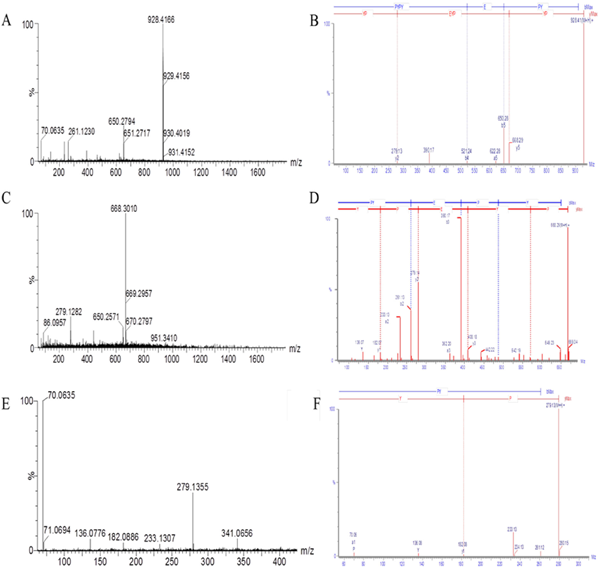
Molecular mass and amino acid sequence of the peptides from the sample of PYPYEPYEPYPY after in vitro gastrointestinal digestion.
Note: A: MS spectrum of the peptide PYPYEPY. B: MS/MS spectrum of the peptide PYPYEPY.
C: MS spectrum of the peptide PYEPY. D: MS/MS spectrum of the peptide PYEPY. E: MS spectrum of the peptide PY. F: MS/MS spectrum of the peptide PY.
Molecular docking simulation Molecular docking is a theoretical simulation method that mainly studies the interaction between molecules (such as ligands and receptors) and predicts its binding mode. The CDOCKER module simulated the molecular docking of peptide PYPYEPYEPYPY and DPP-IV to explore the potential DPP-IV inhibitory effect of peptide. First of all, our molecular docking model was based on the crystal structure of the porcine dipeptidyl peptidase IV, According to the method of Sila et al. (2016). Because most studies that have investigated the in vitro effect of synthetic molecules or compounds of natural origin on DPP-IV have been conducted using the human or porcine enzyme (Lacroix and Li-Chan, 2015). DPP-IV from both species has been widely used to gain knowledge on the enzymes' catalytic and binding properties and to screen compounds for their inhibitory activity. In addition, comparison of the amino acid sequences of porcine and human DPP-IV revealed 92% identity within the catalytic center and an overall identity of 88% between the two species (Wang L et al., 2019). However, human, porcine and mice are mammals, the enzyme sequence is highly conserved among mammalian species. Therefore, the overall identity of porcine and mice should be similar. Additional investigations are needed to identify the similarity of the binding pockets between pigs and mice.
Through docking simulation, Fig. 7A shows the best posture for docking peptide PYPYEPYEPYPY at the active site of DPP-IV. The mutual binding energy was 73.757 kJ/mol. The docking structure Fig. 7B shows that the binding of peptide PYPYEPYEPYPY to the amino acid residues of DPP-IV mainly depended on van der Waals forces and hydrogen bonds. The peptide exists in the narrow channel of the DPP-IV active site and forms 8 hydrogen bonds with DPP-IV. This indicates that the peptide binds tightly to the active site of DPP-IV and helps to enhance its inhibitory activity (Chaudhary et al., 2009). Specifically, the peptide established hydrogen bonds with DPP-IV residues TRP62, THR304 and SER462. Similar to the peptide PYPYEPYEPYPY, the hypoglycemic drug sitaglipin was also found to have the same binding sites at THR304 and SER462, indicating that these amino acids may play an important role in DPP-IV binding (Wu et al., 2016). The active center of DPP-IV is composed of zinc ions and three main active capsules (S1, S2 and S3), so it is Zn (II) and active capsules that matter in molecular docking simulation. Rawendra et al observed that IVRDPNGMGAW with high DPP-IV inhibitory activity cannot interfere with Zn (II) of DPP-IV (Rawendra et al., 2013). However, more studies have shown that active peptides not only interact with the DPP-IV S1 and S2 capsules, but also directly interact with the DPP-IV Zn (II) (Jimsheena and Gowda, 2010). This result may offer an explanation why PYPYEPYEPYPY exhibited high DPP-IV inhibitory activity.

The binding mode of peptide PYPYEPYEPYPY and the active site of DPP-IV.
Note: A: The optimal docking structure diagram of the peptide binding with DPP-IV. B: The 2D diagram of the interaction between the peptide and DPP-IV amino acid residues.
In this study, the novel peptide PYPYEPYEPYPY was identified with antioxidant and hypoglycemic functions from the Yak bone hydrolysate. It could increase the half-lethal rate of cells and showed cytoprotective effect in HepG2 cells. It also reduced blood glucose levels in vivo. Molecular docking predicted the interaction of peptide PYPYEPYEPYPY with DPP-IV residues, and the binding between them mainly depends on van der Waals forces and hydrogen bonds. Therefore, Yak bone peptide shows great potentials to be applied both in food and cosmetic industries.
Acknowledgements This work was supported by the National Natural Science Foundation of China (31771945), the Science Technology Department of Zhejiang Province (2020C02035, 2018C02051), the Key Research and Development Program of Ningxia (2019BDE02002), the Modern Agricultural Technical System Foundation (CARS4225), the Ningbo Natural Science Foundation (2018A610338), the Ningbo Science Technology Department of China (2019C10065).