2021 年 27 巻 3 号 p. 473-481
2021 年 27 巻 3 号 p. 473-481
The current study aimed to investigate the apoptotic and anti-proliferative effect of essential oils obtained from turmeric (Curcuma longa L.) at cellular level. To test the effects and mechanism, H1299 and HepG2 cells were selected for in vitro experiments. Cytotoxicity assay and morphology of cells analyses were performed to estimate the effect of turmeric essential oil (TEO) on the viability and cell growth. Proapoptotic and anti-proliferative effects of TEO were investigated using flow cytometry. Subsequent experiments assessed cell migration and colony formation of cells to evaluate the anti-metastatic effects of TEO by in vitro wound-healing assays and colony formation assays. The results indicated that treatment with more than 50 mg/L TEO for 48 h could significantly inhibit the proliferation of H1299 and HepG2 cells and could significantly inhibit cell migration and colony formation of cells (p < 0.05).
Cancer is a multifactorial syndrome characterized by uncontrolled cellular growth, local tissue invasion, and distant metastasis of abnormal cells, which has been one of the most alarming diseases throughout the world (Dan, Jin et al., 2019). The combination of radiotherapy and chemotherapy was used as the traditional method for the cure of cancer disease. However, these treatments have a strong adverse effect on the human body (Bagher, Farhood et al., 2018). Nowadays, natural products, including an assortment of nutrients, non-nutrients, and bioactive food components, for cancer prevention and cure have attracted broad attention due to their relatively less toxic and ingestive nature (Rad et al.).
Turmeric (Curcuma longa L.) belongs to genus Curcuma in family Zingiberaceae, is an aromatic medicinal herb (Kundu 2005). Turmeric distributes throughout the tropical and subtropical regions in the world, and widely cultivates in Asian countries, especially in China, India and Japan. In Chinese clinical practice, turmeric has been widely prescribed such as antioxidative, antimicrobial, anti-inflammatory, anticancer antiangiogenic, cardiovascular diseases (Lee 2009, Kuttan et al. 2011, Bagher et al. 2018, Marliyana et al., 2019). In recent years, research related to curcumin obtained from turmeric had been widely performed, including evaluation of its effect on inhibiting gastric cancer, liver cancer, lung cancer, colon cancer, cervical cancer and lymphoma (Ramadasan et al., 2018; Soleimani et al., 2018).
However, there are few studies involved the evaluation of turmeric essential oils. As is well known, lipophicity of essential oil enables them to pass through the cell membrane and reach the cell interior, indicating the potential functional activity of TEO in anti-cancer effect (Alikhani-Koupaei 2014; Ben Hsouna et al., 2017). Besides, the effects of TEO on anti-inflammatory and antioxidant activities had been investigated in our previous study (Lu and Mou 2018). The results showed that TEO has certain scavenging ability of DPPH• and O2−. And TEO could significantly inhibit the degree of mice ear and toe swelling and reduce the content of PGE2 in serum, indicating TEO showed remarkable antioxidant and anti-inflammatory properties. As oxidative stress plays an important role in the pathogenesis of several chronic ailments such as cancer (Ebrahimi et al., 2020). Besides, inflammation is an adaptive host defense mechanism against infection or injury and may lead to various ailments including cancer (Lin et al., 2018). Thus, TEO was speculated to have the potential in intervention of cancer.
Liver and Lung cancer are two of the most common malignancies in the world, and its high incidence and recurrence rate are the leading causes of cancer death. Uncontrolled mitosis and rapid migration and migration of cancer cells are hallmarks of cancer. Thus, targeting inhibition of cell proliferation or promoting apoptosis can provide clues to cancer treatment (Xiao et al., 2006; Rayaprolu, Hettiarachchy et al., 2013). In this study, the apoptotic and anti-proliferative effect of TEO was investigated on HepG2 and H1299 cells. Cytotoxicity assay, cell morphological analyses and flow cytometry assay were performed to estimate the effect of TEO on the viability and the apoptotic-inducing effects. Wound-healing assays and colony formation assays were performed to investigate the anti-metastatic function of TEO. Collectively, our results provide insights into the mechanism of action of TEO and demonstrate its potential for developing it as an anticancer agent.
Chemicals Turmeric powder was collected in Lanzhou (Gansu, China). Human liver cancer cells (HepG2) and Human lung cancer cell (H1299) were purchased from American Type Culture Collection (Manassas, VA). DMEM/F-12 medium, RPMI 1640 medium and fetal bovine serum (FBS) were purchased from Sigma Aldrich (Taufkirchen, Germany). Acridine orange (AO) staining kits were purchased from Beyotime (Nantong, China) and Annexin V- FITC/PI Apoptosis detection kits were from Yisheng Biotechnology Co., Ltd. (Shanghai, China). The other laboratory chemicals were of analytical grade.
Preparation and characterization of TEO TEO used in the study was prepared according to our previous method (Lu and Mou 2017). Briefly, turmeric powder was extracted with mixed liquor including 18% ionic liquid [BMIM]PF6, 1.4% cellulase and water at 50 °C for 90 min. The ratio of liquid to material was 10:1. Then, the supernatant was evaporated using a volatile oil extractor for 15 min. Finally, TEO was obtained and stored at 4 °C until further use.
Gas chromatography–mass spectrometer (Agilent 6890 N/5973I) coupled with electrospray ionization was used for the characterization of TEO. Helium gas with a flow rate of 1.0 mL/min was used as a carrier, and 1 µL of sample dissolved in ether was injected for analysis and split ratio was 5:1. The oven temperature was initially maintained at 50 °C for 2 min, then gradually increased at the rate of 15 °C/min to 128 °C for 2 min, at the rate of 1 °C/min to 168 °C for 3 min and finally increased at the rate of 15 °C/min to 259 °C for 2 min. The mass spectrometer electrospray ion source operated at 70 eV, and the ion source temperature was 230 °C, quadrupole temperature was 150 °C, solvent delay time 3 min, scanning quality range 45–550 u. Mass spectral correlations were performed using the NIST05.1 and NIST05-ILB database.
Cell culture HepG2 were cultured using DMEM/F-12 medium supplemented with 10% FBS, 0.1% penicillin and streptomycin. And H1299 cells were using RPMI medium 1640 supplemented with 10% FBS, 0.1% penicillin and streptomycin. The cells were maintained at 37 °C under a humidified atmosphere of 5% CO2. TEO was dissolved in cosolvent DMSO prior to cellular processing. 5-fluorouracil is a common anticancer drug and is often used in the treatment of liver and lung cancer. In this study, 5-fluorouracil was used as positive control (PC).
Cytotoxicity assay MTT, which works on the principle of reduction of (3-(4, 5- dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) by metabolically active cells with the help of dehydrogenase enzymes, is one of the most commonly used techniques to determine the cell viability. In this study, In vitro cytotoxic effects of TEO were evaluated by MTT assay. HepG2 and H1299 cells were seeded in 96-well plate at a density of 5×104 cells per well and grown overnight. Then cells were treated with different concentrations of TEO (25, 50, 100 and 200 mg/L) for 24, 48 and 72 h. Cells treated without any drugs were used as normal control (NC), cells treated with 50 mg/L 5-fluorouracil were used as positive control (PC).
Morphology of cells analyses Morphology of HepG2 and H1299 cells changes were analyzed using fluorescence microscope (Nikon Eclipse Ti) and acridine orange (AO) staining to estimate the effect of TEO on the viability and cell growth. HepG2 and H1299 cells were seeded in 6-well plates at a density of 5 × 105 cells per well and allowed to attach for overnight. Then the cells were treated with 50 and 150 mg/L of TEO, 50 mg/L 5-fluorouracil (PC) or without any drugs (NC) for 48 h, respectively. After incubation, cells were stained with 40 µL of AO (30 mg/L) and incubated in dark for 10 min. Brightfield images of cells were acquired with 100 × objective on an optical microscope equipped with a digital camera.
Cell apoptosis analysis Cell apoptosis assay was conducted using an Annexin V- FITC/PI Apoptosis detection kit and the apoptotic rate was analyzed by flow cytometry (Accuri C6, BD Biosciences). HepG2 and H1299 cells were seeded in 6-well plates at a density of 5 × 105 cells per well and incubated with 50 and 150 mg/L of TEO, 50 mg/L 5-fluorouracil (PC) or without any drugs (NC) for 48 h, respectively. A certain number of cells were then collected and resuspended in 195 µL Annexin V-FITC binding buffer, followed by the addition of 5 µL of annexin v-FITC and 10 µL of PI to mix. Finally, the samples were incubated for 30 min at room temperature in the dark and assayed by flow cytometry.
Wound–healing assays and colony formation assays To further study the anti-metastatic effects of TEO on HepG2 and H1299 cells, wound–healing assays and colony formation assays were conducted. In wound–healing assays, the cells were scratched with a micropipette tip when cells reached 90% monolayer confluent, followed by washing with PBS to remove dead and detached cells. HepG2 cells were subsequently treated with serum-free medium contain 50 and 150 mg/L of TEO, 50 mg/L 5-fluorouracil (PC) or without any drugs (NC) for 12h, and H1299 cells were treated for 24h because different cells have different growth cycles. The extent of space filling, representing the cell migration, was evaluated using an inverted microscope (EVOS fl, Digital microscopy group, USA). The size of cell-free wounds was then measured using Leica QW in image analysis software (Leica Microsystems Inc., Buffalo Grove, Illinois), and the migratory capacity of cells was calculated as (scratch width of the treatment group-scratch width of the control group)/scratch width of the control group × 100(Raineri et al., 2019).
In colony formation assay, 1 000 cells/well were first seeded in 6-well plates for 24 h. Subsequently, attached cells were treated with various concentrations of TEO (50, 100 and 150 mg/L), 50 mg/L 5-fluorouracil (PC) or without any drugs (NC) for 48h. Then medium was aspirated, cells were washed twice with PBS, and treated with fresh serum-free medium without any test sample/drugs. After 10 days incubation, the cells were fixed with 4% paraformaldehyde and stained with 0.2% crystal violet for 30 min at room temperature and photographed with a camera (Fu and Lin 2018).
Statistical analysis Data are presented as mean ± SD. One-way ANOVA was performed using SPSS software to test the differences between groups. Statistical significance is denoted as *p < 0.05 (SPSS version 20.0, Statistical Package for the Social Sciences Software, SPSS Inc., Chicago, IL, USA).
Characterization of TEO In this study, gas chromatography–mass spectrometer was used for the characterization of TEO. As shown in Table 1, 53 compounds were identified in TEO, totally. Most of the compounds are derived from mevalonic acid pathway biogenetically (i.e., mono and sesquiterpenoids). There were 25 kinds of sesquiterpenoids and 10 kinds of monoterpenoids, and the major components of essential oil were sesquiterpenoid, accounting for 84.57% in total. The main constituents in TEO were ar-turmerone (39.37%), curlone (15.02%), 1-(1,5-dimethyl-4-hexen-1-yl)-4-methyl-benzene (9.12%) and turmerone (5.99%) whereas cyclohexene, 3-(1,5-dimethyl-4-hexenyl)-6-methylene-, [S-(R*,S*)]-, 2-ethyl-1,4-dimethylbenzene, and ethyl-tetramethylcyclopentadiene were the minor. Similar results obtained by Flavio Dias Ferreira et al. demonstrated ar-turmerone was one of the main components in essential oil from turmeric. Modern pharmacological studies have demonstrated that ar-turmerone possesses various pharmacological activities such as immunomodulatory, antinociceptive, anti-tumor, and anti-inflammatory properties. Sen et al. have found that ar-turmerone could suppress cell proliferative ability and attenuated inflammatory cytokine expression in HaCaT cells. Mingjie et al. (Ji, Choi et al., 2004) have found that ar-turmerone could promote apoptosis of K562, L1210, U937 and RBL-2H3 cancer cells. Thus, it can be speculated that the apoptotic and anti-proliferative effect of TEO on HepG2 and H1299 cells might be due to ar-turmerone.
| Serial number | Identified compounds | Molecular formula | Relative (%) |
|---|---|---|---|
| 1 | urea, N,N-dimethyl- | C3H8N2O | 0.19 |
| 2 | 2-butenoic acid, 3-methyl-, methylester | C6H10O2 | 0.20 |
| 3 | cyclopentene, 3-ethenyl- | C7H10 | 0.71 |
| 4 | 5-benzaldehyde, 3-hydroxy-, oxime (9CI) | C7H7NO2 | 0.83 |
| 5 | 3,4-dimethyl-O-phenylenediamine | C8H12N2 | 0.10 |
| 6 | 2-hydroxy-5-methylbenzaldehyde | C8H8O2 | 1.20 |
| 7 | 1,2,3-trimethyl-benzene | C9H12 | 0.39 |
| 8 | α-bromomesitylene | C9H11Br | 0.20 |
| 9 | benzenamine, 2-(1-methylethyl)- | C9H13N | 0.18 |
| 10 | 2-cyclohexen-1-one,3,4,4-trimethyl- | C9H14O | 0.28 |
| 11 | 4-isopropylaniline | C9H13N | 0.30 |
| 12 | 3′-methylacetophenone | C9H10O | 0.04 |
| 13 | 4-acetyl-1-methyl-1-cyclohexene | C9H14O | 0.01 |
| 14 | terpinen-4-ol | C10H18O | 0.01 |
| 15 | α-terpineol | C10H18O | 0.03 |
| 16 | 5-isopropyl-2-methylphenol | C10H14O | 0.03 |
| 17 | 2,4,6-octatriene, 2,6-dimethyl- | C10H16 | 0.13 |
| 18 | benzene, 2-ethyl-1,4-dimethyl- | C10H14 | 2.58 |
| 19 | 3-cyclohexadiene, 2-methyl-5-(1-methylethyl)-1 | C10H16 | 0.10 |
| 20 | spiro[4.5]decane | C10H18 | 0.24 |
| 21 | 2-cyclohexen-1-ol,1-methyl-4-(1-methylethenyl)-, (1R,4R)-rel- | C10H16O | 0.13 |
| 22 | p-isopropyltoluene | C10H14 | 0.12 |
| 23 | benzene, 1-methyl-3-(1-methylethyl)- | C10H14 | 0.26 |
| 24 | ethyl-tetramethylcyclopentadiene | C11H18 | 2.27 |
| 25 | cyclohexene, 2-ethenyl-1,3,3-trimethyl | C11H18 | 0.35 |
| 26 | 2-undecanone | C11H22O | 0.02 |
| 27 | 1,3-cyclohexadiene, 5-(1,5-dimethyl-4-hexenyl)-2-methyl-, [S-(R*,S*)]- | C15H24 | 1.35 |
| 28 | α-cedrene | C15H24 | 0.35 |
| 29 | 1,3,6,10-dodecatetraene,3,7,11-trimethyl-, (3Z,6E)- | C15H24 | 0.67 |
| 30 | l-caryophyllene | C15H24 | 0.18 |
| 31 | 6,10-dodecadien-1-yn-3-ol,3,7,11-trimethyl- | C15H24O | 0.61 |
| 32 | 1H-benzocycloheptene,2,4a,5,6,7,8-hexahydro-3,5,5,9-tetramethyl-, (4aR)- | C15H24 | 0.14 |
| 33 | 1,6,10-dodecatriene,7,11-dimethyl-3-methylene-, (6E)- | C15H24 | 0.14 |
| 34 | azulene,1,2,3,3a,4,5,6,7-octahydro-1,4-dimethyl-7-(1-methylethenyl)-, (1R,3aR,4R,7R) | C15H24 | 0.10 |
| 35 | tricyclo[5.4.0.02,8]undec-9-ene,2,6,6,9-tetramethyl-, (1R,2S,7R,8R) | C15H24 | 0.14 |
| 36 | benzene,1-(1,5-dimethyl-4-hexen-1-yl)-4-methyl- | C15H22 | 9.12 |
| 37 | cyclohexene,6-ethenyl-6-methyl-1-(1-methylethyl)-3-(1-methylethylidene)-, (6S) | C15H24 | 0.16 |
| 38 | 2,4,4,6-tetramethyl-6-phenyl-1-heptene | C15H24 | 0.62 |
| 39 | β-bisabolene | C15H24 | 1.34 |
| 40 | (Z,Z)-α-farnesene | C15H24 | 0.41 |
| 41 | 1h-3a,7-methanoazulene,2,3,4,7,8,8a-hexahydro-3,6,8,8-tetramethyl-, (3R,3aR,7R,8aS)- | C15H24 | 0.21 |
| 42 | 3-methyl-2-butenoic acid, 2,7-dimethyloct-7-en-5-yn-4-yl ester | C15H22O2 | 0.41 |
| 43 | 2-penten-1-ol,5-[(1R,3R,6S)-2,3-dimethyltricyclo[2.2.1.02,6]hept-3-yl]-2-methyl-, (2E)- | C15H24O | 0.28 |
| 44 | eudesma-3,7(11)-diene | C15H24 | 1.89 |
| 45 | ar-tumerone | C15H20O | 39.37 |
| 46 | tumerone | C15H20O | 5.99 |
| 47 | β-curcumene | C15H22 | 0.72 |
| 48 | curlone | C15H22O | 15.02 |
| 49 | tricyclo[3.2.2.0(2,4)]non-8-ene-6- carboxylic acid, 7-[(phenylamino)carbonyl]- | C15H24 | 0.05 |
| 50 | cyclohexene, 3-(1,5-dimethyl-4-hexenyl)-6-methylene-, [S-(R*,S*)]- | C15H24 | 4.95 |
| 51 | 7-epi-cis-sesquisabinene hydrate | C15H26O | 0.26 |
| 52 | 23-(nonylphenoxy)-3,6,9,12,15,18,21-Heptaoxatricosan-1-ol | C16H34O9 | 0.11 |
| 53 | methyl stearidonate | C19H30O | 0.16 |
Furthermore, preliminary exploration about the comparation of anti-proliferative effect of ar-turmerone and TEO on HepG2 and H1299 cells were conducted by using MTT, since studies (Gill et al., 2002) have shown that essential oils, as a whole, exhibit higher activity than their major compounds individually. As shown in Fig. S1, cells treated with 50 mg/L TEO showed almost the same growth inhibition capacity as 50 mg/L ar-turmerone of both HepG2 and H1299 cells, suggesting that minor compounds in TEO might have an important additive or synergistic role. Therefore, additional studies on the components of TEO will be necessary to elucidate its anticancer action in our future studies.

Comparation of anti-proliferative effect of TEO and ar-turmerone on HepG2 (A) and H1299 cells (B). HepG2 and H1299 cells were seeded in 96-well plate at a density of 5×104 cells per well and grown overnight. Then cells treated with 50 mg/L TEO and 50 mg/L ar-tumerone for 24, 48 and 72 h. The viability of HepG2 and H1299 cells were evaluated by MTT assay.
Effect of TEO on HepG2 and H1299 cells viability Cytotoxicity of TEO to HepG2 and H1299 cells was measured using MTT assay. As shown in Fig. 1A, the viability of both HepG2 and H1299 cells was inhibited by TEO in a dose-dependent and duration-dependent manner. At 72 h of incubation, low concentrations of TEO significantly inhibited cell proliferation, whereas high concentrations were required for significant cell growth inhibition at 24 h. The IC50 value of TEO for HepG2 cells were 91.4 mg/L for 24 h, 59.0 mg/L for 48 h, 30 mg/L for 72 h, respectively. The IC50 value of TEO for H1299 cells were 200 mg/L for 24 h, 95.1 mg/L for 48 h, 50.0 mg/L for 72 h, respectively. To study the cytotoxicity of essential oils, an IC50 value which less than 50 mg/L represents a strong cytotoxic activity. In addition, IC50 values between 50–100, 100–200 and 200–300 mg/L represent moderate, weak and very weak cytotoxicity, respectively. With this in mind, the IC50 value of TEO in this study was 30 mg/L for HepG2 cells and 50.0 mg/L for H1299 cells when treated for 72 h, indicating strong cytotoxicity against cancer cells.

TEO inhibited cells viability and induced morphology changes of HepG2 and H1299 Cells. The viability of HepG2 and H1299 cells were evaluated by MTT assay (A), in which cells were treated with different concentrations of TEO (25, 50, 100 and 200 mg/L), 50 mg/L 5-fluorouracil (PC) and without any drugs (NC) for 24, 48 and 72 h. Morphology changes of HepG2 and H1299 Cells were analyzed using fluorescence microscope (B), in which cells were treated with TEO (50 and 150 mg/L), 50 mg/L 5-fluorouracil (PC) and without any drug (NC) for 48h. Brightfield images of HepG2 and H1299 cells were then acquired with 100× objective on an optical microscope equipped with a digital camera. The values are the mean ± SD and similar results were obtained in at least 3 independent experiments *p < 0.05 vs. group NC.
Effect of TEO on morphology of HepG2 and H1299 cells After treated with 50, 150 mg/L TEO, 50 mg/L 5-fluorouracil or without any drugs for 48 h, the cells were observed for morphology changes using AO staining. As shown in Fig. 1B, the fluorescent microscopic observations of HepG2 and H1299 cells revealed that treatment with TEO was associated with various cytological features, such as shrinkage of the cell, membrane blebbing, cell membrane disruption, condensation of nuclear chromatin and formation of apoptotic bodies. These features are hallmark biochemical makers of apoptotic cell death. Moreover, observations using fluorescence microscopy suggested that after being treated with TEO for 48 h, approximately 59.03 ± 4.15% of the HepG2 cells and 48.67 ± 7.13% of the H1299 cells had an apoptotic morphotype. And same inhibition effect can be achieved under groups treated by 50 mg/L 5-fluorouracil and 150 mg/L TEO. These cytological changes clearly indicated that the cells treated with TEO were committed to specific modes of cell death, apoptosis and necrosis.
Effect of TEO on apoptosis of HepG2 and H1299 cells The effect of TEO on inducing programmed apoptosis of HepG2 and H1299 cancer cell was assessed by flow cytometry. Annexin V (AV) is a phospholipid binding protein that can selectively bind phosphatidylserine and is one of the sensitive indexes of early apoptosis. Propidium iodide (PI) can stain dead cells' DNA and cells that have lost the integrity of their membranes at a late stage of apoptosis. According to the specificity of these two dyes, cancer cells treated with TEO resulted in four types of cell population, namely viable cells (AV-/PI-), early apoptotic (AV+/PI-), late apoptosis (AV+/PI+) and necrotic cells (AV-/ PI+). As shown in Fig. 2, apoptosis rate was significantly increased after H1299 and HepG2 cells were treated with 50 mg/L TEO by the contrast of the 0 mg/L group. And as the concentration increases, the number of cell apoptosis increases in a concentration-dependent manner. Especially for HepG2 cells, the number of viable cells decreased to 56.2% after the treatment of 150 mg/L TEO whereas the early and late apoptotic cells rose to 11.6% and 32.2%, respectively. These observations confirmed that cytotoxic effect caused by TEO on H1299 and HepG2 cells was due to induction of apoptosis (Sahai et al., 2020).

TEO promoted HepG2 (A) and H1299 (B) cells apoptosis in a dose-dependent manner. The apoptosis of HepG2 and H1299 cells treated without any drugs (NC), 50 and 150 mg/L TEO, 50 mg/L 5-fluorouracil (PC) for 48 h was detected and quantified by flow cytometry.
Effect of TEO on migration and colony formation of HepG2 and H1299 cells Tissue invasion and metastasis are vital pathway in the escape and dissemination of tumor cells from the primary site into distant organs. Aberrant regulation of cell migration contributes to the progression of many diseases, including cancer cell invasion and metastasis. The ability of a cancer cell to migrate and invade enables it to change position within tissues and break free from the primary tumor, thus spreading the disease. In this study, the migratory capacity of HepG2 and H1299 cells was tested with a wound-healing assay. As shown in Fig. 3, significant inhibition of wound-healing was observed when HepG2 and H1299 cells treated with more than 50 mg/L TEO (p < 0.05). The wound-healing rates of the HepG2 cells treated with 50, 150 mg/L TEO and 50 mg/L 5-fluorouracil for 12 h were 58.8 ± 1.0%, 23.5 ± 1.0% and 44.1 ± 2.6%, respectively. Due to different growth rate for different cells, H1299 cells were cultured for 24 h, and the wound-healing rates treated with 50, 150 mg/L TEO and 50 mg/L 5-fluorouracil for 24 h were 44.8 ± 1.8%, 10.3 ± 2.0% and 65.5 ± 0.9%, respectively. These results demonstrated that treatment with TEO induced an inhibition of migratory of HepG2 and H1299 cells in a concentration-dependent and duration-dependent manner, suggesting an inhibitory effect on metastasis.

TEO inhibits the migratory of HepG2 (A) and H1299 (B) cells. Cells were treated without any drugs (NC), 50 and 150 mg/L TEO, 50 mg/L 5-fluorouracil (PC) and artificial scratches were done with sterile pipette. Photographs were taken after treatment.
Effect of TEO on the colony formation of HepG2 and H1299 cells were performed to further confirm the anti-proliferative effect of TEO. As shown in Fig. 4, compared with group NC, colony formation of cancer cells had been significantly inhibited by treatment of 50, 100, 150 mg/L of TEO. Almost the same inhibition can be reached when the cells treated with 150 mg/L TEO and 50 mg/L 5-fluorouracil. The results of colony formation assay suggested that TEO can significantly obstructed colonization of HepG2 and H1299 cells.
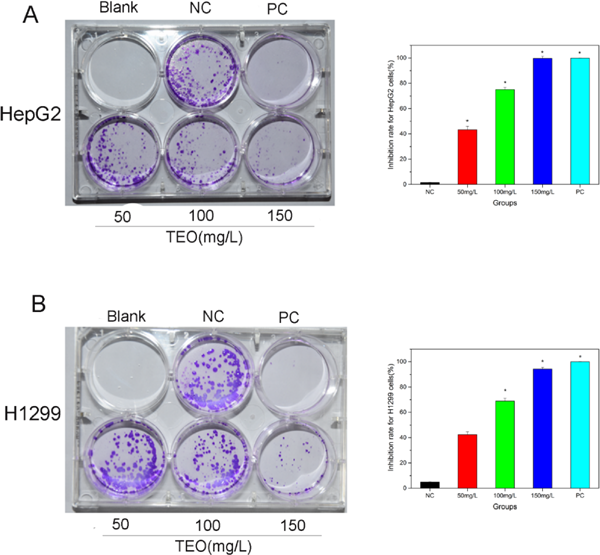
TEO inhibits the colony formation of HepG2 (A) and H1299 (B) cells. Cells were treated with various concentrations of TEO (50, 100 and 150 mg/L), 50 mg/L 5-fluorouracil (PC) and without any drugs (NC) for 48h. Then medium was aspirated and treated with fresh medium without any test sample/drugs. After 10 days incubation, the cells were fixed with 4% paraformaldehyde and stained with 0.2% crystal violet for 30 min and photographed with a camera. The values are the mean ± SD and similar results were obtained in at least 3 independent experiments *p < 0.05 vs. group NC.
In this study, the essential oil of turmeric was investigated on the apoptotic and proliferation of HepG2 and H1299 cells. The results demonstrated that treatment with more than 50 mg/L TEO for 48 h could significantly inhibit the proliferation of H1299 and HepG2 cells and could significantly inhibit cell migration and colony formation of cells (p < 0.05). To further explore the anti-cancer ability of TEO, the different effects of TEO on these two cell lines were investigated. The results demonstrated that treatment with TEO can both induce apoptosis and obstructed colonization of HepG2 and H1299 cells in a concentration-dependent and duration-dependent manner, and TEO showed better effects on HepG2 than that of H1299. It could preliminarily prove that the TEO can induce apoptotic and anti-proliferative effect on tumor cells selectively.
Our previous study found that the main constituents in TEO were ar-turmerone which could promote apoptosis of kinds of cancer cells. Thus, it can be speculated that the apoptotic and anti-proliferative effect of TEO on HepG2 and H1299 cells might be due to ar-turmerone. Through the literature research, we found that ar-turmerone induced apoptosis of different tumor cells in diverse signaling pathways, such as caspase-3, caspase-8 or caspase-9. And arturmerone may run cell death receptor pathways through exogenous or endogenous signals roles, respectively (Cheng et al., 2012). So TEO may have different mechanisms on Hepg2 and H1299, and more experiments are still needed.
It is worth discussing the results obtained for the PC group, treated with 5-fluorouracil. In these experiments, it was found that the cells in TEO-treated groups presented weaker tendencies in apoptosis compared to those in PC group, but showed better effect on cell migration and colony formation, suggesting TEO, a natural product with fewer side effects compared with artificially synthesized drugs, might have significant anti-metastasis capacity against tumor cells and could be applied to develop nutraceuticals that prevent cancer cells from spreading to other parts of the body.
As a conclusion, TEO could significantly inhibit the proliferation of HepG2 and H1299 cells, indicating its positive role in apoptosis of cancer cells. While the present study was conceived to explore the in vitro mechanistic basis of the anti-proliferative action of TEO, future in vivo studies assessing the findings in tumor models will be required in order to establish it as a viable anti-cancer treatment option.
Acknowledgements This research was supported by Colleges and universities in Hebei province science and technology research project (QN2020177), Science foundation for youths of Hebei province of China (C2019208188), Doctoral research fund project of Hebei university of science and technology (81/1181288), Doctoral research fund project of Hebei university of science and technology (81/1181289) and PT fund project of Hebei university of science and technology (82/1182218).