2021 年 27 巻 3 号 p. 521-527
2021 年 27 巻 3 号 p. 521-527
To determine the effective use of ‘lemon juice squeezed residue’ (LJSR), which contains many functional ingredients, we administered a diet containing 5% LJSR to spontaneously hypertensive rats (SHR) and investigated its hypotensive and oxidative stress-reducing effects. The flavonoid and coumarin contents of LJSR were measured: hesperidin, eriocitrin, and auraptene were detected. Increased systolic blood pressure was gradually reduced in the LJSR-treated group (LJSR group) and was significantly lower when compared with the control group at 4, 6, 8, 10, and 12 weeks. The urinary levels of 8-hydroxy-2′-deoxyguanosine and isoprostane were significantly lower in the LJSR group compared with the control group. The antioxidant activities of LJSR are associated with the actions of flavonoids and coumarin, which act as radical scavengers. These results suggest that LJSR could be used commercially as an antihypertensive food.
Hiroshima Prefecture is known for its production of lemons (Citrus limon Burm. f.), accounting for 60% of Japan's lemon production, and studies to promote their consumption are continuously conducted to show their versatile use (Domoto, 2013). Citrus fruits are reported to have health benefits (Assini et al., 2013; Morin et al., 2008) and lemon juice is often consumed during/after exercise as it contains saccharides such as fructose and glucose, organic acids such as citric acid, vitamins such as ascorbic acid, flavor components such as limonene, and nutritional/functional components such as flavonoids (Kumamoto et al., 1984). Fruit processing generates significant amounts of by-products, which pose a complex waste-disposal problem and additional economic burdens on production; however, valuable compounds are contained within their peels, pulp, and seeds. ‘Lemon juice squeezed residue’ (LJSR) is an under-utilized source of beneficial components that could be treated as a functional food and used in the prevention of lifestyle-related diseases.
LJSR, a food processing-related by-product, contains large amounts of functional components such as carotenoids, flavonoids, coumarins, and dietary fibers. Recent studies have discussed the relationship between reactive oxygen species and cardiovascular diseases (Lacy et al., 1998): the preventive and therapeutic effects of flavonoids and coumarins on cardiovascular diseases have been reported and the involvement of free radicals is speculated (Takemori et al., 2005).
We conducted a study to effectively utilize LJSR: we administered LJSR to spontaneously hypertensive rats (SHR), and investigated its hypotensive effects on increased blood pressure (BP) and oxidative stress-reducing effects. We also investigated its preventive effects on hypertension-related organ disorder to examine the safety and functionality of LJSR.
Animals We used 7-week-old male SHR/Izm rats (Disease Model Cooperative Research Association, Kyoto, Japan). The animal room was maintained at a constant temperature (23 ± 2 °C), humidity (55 ± 10%), and mean ventilation frequency (10 – 13/h) with a 12 h light/dark cycle (light: 7:00 – 19:00 h). The animals were fed a commercial powdered diet (MF, Oriental Yeast Co., Ltd., Tokyo, Japan) and provided with filtered tap water ad libitum. Animal care and experimental procedures were approved by the Animal Research Committee of Shimane University and conducted according to the Regulations for Animal Experimentation at Shimane University (approval number: IZ30 - 95).
Experimental procedure Male rats were divided into two groups (control and LJSR: 10 animals in each) and fed the experimental diets ad libitum during the 12 week experimental period. Commercially available powdered food (MF, Oriental Yeast Co., Ltd., Tokyo, Japan) was fed to the control group. 5% LJSR mixed with the same commercial powdered feed was fed to the experimental group. LJSR was obtained by pressing and pulverizing raw lemons (cultivated variety ‘Lisbon’) at Hiroshima Coop (Hiroshima, Japan). The pressed lemons were then used to produce hot-air-dried fruit juice residue, which was mixed with powdered feed. Food consumption and water intake were measured once every 2 weeks of the experimental period. Using a metabolic cage (CT-10S, Clea Japan Inc., Tokyo, Japan), urine was collected for 24 h after the initiation of feeding and again after 10 weeks. Samples were stored at -80 °C until analysis. After 12 weeks, the rats were fasted for 16 h and blood was collected from the abdominal inferior vena cava under anesthesia with isoflurane.
Determination of component analysis, flavonoids, and coumarins in LJSR The composition of LJSR was measured by the Japan Functional Food Analysis and Research Center (Fukuoka, Japan). Basic components, including energy, protein, total fat, carbohydrate, moisture, and ash, were measured.
Preparation of flavonoids (hesperidin and eriocitrin) from LJSR: 5.0 g of LJSR was added to 5 mL of methanol, and the mixture was extracted by treating with an ultrasonic device (ASU-20, ASONE Corporation, Osaka, Japan) for 10 min. The extract was centrifuged (20 000 × g for 5 min) and the supernatant obtained was diluted 2-fold with solvent (acetonitrile:water = 18:82) to prepare the LJSR sample. The amounts of hesperidin and eriocitrin in the LJSR was determined by HPLC (LC-10AT, Shimadzu Co., Ltd., Kyoto, Japan) (Miyake et al., 1998a). The analytical conditions were: column, COSMOSIL 5C18-AR-2 (Nacalai Tesque Inc., Kyoto, Japan, 4.6 mm × 250 mm); column temperature, room temperature; detection wavelength, 280 nm; flow rate, 1.0 mL/min; injection volume, 10 µL; mobile phase, acetonitrile:water = 18:82.
Preparation of coumarin (auraptene) from LJSR: 5.0 g of LJSR was added to 100 mL of a mixing solution (ethyl acetate:sodium hydroxide solution = 5:3). This solution was diluted 3-fold with acetone:ethanol (1:1) for measurements. The amount of auraptene in the LJSR was determined by HPLC (LC-10AT, Shimadzu Co., Ltd., Kyoto, Japan) (Tosa et al., 1988). The analytical conditions were: column, Wakosil-II 5C18HG (FUJIFILM Wako Pure Chemical Co., Osaka, Japan, 4.6 mm ×150 mm); column temperature, room temperature; detection wavelength, 325 nm; flow rate, 1.0 mL/min; injection volume, 10 µL; mobile phase, methanol:water = 80:20.
DPPH (1, 1-diphenyl-2-picrylhydrazyl) radical-scavenging activity in LJSR The antioxidative activity of LJSR was determined by the DPPH radical scavenging assay. Using a 96-well microplate, eight different LJSR concentrations were prepared by diluting with ethanol; 100 µL of the ethanol solution was then added to 50 µL of a 0.2 mmol/L DPPH ethanol solution. After 30 min incubation at room temperature, the absorbance was recorded at 517 nm using a plate reader (Sunrise Rainbow, Tecan, Ltd., Kanagawa, Japan). Samples were measured in duplicate, and the mean value was evaluated. Results are expressed as the percent decrease with respect to control values (Fujinami et al., 2001). The control sample contained solvent (ethanol or water as the sample solvent) in place of the test sample. α-Tocopherol was used as the reference sample (Tominaga et al., 2005).
Measurement of body weight (BW) and BP BW and BP were measured once every 2 weeks during the experimental period. BW was measured using an electronic balance (HF-3000, A&D Co., Ltd., Tokyo, Japan). Heart rate, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using an automatic sphygmomanometer (BP-98A, Softron Co., Ltd., Tokyo, Japan) at the tail vein, employing the tail-cuff method without anesthesia after warming the rat at 38 °C for 8 min. BP was measured 5 times for each rat and the mean was calculated.
Blood chemistry and urine parameters Collected blood was transferred to a micro blood-sampling tube (Capiject, ethylenediaminetetraacetic acid (EDTA)-2Na, Terumo Co., Ltd., Tokyo, Japan) and centrifuged at 860 × g for 15 min at 4 °C, and the supernatant was collected as a plasma sample. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltranspeptidase (GGT), total cholesterol (T-Cho), high-density lipoprotein cholesterol (HDL-c), triglyceride (TG), glucose (GLU), total protein (T-Pro), albumin (ALB), urea nitrogen (BUN), uric acid (UA), and creatinine (Cre) were measured by the Nagahama Life Science Laboratory (Oriental Yeast Co., Ltd., Shiga, Japan).
Urine total protein (U-TP), urine-sodium (U-Na), and urine-potassium (U-K) were measured by the Nagahama Life Science Laboratory (Oriental Yeast Co., Ltd., Shiga, Japan).
Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) and isoprostane assays After thawing to room temperature, urine samples were centrifuged at 860 × g for 15 min at 4 °C, and the supernatant, excluding precipitate, was used for measurement. 8-OHdG was measured using a commercial enzyme-linked immunosorbent assay kit (8-OHdG Check kit, Japan Institute for the Control of Aging, Nikken SEIL Co., Ltd., Tokyo, Japan). Urinary isoprostane was measured using a commercial enzyme-linked immunosorbent assay kit (urinary isoprostane kit, Japan Institute for the Control of Aging).
Statistical analysis All data are expressed as the mean ± standard error of the mean (SEM). Analysis of variance was used to test the overall effect of LJSR. Unpaired comparisons using Student's t-test were used to determine the significance of differences between specific groups. Analyses were performed using StatView (SAS Institute Inc., Cary, NC, USA). p < 0.05 was considered to indicate a statistically significant difference.
The compositions of the control and LJSR diets are shown in Table 1: there were no significant differences in the compositions of the control and LJSR diets. The flavonoid and coumarin contents of LJSR were measured: hesperidin (0.83%), eriocitrin (0.77%), and auraptene (0.55%) were detected, with hesperidin showing the highest concentration. The antioxidative activity of LJSR, examined using the DPPH radical scavenging assay, was confirmed to be 83.70%: a high scavenging effect, even when compared with α-tocopherol (95.50%).
| Constituent | Control | LJSR |
|---|---|---|
| Energy (kcal / 100g) | 341.0 | 334.0 |
| Proteins (g / 100g) | 22.6 | 21.8 |
| Total fat (g / 100g) | 6.1 | 5.4 |
| Carbohydrates (g / 100g) | 57.5 | 59.1 |
| Moisture (g / 100g) | 8.0 | 8.1 |
| Ash (g / 100g) | 5.8 | 5.6 |
To confirm the antihypertensive effect of LJSR, we compared its antihypertensive effect with the control diet after oral administration to SHR. Changes in heart rate are shown in Fig. 1-A. Heart rate ranged from 320 to 380 bpm in both groups. No significant difference in heart rate was noted between the groups. Changes in SBP are shown in Fig. 1-B. SBP was significantly lower in the LJSR group compared with the control group. SBP elevation was significantly inhibited at 4, 6, 8, 10, and 12 weeks in the LJSR group. Changes in DBP during the experimental period are shown in Fig. 1-C. DBP was also significantly lower in the LJSR group compared with the control group after 12 weeks. These results indicate that LJSR exerts an antihypertensive effect.
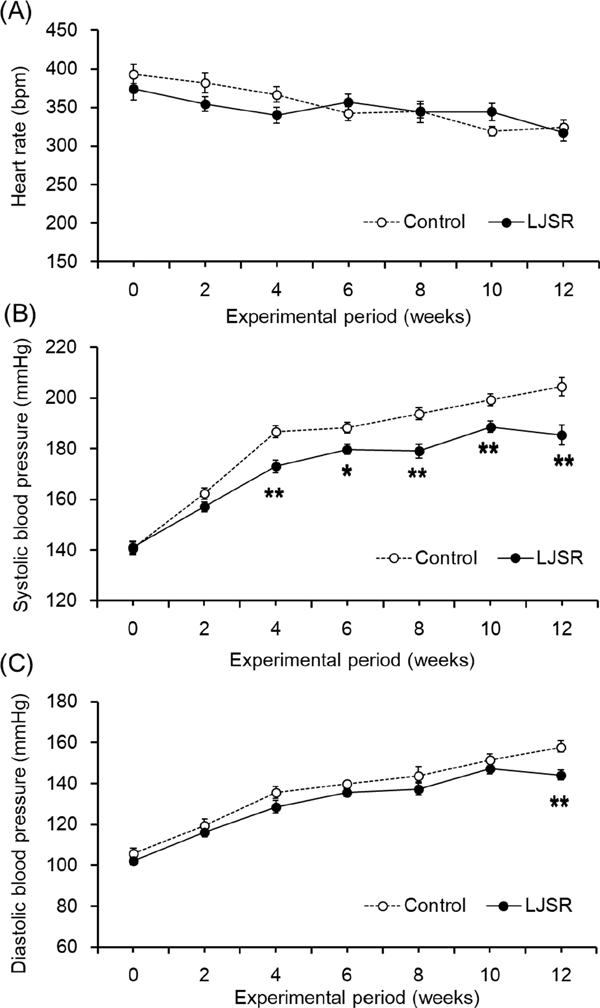
Effects of lemon juice squeezed residue (LJSR) administration on heart rate (A), SBP (B) and DBP (C) in SHR.
Each value represents the mean ± SEM of 10 rats. Significant differences from the control group using Student's t-test, *: p < 0.05, **: p < 0.01.
Measurements of BW and food consumption are useful methods for early detection of the onset of disease or debilitation in animals. LJSR administration did not affect the BW of SHR throughout the experimental period. In addition, there were no significant differences in the mean food intake between the LJSR group (20.60 ± 1.10 g/day) and the control group (21.30 ± 1.30 g/day) or water intake between the LJSR group (33.02 ± 1.59 mL/day) and the control group (34.22 ± 1.66 mL/day) throughout the experimental period. These results suggest that administration of LJSR does not affect the onset of disease or debilitation.
Blood chemistry and urinary parameter analysis results are shown in Table 2. There were no significant differences in blood chemistry measurements between the two groups, suggesting that LJSR does not affect blood chemistry in SHR.
| Control | LJSR | |
|---|---|---|
| Plasma | ||
| AST (IU/L) | 84.30 ± 4.54 | 82.40 ± 4.26 |
| ALT (IU/L) | 22.40 ± 2.70 | 25.60 ± 3.26 |
| GGT (IU/L) | 6.10 ± 0.85 | 5.80 ± 0.79 |
| T-Cho (mg/dL) | 57.70 ± 1.27 | 55.20 ± 2.48 |
| HDL-c (mg/dL) | 23.10 ± 0.38 | 22.20 ± 0.90 |
| TG (mg/dL) | 20.90 ± 1.63 | 26.20 ± 2.49 |
| GLU (mg/dL) | 154.10+ 18.87 | 171.10 ± 14.56 |
| T-Pro (g/dL) | 6.72 ± 0.37 | 6.77 ± 0.45 |
| ALB (g/dL) | 4.33 ± 0.20 | 4.37 ± 0.29 |
| BUN (mg/dL) | 21.86 ± 1.09 | 21.41 ± 1.21 |
| UA (mg/dL) | 2.78 ± 0.39 | 2.07 ± 0.32 |
| Cre (mg/dL) | 0.28 ± 0.01 | 0.27 ± 0.01 |
| Urine | ||
| U-TP (mg/day) | 1.468 ± 0.105 | 1.093 ±0.117* |
| U-Na (mEq/day) | 0.995 ± 0.112 | 0.758 ± 0.113 |
| U-K (mEq/day) | 2.920 ± 0.166 | 2.456 ± 0.254 |
Each value represents mean ± SEM of 10 rats.
U-TP excretion was significantly higher in the control group, but proteinuria was reduced in the LJSR group. There were no significant differences in any other urinary parameter between the two groups.
To investigate whether the inhibition of BP elevation was due to the antioxidative actions of LJSR, urinary 8-OHdG and isoprostane levels were measured (Fig. 2). Urinary 8-OHdG was significantly lower (p < 0.05) in the LJSR group at 1 152.60 ± 96.10 ng/day compared with the control group at 1 620.90 ± 139.10 ng/day. Urinary isoprostane was significantly decreased (p < 0.01) in the LJSR group at 80.90 ± 4.20 ng/day compared with the control group at 129.50 ± 13.20 ng/day. These results suggest that the antihypertensive effect of LJSR might be mediated through the inhibition of oxidative stress.
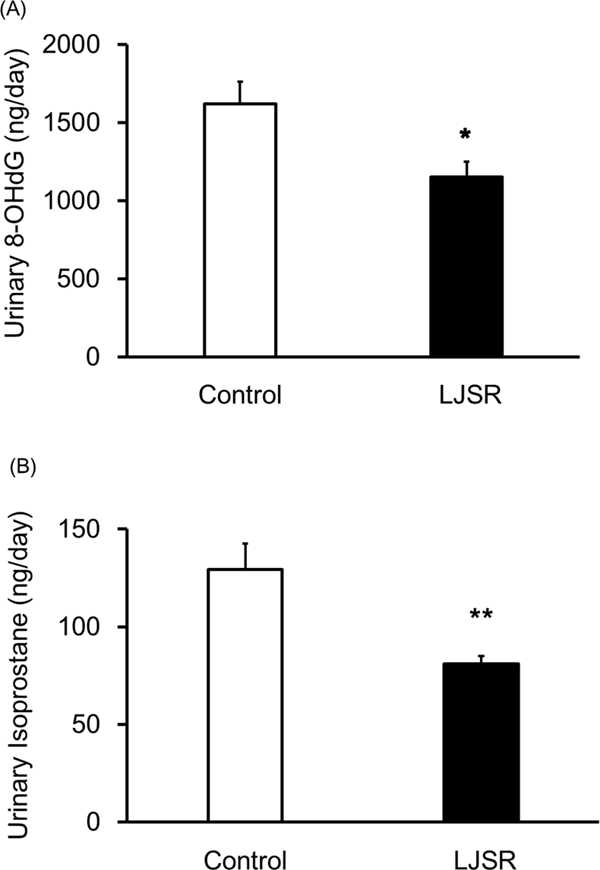
Comparison of urinary 8-OHdG (A) and isoprostane (B) levels in SHR which freely ingested LJSR. Urinary 8-OHdG and isoprostane were measured at 10 weeks after the start of the study, as described in the materials and methods. Each value represents the mean ± SEM of 10 rats. Significant differences from the control group using Student's t-test, *: p < 0.05, **: p < 0.01.
To investigate the effective use of functional ingredients within citrus fruit waste, we administered a diet containing 5% LJSR to rats, based on previous studies. We previously reported that 5% γ-aminobutyric acid (GABA) had BPlowering effects in SHR (Kawakami et al., 2018). In addition, 5% lemon juice was orally administered to SHR to investigate the suppressive effects of lemon juice on BP (Miyake et al., 1998a).
In the LJSR group, increased SBP was gradually reduced, and was significantly lower than the control group at 4, 6, 8, 10, and 12 weeks. Citrus peel contains a high level of flavonoids and coumarins, and many studies have examined their functional properties (Nogata et al., 2006). Hesperidin is the most common flavonoid found in citrus fruits and is suggested to exert a vasodilation effect that reduces BP. Ohtsuki et al (2002) reported that administration of 30 mg/kg hesperidin to SHR inhibited BP elevation. While Yamamoto et al (2008) revealed that single oral administration of hesperidin (10 to 50 mg/kg) induced a dose-dependent reduction in SBP in SHR through enhancement of nitric oxide (NO) mediated vasodilation. We demonstrate that continuous ingestion of approximately 28 mg/kg hesperidin by free feeding LJSR inhibited BP elevation in SHR. Moreover, Razavi et al (2015) reported that chronic administration of auraptene (16 mg/kg) significantly reduced BP in hypertensive rats, attributed to repair activity on smooth muscle cells and inhibition of spontaneous heartbeat induced by calcium. In our study, continuous ingestion of approximately 18 mg/kg auraptene by free feeding LJSR to SHR produced antihypertensive effects that were maintained throughout the study period. Furthermore, a reduction in DBP was observed in the LJSR group after 12 weeks. The reduction in oxidative stress may occur via improvements in peripheral blood flow in SHR fed LJSR.
In this study, urinary excretion of U-TP indicated that components in LJSR reduced proteinuria. This was also reported by Paredes et al (2018), who found that flavonoid treatments reduced proteinuria. Chronic elevation of SBP is associated with the presence of proteinuria and the development of glomerulosclerosis (Mali et al., 2012; Klahr et al., 2001). LJSR has antioxidative activity similar to α-tocopherol; therefore, to investigate whether the antioxidative action was involved in the BP elevation-inhibitory action of LJSR, urinary 8-OHdG and isoprostane were measured: both were significantly lower in the LJSR group compared with the control group. Urinary 8-OHdG and isoprostane are synthesized in tissues and organs under excessive oxidative stress; 8-OHdG is normally cleaved by repair enzymes and finally excreted into urine via the circulation. The result may be due to the actions of flavonoids as radical scavengers; in particular, eriocitrin, a flavanone glycoside which has potent antioxidant properties (Kawaii et al., 1999). Miyake et al (1998b) reported that dietary lemon flavonoids, eriocitrin and hesperidin, have in vivo antioxidant roles. Our results suggest that continuous ingestion of approximately 26.0 mg/kg eriocitrin by free feeding LJSR to SHR improves vascular endothelial function.
Recent studies investigating the association between hypertension and active oxygen indicated that active oxygen caused hypertension (Lacy et al., 1998; Maneesai et al., 2018). Many flavonoids increase the bioavailability of endothelial vasodilatory factors, mainly NO and endothelium-derived hyperpolarizing factor (EDHF), and inhibit the production of pro-inflammatory substances: this considerably improves the function of the vascular endothelium (López-Sepúlveda et al., 2008). Yamamoto et al (2008) reported that potential mechanisms for enhanced NO bioavailability in vessel walls after ingestion of hesperidin are as follows: (1) hesperidin may directly enhance NO production by increasing eNOS activity and/or expression and (2) diminished NO inactivation as a result of decreased O2− levels. Considering the evidence, we suggest that the hypotensive effects of hesperidin, eriocitrin, and auraptene are due to the suppression of NADH/NADPH oxidase, an increase in NO bioavailability, blocking of calcium channels, and alleviation of endothelial dysfunction (Orallo et al., 2004; Yamamoto et al., 2013).
In conclusion, this study provides evidence that flavonoids and coumarins in LJSR might reduce BP elevation in SHR. Our study suggests that citrus fruit waste could be used as a functional material with beneficial health effects, such as preventing hypertension.