Abstract
Koji molds are safe microorganisms belonging to the genus Aspergillus and are used in the production of traditional Japanese fermented foods, such as miso, soy sauce, and sake. Koji molds produce many types of enzymes, including amylase, lipase, and protease, that are used in food processing treatments. To examine the potential of Koji molds for cheese ripening, we measured the lipase and protease activities on cheese curds ripened using five strains of Koji molds (Aspergillus oryzae or A. sojae) and compared them with curds ripened using Penicillium candidum or P. roqueforti. Compared with Penicillium, Koji molds showed similar or higher protease activity on the cheese surface. Lipase activity varied markedly among Koji mold strains; some showed comparable activity to Penicillium, and others showed lower activity. Considering the lipase and protease activities, some Koji molds can be applied to the production of surface mold-ripened cheeses.
Introduction
Aspergillus and Penicillium are sister genera, and many species belonging to these genera are used as sources of enzymes for the food and biotechnology industries (Houbraken et al., 2014). Koji molds (including Aspergillus oryzae, A. sojae and A. luchuensis) are used in Japanese fermented soybean and rice products, such as miso, soy sauce, and sake, and some Penicillium species are used in cheese ripening in European countries. Koji molds are known as safe microorganisms. Their safety can be attributed to gene mutations, including a large deletion in the subtelomeric region containing the biosynthesis gene clusters for toxic compounds such as aflatoxin and cyclopiazonic acid, which are present in the genomes of toxigenic ancestor species such as A. flavus and A. parasiticus (Tominaga et al., 2006; Matsushima et al., 2001). Koji molds produce and secrete large amounts of many types of enzymes, including amylases and proteases that are important for the processing of traditional Japanese fermented foods, as well as lipases (Toida et al., 1995). Proteolysis and lipolysis are crucial biochemical events in cheese ripening that result in the development of the flavor and texture. P. roqueforti proteases degrade milk casein to peptides and amino acids and contribute to the sensory properties of blue cheese (Fernández-Bodega et al., 2009). Lipases produced by P. roqueforti, which liberate volatile fatty acids from milk fat, are key enzymes determining the flavor of Roquefort cheese (Currie, 1914).
Considering the ability of Koji molds to produce proteases and lipases, it is expected that these molds can be used in cheese ripening. Indeed, in 1959 Nakanishi showed that a Koji mold (A. oryzae) could be used for cheese ripening and developed Gouda cheese with A. oryzae mycelial powder kneaded into the curd to enhance and accelerate ripening (Nakanishi and Tokita, 1959a). Nakanishi indexed P. roqueforti protease activity and selected an A. oryzae strain, called “chosen B”, for Gouda cheese ripening from four strains of A. oryzae, two strains of A. niger, and three other strains of Aspergillus species (Nakanishi and Tokita, 1959a). Protease activity of A. oryzae chosen B was comparable to that of P. roqueforti, and the proteases of A. oryzae chosen B were able to degrade casein to small peptides or amino acids equally well as the proteases of P. roqueforti (Nakanishi and Tokita, 1959a). Nakanishi also reported that the cheese fermented by A. oryzae chosen B had a strong rancid and spicy flavor like that of blue cheese, which was derived from volatile short-chain fatty acids; this outcome was ascribed to the high lipase activity of A. oryzae (Nakanishi and Nakazawa, 1963). A. oryzae chosen B was thus an alternative fungus to P. roqueforti for cheese ripening; unfortunately, this strain was lost during the halfcentury following Nakanishi's study.
There are two categories of mold-ripened cheese: blue vein cheeses and cheeses ripened with surface mold (Galli et al., 2016). P. roqueforti is used for the production of blue vein cheeses such as Roquefort and Gorgonzola. P. camemberti and P. candidum are used for the production of surface moldripened cheeses such as Camembert and Brie. In Japan in 2018, consumption of Camembert was 5,400 t and that of blue cheese was 1,000 t (Agriculture & Livestock Industries Corporation, 2019). It is suspected that most Japanese prefer the creamy taste of Camembert to the rancid and spicy flavor of blue cheese. In European countries, suitable fungal species are selected for each fermented food, such as P. roqueforti for blue cheese, P camemberti for Camembert, and P. nalgiovense for sausages (Houbraken et al., 2014). The history of several seed culture companies in Japan is hundreds of years old and they have a huge variety of stock cultures of A. oryzae,A. sojae, and A. luchuensis strains. We hypothesized that some strains of Koji molds (A. oryzae,A. sojae, and A. luchuensis) are suitable for surface mold-ripening of cheeses to produce the mild flavors preferred by most Japanese consumers. A patent for a surface-ripened-type cheese ripened by A. oryzae (P2009-100678A) is held by a Japanese dairy company. To prevent rancid and spicy flavors, the company used low-fat milk to make the cheese curd. When the surface-ripened-type cheese ripened by A. oryzae was made using whole milk, the preference score for their cheese was lower than that of Camembert ripened by P. candidum. The A. oryzae strain the company used is assumed to have excessive lipase activity. Thus, the selection of a strain of Koji mold with sufficient protease activity but low lipase activity will enable the production of a mild flavored Koji cheese from whole milk.
The aim of this work was to assess the cheese ripening ability of molds by determining and comparing the lipase and protease activities in surface-ripened-type soft cheeses fermented by several Koji molds and Penicillium strains.
Materials and Methods
Strains and media A. sojae 2041 and A. oryzae strains B, C, D, and K were stock cultures from Higuchi Matsunosuke Shoten, Japan. P. candidum (Swing PCA 3) and P. roqueforti (Swing PR 4), hereinafter referred to as P. candidum and P. roqueforti, were purchased as commercial products (Chr. Hansen, Hoersholm, Denmark). Potato-dextrose-agar was used for maintaining strains and preincubation to obtain conidia.
Green curd production Forty-five grams of NaCl was dissolved in 3 kg of milk. The milk was heated to 75 °C and immediately cooled to 30 °C. Lactic acid fermentation starter (30 mg, CHN11, Chr. Hansen) was added to the pasteurized milk and incubated for 40 min at 30 °C. Rennet (0.09 g, Fromase 2200 Tl Granulate; DSM Food Specialties, Heerlen, the Netherlands) and 0.3 g of calcium chloride were added. After 30 min of incubation, the coagulated curd was cut into cubes of about 50 cm3 using a curd knife. After standing for 1 h, 2.6 L of 1.5 % (w/v) NaCl was added and the mixture was gently stirred. After standing for 30 min, the curd was then stuffed into three molds (Φ 62 mm × 90 mm H) and the whey was allowed to drain overnight at 30 °C with occasional inversion.
Cube curd fermentation Green curds were cut into 10-mm W × 10-mm D × 10-mm H cubes and inoculated with mold by dipping them in 106/mL conidia of Aspergillus or Penicillium strains. The inoculated cubes were arranged on plastic trays with lids and incubated at 30 °C for Aspergillus strains and 25 °C for Penicillium strains with 90 % relative humidity for 5 days. Fermented cubes were harvested after 3 and 5 days.
Water-soluble compounds of the cube curd were extracted using water. A fermented cube curd or green cube curd was soaked in 10 mL water at 4 °C overnight with periodic agitation, then the mixture was centrifuged at 8000 × g for 15 min. The experiments were conducted in triplicate.
Assay of lipase activity Lipase activity was determined as described by Tamalampudi et al. (Tamalampudi et al., 2007) with slight modification. To assay lipase activity, 50 µL of fermented or green curd extract was suspended in 150 µL of 50 mM Tris-Cl (pH 7.4), 2.1 % (v/v) Triton X-100, and 1 µL substrate solution and incubated at 25 °C for 1 h. To prepare the substrate solution, a 5-µL aliquot of p-nitrophenol butyrate was dissolved in 250 µL of 99 % ethanol. The optical density was measured at 415 nm using a U-1900 spectrophotometer (Hitachi, Japan). One unit of enzyme activity was defined as the amount of enzyme releasing 1 µmol of p-nitrophenol per min under the assay conditions.
Assay of protease activity Protease activity was assayed as described by Maeda et al. (Maeda et al., 2015) with slight modification. Protease activity was assayed using azocasein. The substrate stock solution [160 µL, 1.25 % azocasein dissolved in 100 mM Tris-HCl buffer (pH 7.5) containing 1 mM CaCl2] and 40 µL of fermented or green curd extract were mixed and incubated at 37 °C for 20 h. The reaction was terminated by addition of 200 µL of 10 % trichloroacetic acid and the sample was centrifuged at 21 600 × g, 4 °C, for 10 min. The supernatant (350 µL) was collected and an equal volume of 0.75 M NaOH was added. The optical density was measured at 440 nm using the U-1900 spectrophotometer. One unit of enzyme activity was defined as the amount of enzyme increasing one absorption unit at 440 nm per min under the assay conditions.
Ammonia concentration Ammonia concentration in fermented or green curd extract was measured using an Ammonia Assay Kit (Cell Biolabs, Inc., San Diego, CA, USA) according to the manufacturer's instructions.
pH The pH of fermented or green curd extract was measured using pH test paper (Duotest, Macherey-Nagel, Düren, Germany).
Organic acid measurement Fermented or green curd extract was filtered using an Amicon Ultra ultrafiltration unit (10 kDa molecular weight cut-off; Merck Millipore, Tokyo, Japan). A flow-through sample (10 µL) was injected into a high-performance liquid chromatography system for organic acid analysis, which was conducted as described previously (Hagi et al., 2019). We determined acetic acid, lactic acid, propionic acid, butyric acid, isovaleric acid, and valeric acid.
Results and Discussion
Lipase and protease activities of five Koji molds on cheese curd For an overview of the lipase and protease activities of the five Koji molds, the enzyme activities in cheese curd were measured and compared with those of the control strains of P. candidum and P. roqueforti (Fig. 1).

The lipase activity of green curd (0 day) was undetectable in this study. After 3 days of culture, fungi fully covered the surface of the curd cubes, and ripening of the curd cubes was complete in 5 days. The lipase activity of both Penicillium strains was high (241.5, P. candidum and 187.0 U/g, P. roqueforti); and the lipase activity of A. oryzae strains B (270.6 U/g) and D (210.1 U/g) was comparable to the Penicillium strains. The three Koji molds A. sojae 2041 (38.1 U/g), A. oryzae C (28.3 U/g), and A. oryzae K (57.7 U/g) showed low lipase activity. The protease activity of green curd was 0.11 U/g. Proteases from the starter lactic acid bacteria may contribute to this protease activity. The protease activity of the four Koji molds other than A. oryzae C was higher than that of the Penicillium strains [Koji molds: 2.15 (A. sojae 2041), 1.27 (A. oryzae B), 1.11 (A. oryzae D), and 1.48 U/g (A. oryzae K); Penicillium strains 0.48 (P. candidum) and 0.63 U/g (P. roqueforti)]. A. oryzae C (0.57 U/g) showed comparable protease activity to the Penicillium strains. All the Koji molds showed sufficient protease activity for cheese ripening.
Both the lipase and protease activities of A. oryzae C were distinct from those of all the other fungi studied here. The enzyme activities of the two Penicillium strains and the four Koji molds other than A. oryzae C increased over the 5 days of ripening. However, the enzyme activities of A. oryzae C at 5 days were lower than those at 3 days. It is unknown why the enzyme activities of A. oryzae C decreased during ripening. However, it is possible that the mycelia of A. oryzae C lyse via autophagy in the early ripening period.
Regarding the lipase and protease activities, A. oryzae B and A. oryzae D possess comparable cheese curd ripening ability to P. candidum and P. roqueforti (Fig. 1). Nakanishi measured the protease activity of A. oryzae chosen B using milk casein as a substrate (Nakanishi and Tokita, 1959b). The caseinolytic ability of A. oryzae chosen B was slightly higher than that of P. roqueforti (Nakanishi and Tokita, 1959b). The protease activity of A. oryzae B and A. oryzae D in the present study was approximately double that of P. roqueforti. Since the methods used for protease activity measurement were different, it is impossible to directly compare the protease activity data of Nakanishi and that in the present study. However, it is likely that the protease activity of A. oryzae chosen B in the work of Nakanishi and A. oryzae B and A. oryzae D in the present study is higher than that of P. roqueforti.
Amounts of organic acids and ammonia in cheese curd, and pH of the curd We investigated several properties of the curd fermented by Koji molds. Figure 2 shows the amounts of five volatile short-chain fatty acids— acetic acid, propionic acid, butyric acid, isovaleric acid, and valeric acid. The amount of acetic acid in the green curd was higher than in the fermented curd. This result shows that acetic acid was produced by the starter lactic acid bacteria and then consumed by the fungi during ripening. The volatile short-chain fatty acids with the exception of acetic acid in surface-ripened cheese are generally liberated from milk fat by fungal lipases. The environmental concentration of propionic acid, butyric acid, isovaleric acid, and valeric acid is controlled by Japanese law, as they are specified as offensive odor substances, and Japanese consumers are generally averse to these odors. Propionic and butyric acids were undetectable in the green curd, while isovaleric and valeric acids were detected only in trace amounts. A. oryzae C (0.0014 mmol/g) and P. roqueforti (0.00020 mmol/g) produced trace amounts of propionic acid. The Koji molds were assumed to consume isovaleric or valeric acid contained in the green curd, whereas comparable amounts of isovaleric acid to the green curd were detected in the 3-day fermented curds by P. roqueforti (0.00013 mmol/g) and not detected in the 5-day fermented curds by P. roqueforti. A comparable amount of valeric acid to the green curd was detected in the fermented curd by P candidum (0.00015 mmol/g) and P. roqueforti (0.00014 mmol/g). P. roqueforti produced the highest level of butyric acid (0.014 mmol/g) among the fungi studied here. A. sojae 2041 (0.0047 mmol/g), A. oryzae B (0.0058 mmol/g), A. oryzae D (0.0028 mmol/g), A. oryzae K (0.0041 mmol/g), and P. candidum (0.0018 mmol/g) produced less butyric acid than P. roqueforti. A. oryzae C did not produce a detectable amount of butyric acid as determined by our method.

The lipase activity of the fungi (Fig. 1) and the amounts of the four volatile short-chain fatty acids with the exception of acetic acid showed a weak positive correlation (correlation coefficient 0.310). However, even though they exhibited lower lipase activity than P. candidum, A. sojae 2041 and A. oryzae K produced more butyric acid than P. candidum. These two strains may have another enzyme for milk fat degradation, such as an esterase. The low lipase activity of A. oryzae C was expected to produce a less rancid and spicy curd, as a consequence of lower volatile short-chain fatty acid production, than P. candidum and P. roqueforti. Japanese consumers may prefer such mild cheese to the rancid and spicy cheese ripened by P. candidum and P. roqueforti. Nakanishi measured the concentration of volatile short-chain fatty acids in cheese curd ripened by A. oryzae chosen B (Nakanishi and Nakazawa, 1964). The amount of butyric acid liberated by A. oryzae chosen B during 5 days of ripening was 98 mg/100 g curd (Nakanishi and Nakazawa, 1964), equivalent to 0.011 mmol butyric acid/g. Thus, A. oryzae chosen B in Nakanishi's study produced almost the same amount of butyric acid as P. roqueforti in our study (0.014 mmol/g). Therefore, A. oryzae chosen B would be an alternative fungus to P. roqueforti to ripen rancid and spicy Roquefort cheese, but would not be suitable for ripening of Camembert.
Figure 3 shows the amounts of total organic acids (including lactic acid) and lactic acid, and the calculated value of the average total organic acids minus the average lactic acid for each curd. The amount of total organic acids decreased during ripening for all fungi, and with the exception of A. sojae 2041 and A. oryzae C, the total organic acids level of Koji molds in curd ripened for 5 days was comparable to that for P. candidum and P. roqueforti. The major organic acids component in the cheese curd was lactic acid. Lactic acid is produced by starter lactic acid bacteria during curd production, and fungi consume lactic acid during ripening. A. oryzae B, A. oryzae D, and P. roqueforti consumed most of the lactic acid after 5 days of ripening. The amount of total organic acids excluding lactic acid for A. sojae 2041, A. oryzae B, A. oryzae C, and P. candidum and P. roqueforti in the curd ripened for 5 days was higher than that in the curd ripened for 3 days. This increase in the level of organic acids excluding lactic acid may be partly explained by fatty acid production via lipase activity and tricarboxylic acid production via fungal metabolism. Generally, fungi produce several kinds of organic acids, such as citric acid, malic acid, and succinic acid, during the tricarboxylic acid cycle.

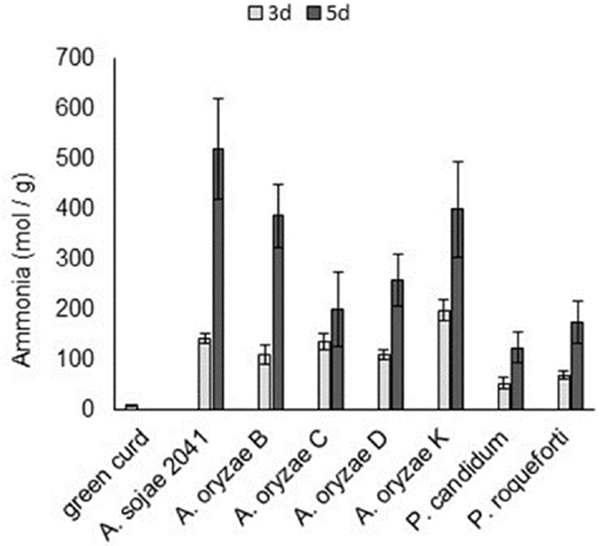
Figure 4 shows the amount of ammonia in the curd fermented by Koji molds. The amount of ammonia increased for all the tested fungi during the ripening period. The protease activity of the fungi (Fig. 1) and the ammonia level showed a strong positive correlation (correlation coefficient 0.863). Protein metabolism is assumed to be a major cause of ammonia production in the curd. Karahadian (Karahadian and Lindsay, 1987) proposed that the consumption of lactic acid and the production of ammonia by fungi cause an elevation of the pH of cheese curd. Figure 5 shows the pH of the curds during ripening in our study. The pH of curd ripened for 5 days by Koji molds was higher than that ripened by P. candidum and P. roqueforti. It is difficult to explain why the curd ripened by A. oryzae C for 5 days had the highest pH among all the strains, as this strain also had the highest level of organic acids of all the strains (Fig. 3) and the lowest level of ammonia production among the A. oryzae strains (Fig. 4).
In conclusion, regarding the lipase and protease activities, Koji molds can be used in cheese ripening. In addition, considering the low lipase activity and low level of volatile short-chain fatty acid production, it is expected that cheese ripened by A. oryzae C will have a milder flavor than Camembert ripened by P. candidum. Our study will contribute to the establishment of novel categories of cheese ripened with surface mold.
Acknowledgements This research was supported by grants from the Project of the NARO Bio-oriented Technology Research Advancement Institution (the special scheme project on vitalizing management entities of agriculture, forestry and fisheries). We thank Edanz Group (https://en-authorservices.edanzgroup.com/ac) for editing a draft of this manuscript.
References
- Agriculture & Livestock Industries Corporation (2019). Investigation of the domestic distribution of butter, skim milk and cheese in Japan. Chikusan no Jouhou, 4, 63-73. (in Japanese)
- Currie, J.N. (1914). Flavor of roquefort cheese. J. Agric. Res. (Washington, D. C.), 2, 1-14.
- Fernández-Bodega, M.A., Mauriz, E., Gómez, A. and Martín, J.F. (2009). Proteolytic activity, mycotoxins and andrastin A in Penicillium roqueforti strains isolated from Cabrales, Valdeón and Bejes-Tresviso local varieties of blue-veined cheeses. Int. J. Food Microbiol., 136, 18-25.
- Galli, B.D., Martin, J.G.P., da Silva, P.P.M., Porto, E. and Spoto, M.H.F. (2016). Sensory quality of Camembert-type cheese: Relationship between starter cultures and ripening molds. Int. J. Food Microbiol., 234, 71-75.
- Hagi, T., Nakagawa, H., Ohmori, H., Sasaki, K., Kobayashi, M., Narita, T. and Nomura, M. (2019). Characterization of unique metabolites in γ-aminobutyric acid-rich cheese by metabolome analysis using liquid chromatography-mass spectrometry. J. Food Biochem., 43, e13039.
- Houbraken, J., de Vries, R. P. and Samson, R. A. (2014) Modern taxonomy of biotechnologically important Aspergillus and Penicillium species, Adv. Appl. Microbiol., 86, 199-249.
- Karahadian, C. and Lindsay, R.C. (1987). Integrated Roles of Lactate, Ammonia, and Calcium in Texture Development of Mold Surface-Ripened Cheese. J. Dairy Sci., 70, 909-918.
- Maeda, H., Nakagawa, K., Murayama, K., Goto, M., Watanabe, K., Takeuchi, M., and Yamagata, Y. (2015). Cloning a neutral protease of Clostridium histolyticum, determining its substrate specificity, and designing a specific substrate. Appl. Microbiol. Biotechnol., 99, 10489-10499.
- Matsushima, K., Chang, P.K., Yu, J., Abe, K., Bhatnagar, D. and Cleveland, T.E. (2001) Pre-termination in aflR of Aspergillus sojae inhibits aflatoxin biosynthesis. Appl. Microbiol. Biotechnol., 55, 585-589.
- Nakanishi, T. and Nakazawa, Y. (1963). Studies on the Cheese Flavors 2. The Relation between the content of the Free Volatile Fatty Acids and the Degree of Ripening of cheese curd with Aspergillus oryzae. Japanese journal of dairy science (Rakunoukagaku no Kenkyu). 12, A148-A154. (in Japanese)
- Nakanishi, T. and Nakazawa, Y. (1964). Studies on the Cheese Flavors 3. The influence of the fat content in cheese curd for liberation of the Free Volatile Fatty Acids during Ripening of cheese curd with Aspergillus oryzae. Animal Science Journal (Nihon Chikusan Gakkaiho). 35, 98-105. (in Japanese)
- Nakanishi, T. and Tokita, F. (1959a). Studies on Cheese Ripening IV Enzyme Action of Aspergillus in Cheeses (1). Animal Science Journal (Nihon Chikusan Gakkaiho). 30, 47-50. (in Japanese)
- Nakanishi, T. and Tokita, F. (1959b). Studies on Cheese Ripening V Enzyme Action of Aspergillus in cheese (2). Animal Science Journal (Nihon Chikusan Gakkaiho). 30, 283-286. (in Japanese)
- Tamalampudi, S., Talukder, M.M.R., Hama, S., Tanino, T., Suzuki, Y., Kondo, A. and Fukuda, H. (2007). Development of recombinant Aspergillus oryzae whole-cell biocatalyst expressing lipase-encoding gene from Candida antarctica. Appl. Microbiol. Biotechnol., 75, 387-395.
- Toida, J., Kondoh, K., Fukuzawa, M., Ohnishi, K., and Sekiguchi, J. (1995). Purification and Characterization of a Lipase from Aspergillus oryzae. Bioschi. Biotech. Biochem., 59, 1199-1203.
- Tominaga, M., Lee, Y.H., Hayashi, R., Suzuki, Y., Yamada, O., Sakamoto, K., Gotoh, K. and Akita, O. (2006). Molecular Analysis of an Inactive Aflatoxin Biosynthesis Gene Cluster in Aspergillus oryzae RIB Strains. Appl. Environ. Microbiol., 72, 484-490.






