2021 年 27 巻 5 号 p. 797-806
2021 年 27 巻 5 号 p. 797-806
The inhibition of the β-glucuronidase activity of the intestinal microbiota could contribute to reducing the risk of colon cancer. This study aimed to investigate the effect of alkyl catechols, which are found in fermented foods and wood smoke utilized in food preservation, on β-glucuronidase activity. In this regard, 65.5% inhibition of β-glucuronidase activity was demonstrated by 4-ethylcatechol (4EC) at 20 µM. The concentration of 4EC, which led to 50% loss of the enzyme activity was 7.57 µM. In addition, a kinetic study was conducted on β-glucuronidase inhibition by 4EC through Lineweaver-Burk analysis and the kinetic profile proposed that 4EC is a competitive inhibitor of β-glucuronidase. Analysis of the structure-activity relationship revealed the importance of the ethyl moiety and catechol structure of 4EC for the β-glucuronidase inhibitory activity.
In recent times, studies have shown that excessive oxidative stress, mediated by reactive oxygen species and free radicals, contribute greatly to the pathology of many common diseases, such as cancer (Halliwell, 2006; Wells et al., 2009; Dalleau et al., 2013), metabolic syndrome (Furukawa et al., 2004; Dalleau et al., 2013), coronary artery disease (Heitzer et al., 2001; Dalleau et al., 2013), and neuro-degeneration (Lin and Beal, 2006; Johnson et al., 2008; Calkins et al., 2009).
In mammalian cells, the nuclear factor-erythroid 2-related factor 2 (Nrf2) plays a vital role in reducing the damages caused by oxidative stress and toxic chemical substances (Motohashi and Yamamoto, 2004; Kensler et al., 2007). The expression of antioxidant and detoxifying enzymes is induced by activated Nrf2, which is being activated by oxidative stress (Hayes and Dinkova-Kostova, 2014; Dinkova-Kostova and Abramov, 2015). Therefore, as co-factors, cancer-protective compounds enhance the activation of the Nrf2 signaling pathway induced by oxidative stress (Balogun et al., 2003; Xu et al., 2006; Itoh et al., 2010). In addition, dietary factors, such as sulforaphane, are expected to provide effective strategies for the prevention of cancer (Xu et al., 2006; Cornblatt et al., 2007). Although a variety of chemical compounds are found to be co-factors, all of them are redox-sensitive compounds and are prone to oxidation-reduction reactions (Prochaska et al., 1985). Alkyl catechols are found in fermented foods and wood smoke used for food preservation (Senger et al., 2016). Recently, alkyl catechols have received attention as potential activators of Nrf2 signaling pathway. Senger et al. (2016) reported that 4-ethylcatechol (4EC) activates Nrf2 signaling pathway, thus providing protection against common diseases, including cancer, both in vitro and in vivo.
Detoxification of carcinogens plays a vital role in prevention of cancer, despite the Nrf2 defense mechanism. Conjugation of carcinogens, including glucuronidation, is among the major processes of detoxification. Carcinogens taken into the body are conjugated with glucuronic acid in the liver and excreted into the intestine (Kavak et al., 2010). Furthermore, there is a reduction in reabsorption of glucuronide carcinogens, which makes them to be easily eliminated from the body (Beaud et al., 2005). However, glucuronide conjugates are good sources of carbon for several intestinal microbiota that have β-glucuronidase (Dashnyam et al., 2018). Bacterial β-glucuronidase hydrolyzes glucuronide, liberates glucuronic acid, which is then utilized as a carbon source, and regenerates carcinogens in the intestinal tract (Goldin, 1986). In an animal model, high fecal β-glucuronidase activities were reported to increase the risk of colorectal cancer (Kim and Jin, 2001; Rafter et al., 2004). Kim and Jin (2001) discovered the increase in fecal β-glucuronidase activity in patients with colon cancer, thus suggesting a vital role of microbial β-glucuronidase in the pathology of cancer. Therefore, inhibition of β-glucuronidase activity in the intestinal tract is expected to prevent colon carcinogenesis. Hence, the discovery of β-glucuronidase inhibitor, especially from dietary factors, has been carefully considered. Methyl methanethiosulfinate is present in leaves of Allium tuberosum Rottler, which was discovered to be an inhibitor of β-glucuronidase (Watanabe et al., 2013). Sun et al. (2020) reported the inhibitory effect of natural polyphenols, including demethylbellidifolin and gentisin, on β-glucuronidase. In addition, D-saccharic acid 1,4-lactone (SL) was reported to show the inhibitory effect (Kim et al., 1995). However, to the best of our knowledge, it has not been reported that β-glucuronidase activity could be inhibited by 4EC. In this regard, the effect of 4EC on β-glucuronidase activity was evaluated.
Chemicals β-glucuronidase from Escherichia coli, p-nitrophenyl-β-D-glucuronide (PNPG), 4EC, and SL were purchased from Sigma-Aldrich Japan (Tokyo, Japan). Catechol (Cat) was obtained from Kanto Chemical Co., Inc. (Tokyo, Japan). 4-Ethyl-2-methoxyphenol (4E2M) was purchased from FUJIFILM Wako Pure Chemical Co. (Osaka, Japan). All other chemicals were of analytical grade.
β-glucuronidase assay protocol β-Glucuronidase activity was assayed by monitoring the p-nitrophenol produced from PNPG following the method described by Hashimoto et al. (2013). In brief, 50 µL of assay sample solution in 20 mM phosphate buffer (pH 7.0) and β-glucuronidase (12 U/mL) was added to a 96-well microplate and incubated for 10 min at 37 °C. Then, 50 µL of 0.6 mM PNPG was added to each well to initiate the reaction. The microplate was read with a Sunrise microplate reader (Tecan Japan, Kanagawa, Japan) at 405 nm and 37 °C for 5 min. The enzyme activity was expressed as the change in absorbance at 405 nm/min (slope), which was calculated using the Magellan software (Tecan Japan). The inhibitory activity was calculated using the formula: (C −T)/C × 100, where C denotes control, which is enzyme activity without the assay sample, and T denotes tested, which is enzyme activity with the assay sample.
Statistical analysis All experimental data were expressed as mean ± standard error (mean ± SE, n = 4). Statistical analysis was by Tukey's test. Differences were considered statistically significant at p < 0.05.
Effect of 4EC on β-glucuronidase activity The inhibitory effect of 4EC at different concentrations is shown in Fig. 1. We observed that 4EC significantly inhibited β-glucuronidase activity, even at 1 µM. Its inhibitory activity increased with increasing concentration of 4EC and gradually leveled off. When the concentration of 4EC was 20 µM, there was a reduction in β-glucuronidase activity by 65.5%. In addition, when the concentration of 4EC was 7.57 µM, 50% activity loss was recorded.

Inhibition of β-glucuronidase activity by 4EC
Results are presented as mean ± SE of quadruplicate measurements, unless the error bar is smaller than the symbol. The mean values were compared using the Tukey's test (p < 0.05). Significant differences are indicated by different alphabets.
Inhibition kinetics of 4EC toward β-glucuronidase The inhibitory action of 4EC against β-glucuronidase was evaluated using Lineweaver-Burk plots (Fig. 2). In the process, there was an increase in the Km value at increasing concentrations of 4EC, while the Vmax value remained constant. This shows that 4EC is a competitive inhibitor and that it binds to the free enzyme instead of the enzyme-substrate complex. The value of the equilibrium constant for the binding of the inhibitor with the free enzyme, Ki, was calculated from a secondary replot of the slopes of straight lines in the Lineweaver-Burk plots (Fig. 2) versus the 4EC concentrations (Fig. 3) and the resultant value of Ki was 5.81 µM. The Ki value of the heat-treated 3-(3′,4′-dihydroxyphenyl)-l-alanine was reported to be 1.42 µM (Hashimoto et al., 2013). Wallace et al. (2010) discovered that the effective inhibitors of β-glucuronidase via high-throughput screening as well as the Ki value of those inhibitors ranging from 164 nM to 1.38 µM were similar to that of 4EC.

Lineweaver-Burk plots of β-glucuronidase in the presence of 4EC
●, Control; ○, 1 µM 4EC; ▲, 2 µM 4EC; △, 5 µM 4EC; ◆, 10 µM 4EC; ◊, 20 µM 4EC. Results are presented as the mean of quadruplicate measurements.
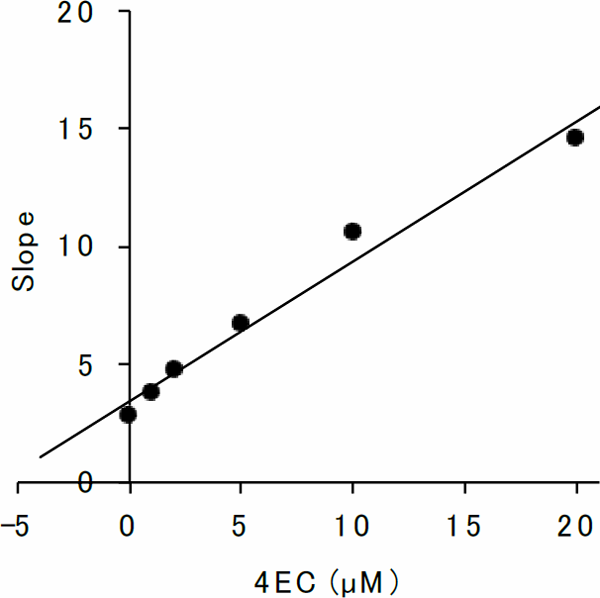
Secondary replot of the Lineweaver-Burk plots of β-glucuronidase in the presence of 4EC
Ki was obtained by plotting the slopes of the straight lines in the Lineweaver-Burk plots in Fig. 2 versus 4EC concentrations.
Structure-activity relationship The inhibitory effects of the following catechol derivatives on β-glucuronidase were studied to elucidate their structure–activity relationship: 4EC, Cat, and 4E2M. The structures of these compounds are shown in Fig. 4. The assay showed that 4EC at 20 µM performed best in inhibiting β-glucuronidase (Fig. 5). In this regard, Cat at 20 µM showed lower but significant inhibition. In 4EC, the hydrogen at the fourth position of Cat was substituted with an ethyl group. 4EC had a much stronger inhibitory effect than Cat (Fig. 5), showing that the presence of a bulky group at the fourth position enhanced the inhibitory activity of β-glucuronidase.
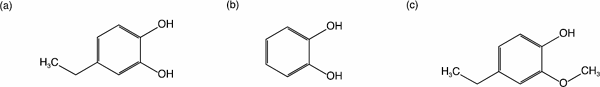
Structure of the assayed compounds
(a) 4EC, (b) Cat, (c) 4E2M
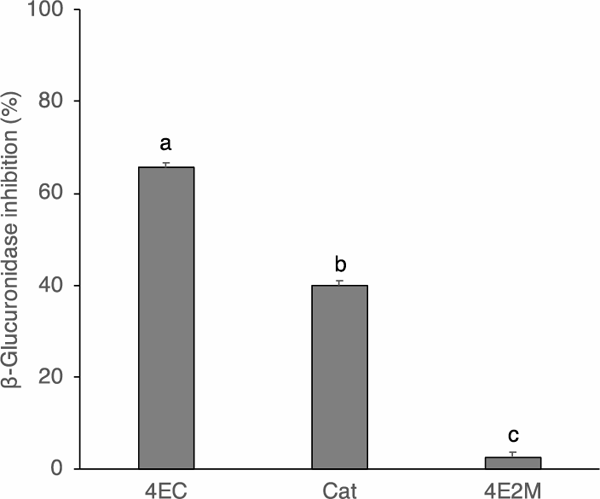
Inhibition of β-glucuronidase activity by the catechol derivatives at 20 µM
Results are presented as mean ± SE of quadruplicate measurements. The mean values were compared using the Tukey's test (p < 0.05). Significant differences are indicated by different alphabets.
However, 4E2M bears a methoxy group at the second position, instead of a hydroxy group, as in the case of 4EC. Consequently, there was no significant inhibitory activity of β-glucuronidase by 4E2M (Fig. 5). These results suggest that a catechol group plays a pivotal role in inhibitory activity. Therefore, we evaluated the inhibition properties of Cat. The inhibitory effect of Cat at different concentrations is shown in Fig. 6. The inhibitory activity increased with the increasing Cat concentration and gradually leveled off. However, inhibition activity did not reach 50%. The inhibitory action of Cat against β-glucuronidase was evaluated using Lineweaver-Burk plots (Fig. 7). Cat was shown to be a competitive inhibitor. The Ki value was calculated from a secondary replot of the slopes of straight lines in the Lineweaver-Burk plots (Fig. 7) versus the Cat concentrations and the resultant value of Ki was 78.6 µM (Fig. 8). Cat is a competitive inhibitor, although its inhibitory activity is weak. Therefore, although the catechol group seemed to play an important role in the competitive inhibition, the substitution with the alkyl group is needed to show the enhanced inhibitory activity.
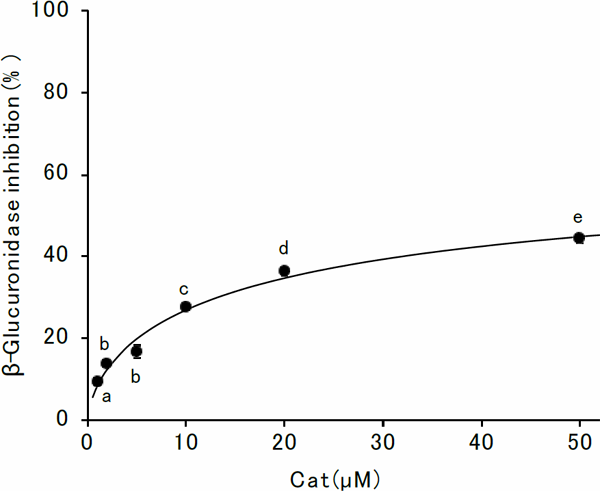
Inhibition of β-glucuronidase activity by Cat
Results are presented as mean ± SE of quadruplicate measurements, unless the error bar is smaller than the symbol. The mean values were compared using the Tukey's test (p < 0.05). Significant differences are indicated by different alphabets.

Lineweaver-Burk plots of β-glucuronidase in the presence of Cat
●, Control; ○, 5 µM Cat; ▲, 10 µM Cat; △, 20 µM Cat; ◆, 50 µM Cat. Results are presented as the mean of quadruplicate measurements.
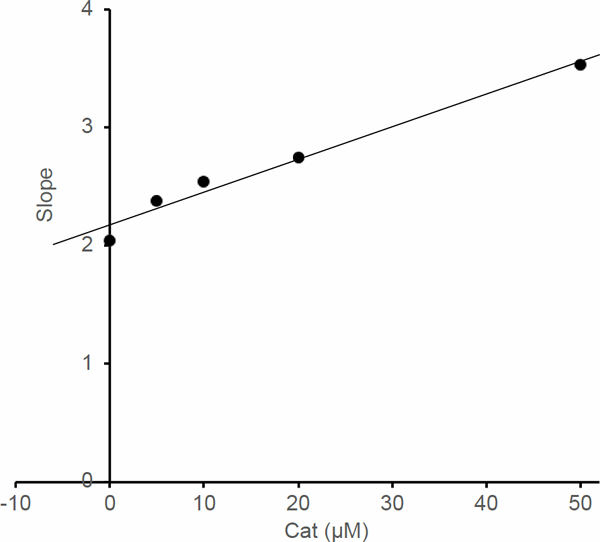
Secondary replot of the Lineweaver-Burk plots of β-glucuronidase in the presence of Cat
Ki was obtained by plotting the slopes of the straight lines in the Lineweaver-Burk plots in Fig. 7 versus Cat concentrations.
Methyl methanethiosulfinate was reported to show the inhibitory effect (Watanabe et al., 2013). The value of IC50 was 3.6 µM, similar to that of 4EC. Interestingly, it was an uncompetitive inhibitor of β-glucuronidase. They suggested the importance of the thiosulfinate group comparing to the disulfide group. The inhibitory mechanisms by the thiosulfinate seemed to be different from the mechanisms by the alkyl catechol.
Demethylbellidifolin and gentisin were reported to be a mixed-type inhibitors, the IC50 values being comparable to 0.91 µM and 0.68 µM, respectively (Sun et al., 2020). Both of them have phenolic hydroxyl groups, like 4EC. However, they are more potent inhibitors compared to 4EC. Sun et al. (2020) showed the interaction between demethylbellidifolin and gentisin with β-glucuronidase by the hydrogen bonds of their hydroxy groups with the amino acid residues of β-glucuronidase. SL, which is also a polyhydroxy compound, was reported to be a competitive inhibitor (Kim et al., 1995). The IC50 value was 19.8 µM (Fig. 9), similar order to 4EC. The presence of the hydroxy groups and the hydrogen bond between the inhibitor and the enzyme seemed to be crucial to inhibit the β-glucuronidase. Further investigations are necessary to evaluate the interaction between the hydroxy groups of 4EC and the β-glucuronidase.
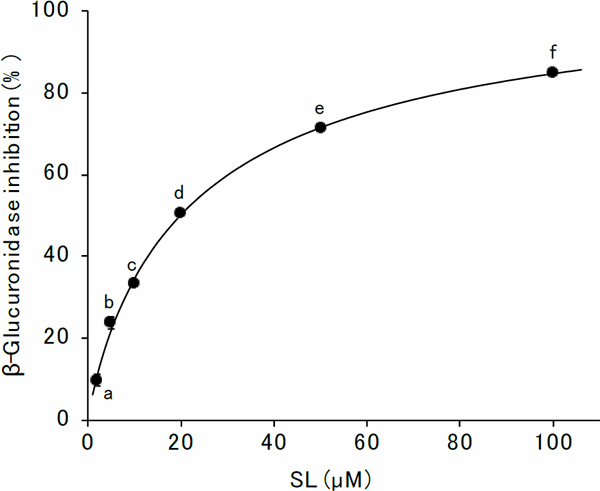
Inhibition of β-glucuronidase activity by SL
Results are presented as mean ± SE of quadruplicate measurements, unless the error bar is smaller than the symbol. The mean values were compared using the Tukey's test (p < 0.05). Significant differences are indicated by different alphabets.
Potential food sources of 4EC are fermented foods and foods smoked with wood chips (Senger et al., 2016). 4EC in fermented foods were produced by lactobacilli, including Lactobacillus collinoides (Senger et al., 2016). The 4EC was produced from dietary phenolics through multi-enzyme conversion. For instance, chlorogenic acid, which was found in a variety of vegetables and fruits (Olthof et al., 2001), was hydrolyzed and caffeic acid was liberated by cinnamoyl esterase. Phenolic acid decarboxylase catalyzed the conversion of caffeic acid to 4-vinylcatechol. In addition, 4-vinylcatechol was reduced to 4EC by phenolic acid reductase (Buron et al., 2011). Cider, made by fermentation of apple juices, contains 4EC. The maturation of ciders significantly increased the level of 4EC (Lobo et al., 2016). The young cider contains ca. 20 µM 4EC. While the matured cider contains ca. 40 µM 4EC. However, in modern times alkyl catechols, such as 4EC, were treated as undesirable off-flavors of fermented foods and efforts were made to eliminate such compounds (Senger et al., 2016). Larcher et al. (2008) determined the 4EC content in wine. They tested the 153 samples of red wine, and 75% of samples were below 640 nM. The contents of the alkyl catechols, such as 4-methylcatechol, 4-vinylcatechol, and 4EC, dramatically reduced in modern Western foods, because of extensive changes in food preservation and preparation (Senger et al., 2016).
Although the content of 4EC in foods was decreased, 4EC was suggested to be produced by microbiota in the intestinal tract. The presence of 4EC was detected in the urine when crude vegetable diets were fed to rats (Bakke, 1969). The presence of 4EC was detected in the urine of rats that fed on caffeic acid, but was not found in germ-free rats (Peppercorn and Goldman, 1972). In addition to this, Rogozinska et al. (2021) showed that Lactobacillus plantarum 299v, probiotic Lactobacillus strain, degraded caffeic acid to 4EC, 4-vinylcatechol, and dihydrocaffeic acid. Therefore, dietary phenolics in vegetables and fruit are expected to be fermented in the intestine by the intestinal microbiota to metabolites including 4EC. In fact, 4EC was detected in human feces at 0.21 µg/g feces (Guadamuro et al., 2015). Using the value of the specific weight of feces, 1.03 g/mL (Kasper et al., 1984), the concentration of 4EC in the feces was estimated to 1.6 µM. At that concentration, the β-glucuronidase inhibitory activity of 4EC is expected to only 20% (Fig. 1). One hundred mL of matured cider contains ca. 4 µmol of 4EC (Lobo et al., 2016), and ca. 580 mL of luminal contents are estimated to transit through the colon per day (Maurer et al., 2015; Sloan et al., 2018; Lam et al., 2019). Therefore, assuming that all of the ingested 4EC, contained in 100 mL of matured cider, reached the intestinal tract, the concentration of 4EC at the colon is expected to 6.9 µM. Considering the 4EC concentration in the feces as the basal 4EC concentration in the colon, 1.6 µM as mentioned above, 4EC concentration is expected to reach 8.5 µM by ingesting 100 mL of matured cider per day. At that concentration, more than 50% of inhibition of the β-glucuronidase activity is expected. Further investigations are necessary to evaluate the effect of 4EC ingested as food on the inhibition of the β-glucuronidase in the colon.
4EC is expected to play a vital role in preventing the utilization of the xenobiotics-glucuronide as a carbon source. In addition to this, it might inhibit the regeneration of carcinogens in the intestinal tract and prevent colon carcinogenesis. Anticancer drugs, including irinotecan, are detoxified by conjugation. However, microbial β-glucuronidase in the intestine hydrolyzes the detoxified drugs and enhances the drug toxicity. Since β-glucuronidase inhibitors could play a vital role in reducing the anticancer-drug toxicity (Gupta et al., 1994; Saitta et al., 2014), 4EC is also expected to reduce the toxicity of anticancer-drug through its inhibitory activity on β-glucuronidase.
In this study, β-glucuronidase from E. coli was used in evaluating the effect of phytochemicals on β-glucuronidase activity. Moreover, β-glucuronidase from E. coli has been widely used (Kavak et al., 2010; Li et al., 2020; Sun et al., 2020). Although E. coli is not the only source for β-glucuronidase in the intestinal tract (Wallace et al., 2010), further investigations are necessary to evaluate the effect of 4EC on β-glucuronidase of other intestinal microbiota.
Evidently, 4EC was found to be a competitive inhibitor of β-glucuronidase. The 4EC present in fermented food was seen to cause off-flavor, which adversely reduced the content of 4EC in fermented foods due to quality improvement. However, 4EC was suggested to be produced by microbiota in the intestinal tract from dietary phenolic compounds. The results obtained in this study suggested that the intake of vegetables and fruit was promising in reducing the risk of colon cancer through inhibition of β-glucuronidase activity.
Acknowledgements The authors are deeply grateful for the English language review provided by Enago (www.enago.jp).
Conflict of interest There are no conflicts of interest to declare.