2021 Volume 27 Issue 6 Pages 923-931
2021 Volume 27 Issue 6 Pages 923-931
Dry-heat-treated sodium caseinate improves yogurt curd strength, but its production is time-consuming. Thus, a simpler pretreatment is needed. We conducted this study to investigate the effects of dry-heat treatment (65 °C, RH 8 %, 2–6 days) of skim milk powder on acid-induced gelation and identify improvement factors. Acid-induced gels were formed by the addition of 1.84 % (w/v) glucono-δ-lactone to skim milk powder dispersions (protein conc., 40 mg/mL). Gel strength improved proportionally to dry-heat treatment duration. We examined the relationship between the improvement of curd strength and protein denaturation induced upon heat treatment of skim milk powder. We observed improved curd strength due to a decrease in zeta potential and an increase in the surface hydrophobicity of casein micelles as well as cross-linking of caseins, which were a result of Maillard reactions. These results suggest that dry-heat treatment of skim milk powder is useful for yogurt manufacturing.
During yogurt production, skim milk powder, milk powder, whey protein, or sodium caseinate are usually added to the yogurt mix. These auxiliary materials are used to maintain the total amount of milk solids and enhance the physical characteristics of yogurt curd (Kaminogawa et al., 2009). Moreover, dry-heat treatment of sodium caseinate with lactose improves the physical characteristics of acid-induced casein gels (Hannß et al., 2018; 2020). It is speculated that the improvement of acid-induced gelation is due to glycation of caseins (El-Salam and El-Shibiny, 2020) and cross-linking of caseins with lysinoalanine (Hannß et al., 2018; 2020) via Maillard reactions. However, the use of sodium caseinate requires dispersion of both sodium caseinate and reducing sugar in water prior to dry-heat treatment, and this is followed by freeze-drying or spray-drying for powdering again. Thus, the preparation is time-consuming, and it is necessary to develop a simpler pretreatment.
Therefore, in this study, we focused on skim milk powder. Since skim milk powder is already in a state where protein molecules are mixed with lactose, it is not necessary to perform operations such as hydration, mixing of proteins with reducing sugars, freeze-drying, or spray-drying before dry-heat treatment. Thus, skim milk powder is more likely to improve acid-induced gelation by dry-heat treatment than sodium caseinate. On the contrary, unlike sodium caseinate, skim milk powder contains both whey protein and casein; thus, it is possible that these two proteins may interact with each other in addition to Maillard reactions during dry-heat treatment.
Heat denaturation of milk proteins that contribute to improved yogurt curd strength is often discussed for the SS bonds formed between κ-casein (κ-CN) and β-lactoglobulin (β-Lg) by heating milk. SS bonds between κ-CN and β-Lg cause κ-CN dissociation from casein micelles and whey protein/κ-CN complexes are formed, which consist of κ-CN, β-Lg, and α-lactalbumin (α-La) (Anema, 2007; 2008). Heat denaturation improves acid-induced gelation (Donato et al., 2007; Mahomud et al., 2017; Morand et al., 2011). Similarly, we previously reported that κ-CN dissociation from casein micelles causes a decrease in zeta potential and an increase in surface hydrophobicity of casein micelles, which improves acid-induced gelation (Oka et al., 2018). Thus, to analyze heat denaturation of milk proteins, which contributes to the improvement of acid-induced gelation, it is important to analyze the dissociation of κ-CN from casein micelles and the formation of whey protein/κ-CN complexes. Heat denaturation may occur upon dry-heat treatment of skim milk powder.
Based on the above background, we conducted this study to examine the effect of dry-heat treatment of skim milk powder on acid-induced gelation as well as the heat denaturation of milk proteins caused by dry-heat treatment. Specifically, we examined the glycation of proteins, cross-linking of caseins, dissociation of κ-CN from casein micelles, and formation of whey protein/κ-CN complexes.
Preparation of samples Low-heated skim milk powder (SMP) was obtained from Megmilk Snow Brand Co., Ltd. (Tokyo, Japan). The SMP was dry-heated in a dry-heat oven (FC-410, Advantec Toyo Co., Ltd., Tokyo, Japan) at 65 °C and RH 8 % for 2–6 days.
Solubility of SMP SMP was dispersed in pure water to a final concentration of 12 % (w/v). The dispersions (20 mL) were stirred at room temperature for 1 h and then centrifuged at 2 000 × g and 20 °C for 5 min to separate the insoluble fraction. The supernatant was collected for determination of protein concentration using the Lowry method. The result is shown as a relative value with non-treated as 100 %.
Physical characteristics of acid-induced gel For the solubility test, the supernatant was adjusted to a protein concentration of 40 mg/mL using pure water. The solution (5 mL) was transferred to φ 12.6 mm × 61 mm plastic tubes and then acidified with 1.84 % (w/v) glucono-δ-lactone at 37 °C for 12 h. The strength of acid gels was measured using a creep meter (RE2-33005C, Yamaden Co., Ltd., Tokyo, Japan). The acid gels were compressed with a φ 3 mm plunger at a compression speed of 1 mm/s to a compression rate of 30 %, and the breaking load (mN) was considered as the curd strength. The water-holding capacity (WHC) of acid gels was measured using centrifugal methods. The acid gels were centrifuged at 2 000 × g and 20 °C for 10 min. Immediately after centrifugation, the supernatant was removed from the plastic tube and weighed. WHC (%) was calculated using the equation (A – B)/A ×100, where A is the total mass of the gel before centrifugation and B is the total mass of the supernatant.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) Polyacrylamide gels were prepared at a concentration of 15 % (w/v). Samples were dissolved in 0.1 M phosphate buffer (pH 6.7) at a protein concentration of 1.5 mg/mL, and the solutions were treated with sample buffer with or without 2-mercaptoethanol. Samples (10 µL) were injected into the gels. Electrophoresis was performed at 300 V and 20 mA for 90 min. Subsequently, gels were stained with CBB R-250 and analyzed using Lumino Graph WSE-6100 and CS Analyzer 4 (both from ATTO Co., Ltd., Tokyo, Japan).
Free amino group content The free amino group content of SMP was determined as previously described (Liu et al., 2013). SMP was dispersed in 1 % (w/v) SDS to a final concentration of 3 % (w/v). The dispersions (125 µL) were mixed with 2 mL of 0.2125 M phosphate buffer (pH 8.2) and 1 mL of 0.01 % (w/v) trinitrobenzenesulfonic acid (TNBS). The solutions were mixed thoroughly prior to being placed in a water bath at 50 °C for 30 min in the dark. The reaction was terminated by adding 2 mL of 0.1 M sodium sulfate. The mixtures were then cooled to room temperature for 15 min. The absorbance was measured at 420 nm. The result is shown as a relative value with non-treated as 100 %.
In-gel tryptic digestion SDS-PAGE gels were cut in the range of > 75 kDa or 50–75 kDa. The gel pieces were decolorized with 25 mM ammonium bicarbonate containing 30 % (v/v) acetonitrile. Next, the gel pieces were reduced using 25 mM ammonium bicarbonate containing 10 mM DTT, and the SH group was modified using 25 mM ammonium bicarbonate containing 55 mM iodine acetamide. The gel pieces were dehydrated with 25 mM ammonium bicarbonate containing 50 % (v/v) acetonitrile, and then rehydrated with 0.1 µg/mL sequencing grade modified trypsin (Promega Japan Co., Ltd., Tokyo, Japan). The enzyme reaction was performed at 37 °C for 12 h. Digested samples were extracted by adding 50 % (v/v) acetonitrile containing 5 % (v/v) trifluoroacetic acid (TFA).
Identification of proteins The peptides of digested samples were identified using an Agilent 1260 Infinity series-MS/MS 6563 Accurate-MASS Q-TOF LC/MS (Agilent Technologies Japan Co., Ltd., Tokyo, Japan). An AdvanceBio Peptide Mapping (Agilent Technologies Japan Co., Ltd.) column was used at an oven temperature of 60 °C, and the samples were eluted at a flow rate of 0.4 mL/min with a gradient of two solvents. Solvent A consisted of 0.1 % (v/v) formic acid, and solvent B consisted of acetonitrile with 0.1 % (v/v) formic acid. Samples were filtered with a 0.45 µm filter, and 5 µL samples were injected into the HPLC system. Equilibration was performed at 0 % concentration of B. Elution was first performed with 40 % B from 0 to 15 min, and the concentration was subsequently increased to 80 % from 15–18 min to remove the adsorbed substances. Q-TOF conditions were set as follows: ionization was performed in ESI positive ion reflector mode, nebulizer gas was set at 40 psi, dry gas was set at 6 L/min and 300 °C, and the fragmenter was set at 150 V. MS/MS analysis was conducted on peptides charged with divalent ions or higher. Peptides were identified using Mascot Server (Matrix Science Inc., Boston, MA, USA) with the Swiss-Prot database (Swiss Institute of Bioinformatics, Lausanne, Switzerland), and the selected peptides significantly matched with cow milk proteins at p < 0.05.
Separation of casein micelle and whey fractions Casein micelle and whey fractions were separated as previously described (Jean et al., 2006; Ono et al., 2017). Next, SMP dispersions (protein conc., 40 mg/mL), which were used for the preparation of the acid-induced gel, were ultracentrifuged at 33 000 × g and 20 °C for 65 min to obtain the supernatant (whey fraction). The precipitate was dispersed with synthetic milk ultrafiltrate (SMUF, pH 6.7) to remove the remaining whey proteins and to maintain the structure of casein micelles, which were then ultracentrifuged at 33 000 × g and 20 °C for 65 min to obtain the precipitate (casein micelle fraction). SMUF was prepared according to previously described methods (Jenness and Koops, 1962; Rosmaninho and Melo, 2006).
Quantification of dissociated κ-CN from casein micelles The dissociated κ-CN from casein micelles was considered as the content of κ-CN in the whey fraction. The whey fraction was diluted using 0.1 M phosphate buffer (pH 6.7) to a protein concentration of 2 mg/mL. For the reduction, 50 µL of 0.1 M phosphate buffer (pH 6.7) containing 50 mM EDTA and 500 mM DTT was added to 850 µL of the diluted sample. After the reduction, the SH group was modified by adding 100 µL of 0.1 M phosphate buffer (pH 6.7) containing 500 mM iodine acetamide. Samples were filtered with a 0.45 µm filter, and RP-HPLC was conducted to determine the κ-CN content. The prepared sample (50 µL) was loaded on a ZORBAX 300SB-C8 column (Agilent Technologies Japan Co., Ltd.) at an oven temperature of 45 °C, and then eluted at 0.5 mL/min using a gradient of two solvents. Solvent A consisted of 0.1 % (v/v) TFA in water, and solvent B consisted of 0.1 % (v/v) TFA in acetonitrile. Separation was performed using the following program: 0–8 min, linear gradient from 30 % B to 37 % B; 8–12 min, isocratic elution of 37 % B; 12–18 min, linear gradient from 37 % B to 40 % B; 18–23 min, isocratic elution of 40 % B; 23–27 min, linear gradient from 40 % B to 80 % B; 27–31 min, isocratic elution of 80 % B; 31–34 min, linear gradient from 80 % B to 30 % B, and 34–36 min, isocratic elution under the initial conditions. Thus, the total duration of a single run, including column equilibration, was 40 min. Detection was performed at a wavelength of 214 nm. The κ-CN peak was determined by fitting to the peak of a standard κ-CN solution (Sigma-Aldrich Japan Co., Ltd., Tokyo, Japan). The κ-CN content of the whey fraction was calculated as a relative ratio against the amount of κ-CN in SMP, which was considered to be 100 %.
Measurement of particle size and zeta-potential The particle size and zeta-potential of casein micelles were analyzed. The casein micelle fraction was dispersed in SMUF (pH 6.7) to a protein concentration of 0.1 mg/mL. The solutions were filtered with a 0.45 µm filter, and they were analyzed using the zeta-potential analyzer Mobius coupled to the pressurizer Atlas (both from Wyatt Technology Co., Ltd., Santa Barbara, CA, USA). Detection was conducted as previously described (Oka et al., 2018). The average particle radius was measured via dynamic light scattering (DLS), and the obtained data were analyzed using the cumulant method. DLS measurements were performed at 25 °C for 5 s, and they were repeated 15 times. Zeta-potential was measured before and after pressurization to 3 × 106 Pa by applying a voltage of 2.5 V at a frequency of 10 Hz for 15 s. The measurements were repeated five times.
Measurement of surface hydrophobicity The surface hydrophobicity of casein micelles was measured as previously described (Hayakawa and Nakai, 1985; Oka et al., 2018). The casein micelle fraction was dispersed in 0.1 M phosphate buffer (pH 6.7) to a protein concentration of 0.1 mg/mL. Next, 40 µL of 2 mM ANS solution was added to 2 mL of the dispersions, and the solutions were mixed and stored in the dark at room temperature for 30 min. The fluorescence intensities of solutions were measured using an RF-5300 (Shimadzu Co., Ltd., Kyoto, Japan). The excitation wavelength was set at 380 nm, and the emission spectra were measured at 480 nm. Fluorescence intensity per milligram of protein was used to indicate the degree of surface hydrophobicity (F.I./mg protein).
Statistical analysis The results represent the mean ± SD of at least triplicate samples measured independently. Statistical analysis was conducted using one-way analysis of variance with the Tukey-Kramer test. Differences were considered statistically significant at p < 0.05.
Solubility of SMP Analysis of solubility was needed because dry-heat treatment induces Maillard reactions that decrease the solubility of milk proteins. Specific examples of this phenomenon are micellar casein isolate (MCI), whey protein concentrate, milk protein concentrate, and SMP (Thomas et al., 2004; Wu et al., 2021; Zenker et al., 2020). In turn, acid-induced gelation depends on the concentration of milk proteins, which is directly related to protein solubility. Thus, we examined the solubility of SMP in this study. The effect of dry-heat treatment (65 °C, RH 8 %) on SMP solubility was analyzed using centrifugal methods. The results are presented in Table 1. Each SMP exhibited a similar solubility.
| Samples | Relative Solubility (%) |
|---|---|
| 0-Days | 100.0 ± 6.4 a |
| 2-Days | 99.8 ± 2.7 a |
| 4-Days | 102.3 ± 4.9 a |
| 6-Days | 104.6 ± 4.1 a |
SMP was dispersed in pure water (SMP 12 % (w/v) and centrifuged (2 000 × g, 20 °C, 5 min)). The supernatant was then analyzed using the Lowry method. Analysis was conducted on non-treated SMP (0-Days), dry-heat-treated SMP at 65 °C for 2 days (2-Days), dry-heat-treated SMP at 65 °C for 4 days (4-Days), and dry-heat-treated SMP at 65 °C for 6 days (6-Days). The solubility of non-treated samples was 100 %. The values are represented as mean ± SD. Values with different superscripts are significantly different (Tukey-Kramer, p < 0.05).
Differences in SMP solubility from another study were considered in terms of RH conditions. For example, according to the conditions of Wu et al. (2021), SMP was dry-heated at 60 °C and RH 74 %, which can promote Maillard reactions. Generally, high RH conditions promote Maillard reactions (Le et al., 2012). Therefore, under the low RH conditions in this study (65 °C, RH 8 %), the progress of Maillard reactions was slower than in the other studies, and did not cause a decline in solubility.
Physical characteristics of acid-induced gels Acid-induced gels were prepared using glucono-δ-lactone with a protein concentration of 40 mg/mL. The curd strength and WHC of acid-induced gels were analyzed. The curd strength of gels is shown in Fig. 1A, and WHC of gels is shown in Fig. 1B. The curd strength of untreated SMP was 76 mN. The strength of dry-heat-treated SMP was significantly improved compared to non-treated SMP following dry-heat treatment; at 6 days of treatment, the curd strength was 150 mN. Similarly, WHC was significantly improved in the dry-heat-treated group than in the non-treated sample. These results clearly indicate that the dry-heat treatment of SMP improves acid-induced gelation as well as for sodium caseinate (Hannß et al., 2018; 2020). Therefore, we examined the heat denaturation of milk proteins, which contributes to the improvement of curd strength.
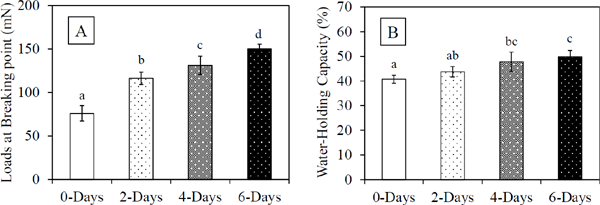
Breaking point loads (A) and water-holding capacity (B) of SMP gels (protein conc., 40 mg/mL).
Gels were acidified with 1.84% (w/v) glucono-δ-lactone at 37 °C for 12 h. Gels were prepared from non-treated SMP (0-Days), dry-heat-treated SMP at 65 °C for 2 days (2-Days), dry-heat-treated SMP at 65 °C for 4 days (4-Days), and dry-heat-treated SMP at 65 °C for 6 days (6-Days). Error bars indicate standard deviation. Values with different letters are significantly different (Tukey-Kramer, p < 0.05).
Analysis of denatured milk proteins Dry-heat treatment of SMP likely causes a mutual interaction between whey proteins and casein micelles, in addition to protein denaturation because of Maillard reactions. Herein, these interactions and protein denaturation were analyzed using SDS-PAGE. SMP dispersions (protein conc., 40 mg/mL), which were used for the preparation of acid-induced gels, were loaded onto reducing and non-reducing SDS-PAGE. After electrophoresis, the molecular weight of protein bands was analyzed. The results are presented in Fig. 2 and Table 2. In reducing SDS-PAGE, each protein band (α-La, β-Lg, κ-CN, β-casein (β-CN), and αs-casein (αs-CN)) of dry-heat-treated SMP was slightly shifted to a higher molecular weight compared to the non-treated samples. Moreover, smeared bands were generated in areas > 50 kDa following dry-heat treatment. In non-reducing SDS-PAGE, protein aggregate bands caused by SS bonds were detected in the stacking gel and in areas > 50 kDa in all samples, suggesting that SMP had already formed SS bonds between κ-CN and β-Lg during the manufacturing process. Furthermore, in non-reducing SDS-PAGE, κ-CN and β-Lg bands showed a similar intensity among all samples, thereby suggesting that SS bond formation between κ-CN and β-Lg was not increased by the dry-heat treatment of SMP.
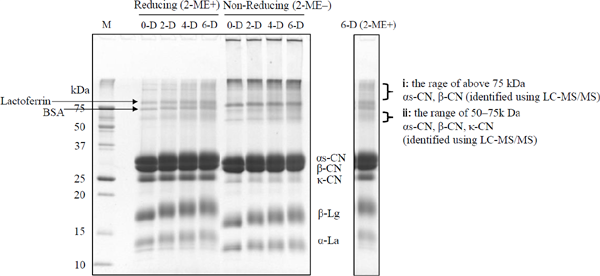
SDS-PAGE (T = 15 %) image of SMP.
Individual lanes were non-treated (0-D), dry-heat-treated at 65 °C for 2 days (2-D), dry-heat-treated at 65 °C for 4 days (4-D), and dry-heat-treated at 65 °C for 6 days (6-D); molecular weight marker (M). Clear bands were identified as previously described (Zenker et al., 2020). The SDS-PAGE gel was stained with CBB. Left image: reducing (2-ME+) and non-reducing SDS-PAGE (2-ME−). The molecular weight of each protein band is shown in Table 2. Right image: reducing SDS-PAGE of dry-heat-treated SMP at 65 °C for 6 days (6-D (2-ME+)). Proteins > 75 kDa and 50–75 kDa were identified using LC-MS/MS. The identified milk proteins are shown in Table 3.
| Samples | αs-CN (Da) | β-CN (Da) | κ-CN (Da) | β-Lg (Da) | α-La (Da) |
|---|---|---|---|---|---|
| 0-Days | 30 902 | 28 738 | 24 908 | 17 190 | 13 612 |
| 2-Days | 31 394 | 28 837 | 25 296 | 17 774 | 14 213 |
| 4-Days | 31 591 | 29 230 | 25 394 | 18 103 | 14 491 |
| 6-Days | 31 689 | 29 525 | 25 492 | 18 249 | 14 538 |
The molecular weight of each protein band was analyzed with the CS Analyzer 4 (ATTO Co., Ltd., Tokyo, Japan) using reducing SDS-PAGE (Fig. 2) of non-treated (0-Days), dry-heat-treated at 65 °C for 2 days (2-Days), dry-heat-treated at 65 °C for 4 days (4-Days), and dry-heat-treated at 65 °C for 6 days (6-Days).
One of the factors that improve curd strength is the increase in whey protein/κ-CN complexes formed by heat denaturation of milk proteins (Mahomud et al., 2017; Morand et al., 2011). The complex is composed of an SS bond between β-Lg and κ-CN. In this analysis, it seemed that the complex was not formed during dry-heat treatment of SMP. Thus, these results suggest that improvements in curd strength were attributable to factors other than β-Lg/κ-CN complex formation.
It was inferred that smeared bands > 50 kDa (reducing SDS-PAGE) were formed by covalent bonds other than SS bonds. Previously, covalent bonds other than SS bonds between milk proteins have been reported, for example, in MCI and sodium caseinate. These covalent bonds are caused by Maillard reactions, and they have been reported to be lanthionine and lysinoalanine (Hannß et al., 2018; 2020; Nielsen et al., 2020). Therefore, for samples subjected to dry-heat treatment for 6 days, the proteins of smeared bands were identified using LC-MS/MS. The results are shown in Fig. 2 and Table 3. In areas > 75 kDa, smeared bands were composed of αs-CN and β-CN. In the 50–75 kDa area, smeared bands were composed of κ-CN, β-CN, and αs-CN. These results suggest that caseins are covalently bonded to each other, except for SS bonds. Our results are similar to those of previous studies.
| Gel fragment | No. | Accession | Score | Mass | Matches | Sequences | emPAI |
|---|---|---|---|---|---|---|---|
| i | 1 | CASA1_BOVIN | 104 | 24 570 | 6 | 3 | 0.64 |
| 2 | CASB_BOVIN | 54 | 25 148 | 1 | 1 | 0.17 | |
| ii | 1 | CASK_BOVIN | 108 | 21 370 | 4 | 2 | 0.54 |
| 2 | CASB_BOVIN | 41 | 25 148 | 2 | 1 | 0.20 | |
| 3 | CASA1_BOVIN | 27 | 24 570 | 1 | 1 | 0.21 |
Milk proteins > 75 kDa (i) and 50–75 kDa (ii) were subjected to dry-heat treatment at 65 °C for 6 days. Protein identification was performed using LC-MS/MS, and significant peptide matches (p < 0.05) were analyzed using Mascot Server (Matrix Science Inc., Boston, MA, USA) with the Swiss-Prot database (Swiss Institute of Bioinformatics, Lausanne, Switzerland). Matched cow milk proteins are shown. Score indicates certainty of protein identification. Matches indicate the number of matched peptides. Sequences indicate the number of matched amino acid sequences. emPAI represents Exponentially Modified Protein Abundance Index, which means relative quantitation of the matched protein. These explanations were referenced from the mascot server (http://www.matrixscience.com/help.html).
It was inferred that the bands shifted slightly to higher molecular weights, and they were involved in the glycation of proteins caused by Maillard reactions. Thus, the glycation of proteins was analyzed using TNBS methods, as described by Liu et al. (2013). The results are presented in Fig. 3. The free amino groups of proteins were significantly decreased by dry-heat treatment of SMP. These results suggest that free amino groups were glycated by Maillard reactions; thus, proteins were shifted to higher molecular weights. Protein glycation was also observed by Liu et al. (2013), Raak et al. (2018), and Wu et al. (2021).

Free amino group content of SMP.
Free amino group content of SMP was detected using TNBS methods, as previously described (Liu et al., 2013). Analysis was conducted on non-treated SMP (0-Days), dry-heat-treated SMP at 65 °C for 2 days (2-Days), dry-heat-treated SMP at 65 °C for 4 days (4-Days), and dry-heat-treated SMP at 65 °C for 6 days (6-Days). The free amino group content of the non-treated sample was 100 %. Error bars indicate standard deviation. Values with different letters are significantly different (Tukey-Kramer, p < 0.05).
To summarize the results, heat denaturation of milk proteins using dry-heat treatment of SMP led to glycation of proteins and covalent cross-linking of caseins caused by Maillard reactions rather than interaction between whey protein and casein micelles.
Quantification of dissociated κ-CN from casein micelles One of the factors other than whey protein/κ-CN complex formation that improves curd strength is κ-CN dissociation from casein micelles (Donato et al., 2007; Morand et al., 2011). Similarly, we reported that κ-CN dissociation from casein micelles causes an increase in surface hydrophobicity and a decrease in zeta potential of casein micelles, improving curd strength (Oka et al., 2018). Therefore, we examined the dissociation of κ-CN from casein micelles. To quantify dissociated κ-CN from casein micelles, SMP dispersions (protein conc., 40 mg/mL), which were used for preparation of the acid-induced gel, were ultracentrifuged and fractioned into casein micelle and whey fractions. Next, κ-CN in the whey fraction was quantified using RP-HPLC. The casein micelle and whey fractions were analyzed using SDS-PAGE, and the results are presented in Table 4 and Fig. 4. The RP-HPLC results (Table 4) showed that the amount of κ-CN in the whey fraction did not change after dry-heat treatment. Moreover, κ-CN and β-Lg bands in the casein micelle and whey fractions detected using SDS-PAGE (Fig. 4) indicated similar intensities among all samples. κ-CN dissociation from casein micelles is similar to whey protein/κ-CN complex formation, which is caused by SS bonds between κ-CN and β-Lg (Anema, 2007; 2008; Donato et al., 2007; Morand et al., 2011). These results suggest that κ-CN dissociation from casein micelles and whey protein/κ-CN complex formation were not improved by dry-heat treatment of SMP. In this study, it was suggested that improving the curd strength by dry heat treatment of SMP did not involve κ-CN dissociation from casein micelles and formation of whey protein/κ-CN complexes.
| Samples | Content of κ-CN (%) |
|---|---|
| 0-Days | 9.65 ± 1.46 a |
| 2-Days | 9.78 ± 0.72 a |
| 4-Days | 10.97 ± 0.26 a |
| 6-Days | 11.36 ± 0.47 a |
Whey fractions were prepared by ultracentrifugation (33 000 × g, 20 °C, 65 min) and analyzed using RP-HPLC. Analysis was conducted on non-treated SMP (0-Days), dry-heat-treated SMP at 65 °C for 2 days (2-Days), dry-heat-treated SMP at 65 °C for 4 days (4-Days), and dry-heat-treated SMP at 65 °C for 6 days (6-Days). The amount of κ-CN in SMP was considered as 100 %. The values are represented as mean ± SD. Values with different superscripts are significantly different (Tukey-Kramer, p < 0.05).

SDS-PAGE (T = 15 %) image of supernatant and precipitation (33 000 × g, 20 °C, 65 min).
Individual lanes were non-treated (0-D), dry-heat-treated at 65 °C for 2 days (2-D), dry-heat-treated at 65 °C for 4 days (4-D), and dry-heat-treated at 65 °C for 6 days (6-D); molecular weight marker (M). The SDS-PAGE gel was stained with CBB. Left image: supernatant of reducing (2-ME+) and non-reducing SDS-PAGE (2-ME−). Right image: precipitation of reducing (2-ME+) and non-reducing SDS-PAGE (2-ME−).
Therefore, it was speculated that improvement in curd strength involved glycation of proteins (Figs. 2 and 3) and covalent cross-linking other than SS bonds of caseins (Fig. 2, Table 3) caused by Maillard reactions. Covalent cross-linking other than SS bonds of caseins improves curd strength. Lauber et al. (2000) and Jaros et al. (2014) reported the relationship between the degree of casein polymerization and curd strength by cross-linking casein with isopeptide bonds by transglutaminase using skim milk or sodium caseinate. Similarly, Hannß et al. (2018; 2020) reported the relationship between the degree of casein polymerization and curd strength by cross-linking casein with lysinoalanine using dry-heat treatment of sodium caseinate with lactose. Moreover, glycation of milk proteins changes the structure of proteins; for example, α-helix and β-turns of milk proteins decrease after dry-heat treatment of SMP (Wu et al., 2021), and the secondary structure of β-CN changes after dry-heat treatment with glucose (Darewicz and Dziuba, 2001). These structural changes are likely to alter the properties of casein micelles. Thus, we examined the radii, zeta potential values, and surface hydrophobicities of casein micelles.
Properties of casein micelles The average radius, zeta-potential, and surface hydrophobicity of casein micelles were analyzed. For these analyses, the casein micelle fraction was used. The results are presented in Table 5. As cross-linking was observed between caseins (Fig. 2, Table 3), the particle size of casein micelles was measured. Surprisingly, the average radius of casein micelles did not change despite cross-linking between caseins. This result suggests that cross-linking of αs-CN, β-CN, and κ-CN occurred inside the casein micelles. Interestingly, similar to our speculation, Moeckel et al. (2016) reported that cross-linking of caseins preferentially occurs inside the casein micelles (intramolecular) rather than between them (intermolecular). Significant intramolecular cross-linking formation was observed in an experimental system using casein micelles and sodium caseinate (Moeckel et al., 2016).
| Samples | Average radius (nm) | Zeta Potential (mV) | Surface hydrophobicity (F.I./mg) |
|---|---|---|---|
| 0-Days | 95.68±1.98 a | −22.41±0.28 a | 11.97±0.49 a |
| 2-Days | 96.20±1.59 a | −21.26±0.70ab | 12.52±0.35 a |
| 4-Days | 94.74±2.18 a | −20.80±0.78 b | 12.30±0.46 a |
| 6-Days | 95.38±1.45 a | −20.13±0.74 b | 13.57±0.18 b |
Casein micelles were prepared by ultracentrifugation (33 000 × g, 20 °C, 65 min). Average radius and zeta-potential were detected using the zeta potential analyzer Mobius (Wyatt Technology Co., Ltd., Santa Barbara, CA, USA), as previously described (Oka et al., 2018). Surface hydrophobicity was detected using the ANS method (ex.380 nm, em.480 nm), as previously described (Hayakawa and Nakai, 1985). Analysis was conducted on non-treated SMP (0-Days), dry-heat-treated SMP at 65 °C for 2 days (2-Days), dry-heat-treated SMP at 65 °C for 4 days (4-Days), and dry-heat-treated SMP at 65 °C for 6 days (6-Days). The values are represented as mean ± SD. Values with different superscripts are significantly different (Tukey-Kramer, p < 0.05).
Subsequently, the zeta potential and surface hydrophobicity of casein micelles were measured. The zeta-potential was significantly decreased and surface hydrophobicity was significantly increased in dry-heat-treated samples compared to non-treated samples. Decreased zeta potential and increased surface hydrophobicity enhanced the hydrophobic interactions between casein micelles, further improving the curd strength (Oka et al., 2012; 2018). The zeta potential and surface hydrophobicity results are attributed to structural changes in casein micelles caused by protein glycation (Darewicz and Dziuba, 2001; Wu et al., 2021). It was speculated that these changes exposed the internal hydrophobic groups to the surface of casein micelles. Thus, these results suggest that one of the factors that improve the curd strength of dry-heat-treated SMP is a decrease in zeta potential and an increase in surface hydrophobicity of casein micelles due to protein glycation.
The relationships between the improvement of curd strength and heat denaturation of proteins caused by dry-heat treatment of SMP are summarized below: improved curd strength was due to decreased zeta potential and increased surface hydrophobicity of casein micelles resulting from protein glycation. The other factor was covalent cross-linking other than SS bonds of caseins caused by Maillard reactions. These phenomena occurred without interaction between κ-CN and β-Lg; in particularly, κ-CN dissociation from casein micelles and formation of whey protein/κ-CN complexes.
Dry-heat treatment of skim milk powder improved the acid-induced gelation to levels similar to that for sodium caseinate. The factors responsible for improvements in acid-induced gelation are denaturation of proteins caused by Maillard reactions without interaction between κ-CN and β-Lg. Thus, κ-CN dissociation from casein micelles and whey protein/κ-CN complex formation are not increased by dry-heat treatment of skim milk powder. Specific factors for improvement of acid-induced gelation are mediated by decreases in the zeta potential and increases in the surface hydrophobicity of casein micelles caused by protein glycation. Another factor is mediated by covalent cross-linking other than SS bonds of caseins caused by Maillard reactions. The results of this study may promote new applications of skim milk powder, which may be useful in yogurt manufacturing. Additionally, the method is simpler and cleaner than current methods, which involve the dry-heat treatment of sodium caseinate. For these reasons, further studies investigating the functional properties of dry-heat-treated skim milk powder and the conditions of dry-heat treatment are needed.
Conflict of interest There are no conflicts of interest to declare.
κ-casein
β-Lgβ-lactoglobulin
α-Laα-lactalbumin
SMPskim milk powder
WHCwater-holding capacity
SDS-PAGEsodium dodecyl sulfate-polyacrylamide gel electrophoresis
SMUFsynthetic milk ultrafiltrate
DLSdynamic light scattering
MCImicellar casein isolate
β-CNβ-casein
αs-CNαs-casein
TNBStrinitrobenzenesulfonic acid
TFAtrifluoroacetic acid