2022 年 28 巻 1 号 p. 1-12
2022 年 28 巻 1 号 p. 1-12
Food chemistry and biochemistry of enzymatic browning are reviewed with a focus on the author's research and recent information. Enzymatic browning is categorized into two types, immediate and delayed, according to the time required for browning. Apple browning has been extensively examined as an example of the former. The enzyme polyphenol oxidase (PPO) and its substrate polyphenols are separated into plastids and vacuoles, respectively, in cells. Disruption of this compartmentation by cutting, crushing, wounding, and physiological disorders allows interaction of the enzyme and substrate, and the reaction starts to turn brown. For the immediate type of enzymatic browning, regulation of oxidative polymerization of phenolic compounds by PPO is essential to inhibit browning. Browning of cut lettuce and mung bean sprouts are described here as the delayed type, in which phenylalanine ammonia-lyase (PAL) is induced during storage and phenolic compounds are synthesized de novo. The formed phenolic compounds are successively oxidized by PPO to turn brown. Regulation of PAL induction is crucial to inhibit this type of browning. Treatments with mild heat shock and cinnamaldehyde are described here as physical and chemical methods, respectively, to repress PAL induction and browning.
Food often discolors or changes color during processing and storage, typically turning brown. It is easy to visually perceive and recognize this phenomenon, which has implications for the purchasing action of consumers. Therefore, regulating discoloration or browning is crucial for food manufacturers. The chemical reactions of browning are varied and complicated. Brown pigments are likely heterogeneous polymers and identification of the chemical structures of pigments is difficult.
In general, the browning of food is divided into enzymatic and non-enzymatic browning. The former is characterized by the oxidative polymerization of phenolic compounds by polyphenol oxidase (PPO) in the presence of oxygen. The latter is known as the Maillard or amino-carbonyl reaction and begins with the condensation of reducing sugars and amino acids/proteins. Here, studies on the enzymatic browning performed by our group and recent information are described.
An outline of enzymatic browning is shown in Fig. 1. Polyphenols or phenolic compounds (Fig. 2) in food are oxidized in the presence of oxygen by PPO, and the formed quinones are then chemically polymerized to turn brown. Various kinds of phenolic compounds and oxidizing enzymes (PPOs) exist in vegetables and fruits, which easily turn brown during processing, e.g., cutting and crushing, and storage. The regulation of browning is crucial for the quality control of vegetables and fruits (Martinez and Whitaker, 1995)?

Enzymatic browning and polyphenol oxidase (PPO).

Chemical structures of several phenolic substrates of polyphenol oxidase (PPO).
Enzymatic browning is classified into two types, immediate and delayed, according to the time required for browning (Table 1). When an apple is crushed, the juice immediately turns brown, or when an apple is cut, the section turns brown within ten minutes or slightly more. On the other hand, when lettuce leaves are cut, browning occurs several days later. The former is the immediate type, and the latter is the delayed type. When sufficient amounts of both PPO and its substrate exist, browning appears as the immediate type, exemplified by apple juice, sections of apple, potato, eggplant, burdock, and avocado, and injured peel of banana. In the delayed type, either the substrate or enzyme (PPO) is insufficient and is formed during storage to turn brown. Cut lettuce and stored mung bean sprout are examples of the delayed type. In these examples, phenolics are synthesized during storage as described later.
| Types of browning | Turning brown | Amount of substrate and enzyme | Example |
|---|---|---|---|
| Immediate type | Immediately after crashing or within a few 10 min after cutting |
Sufficient | Apple juice Black tea Avocado paste Shredded burdock Cut eggplant |
| Delayed type | Several days after storage | Insufficient and formed during storage | Cut lettuce Cut cabbage Mung bean sprout |
PPO is categorized according to the activity toward its substrate. Monophenol-monooxygenase (EC 1.14.18.1; cresolase, tyrosinase, and phenolase), catechol oxidase (EC 1.10.3.1; catecholase, polyphenol oxidase, o-diphenol oxidase), and laccase (EC 1.10.3.2; p-diphenol oxidase) oxidize monophenol, o-diphenol, and p-diphenol, respectively. The major activity is o-diphenol oxidation, and is typically referred to as PPO. Monophenol-monooxygenase is categorical activity and o-diphenol is not isolated during monophenol oxidation by PPO. Biosynthesis of o-diphenol is catalyzed by other specific biosynthetic enzymes in the presence of a co-enzyme. For example, the biosynthesis of l-dopa or 3,4-dihydroxy-l-phenylalanine is catalyzed by tyrosine 3-monooxygenase (E.C. 1.14.16.2) in the presence of 5,6,7,8-tetrahydrobiopterine and oxygen (Goodwill et al., 1997). The putative reaction mechanism of PPO was first proposed by Lerch (1983) and modified by Whitaker (1995) and Eicken et al. (1999), as shown in Fig. 3. PPO exists in three different oxidative states: Deoxy-, Oxy-, and Met-forms. In the Deoxy-form, copper is monovalent, and oxygen does not combine with the enzyme. When oxygen combines with the Deoxy-form, it turns to an Oxy-state, in which copper is divalent and peroxy-like. The Oxy-form reacts with o-diphenol and forms a complex consisting of enzyme, copper, and o-diphenol. In this complex, electrons are transferred, the enzyme is reduced to the Met-form, in which copper remains divalent, and o-diphenol is oxidized to the corresponding quinone. As the resulting Met-form remains oxidative, it oxidizes another o-diphenol and is reduced to the Deoxy-state. During this cycle, one oxygen molecule oxidizes two o-diphenols. For monophenol-monooxygenase, monophenol combines with the copper of the Oxy-form, and a complex made of enzyme, copper, and o-diphenol is formed, from which o-quinone is released, and the enzyme is reduced to the Deoxy-form.
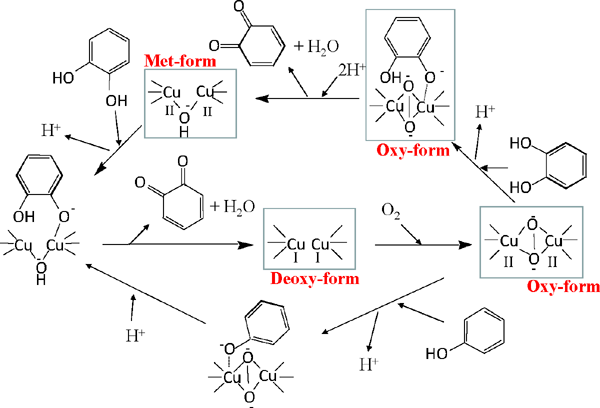
Putative reaction mechanism of polyphenol oxidase (PPO).
This picture is depicted based on the literature of Whitaker (1995) and Eicken et al. (1999) with some modifications.
PPO genes have been cloned from various plants and investigated at the molecular level (Martinez and Whitaker, 1995; Zhang and Sun, 2021). Although the physiological role of PPO in plants is not completely understood, the most plausible role is as part of the defense mechanism against herbivores and pathogens (Zhang and Sun, 2021). The deduced amino acid sequence contained the highly conserved active sites or two copper-binding domains (CuA and CuB). X-ray analyses showed six histidine residues coordinating the two Cu ions (Fig. 4A; Klabunde et al., 1998; Eicken et al., 1999; Virador et al., 2010). In the active site, a unique thioether linkage between cysteine and histidine residues was formed (Fig. 4B; Lerch 1982; Klabunde et al., 1998; Eicken et al., 1999; Virador et al., 2010).

Active site (A) and plausible formation scheme of thioether linkage between cysteine and histidine residues (B) of polyphenol oxidase (PPO).
In many plant foods, o-diphenols such as chlorogenic acids and catechins exist, and they are the major substrate of PPO (Fig. 2). In some plant foods, monophenols such as tyrosine are also a major substrate. For example, the major polyphenol of mature apple is 5-caffeoylquinic acid (5-CQA or 5-chlorogenic acid) and some catechins exist (Murata et al., 1995a, 1995b). For lettuce, in addition to 5-CQA, chicoric acid, an ester formed of two caffeic acids and tartaric acid, is the major polyphenol (Hisaminato et al., 2001).
The amount and type of phenolic compounds have a practical effect on browning. For apple browning, 5-CQA is the most abundant polyphenol in mature apple and shows the highest affinity to apple PPO (Murata et al., 1992). However, catechin, which is generally less abundant than 5-CQA, contributes highly to browning (Murata et al., 1995a). Although mature Japanese pear has a similar level of PPO activity to mature apple, it shows minimal browning because of its low polyphenol content (Tsurutani et al., 2000).
The substrates of PPO, phenolics or polyphenols, are present in vacuoles, while PPO is eventually found in the plastids of fruits or chloroplasts of leaves (Vaughn and Duke, 1984). In normal or young plant cells, the substrate does not come in contact with PPO and browning does not occur in the plants. However, this compartmentation is disrupted by specific circumstances, including food processing, injury by birds and insects, physiological disorders, or maturation, after which contact occurs and browning begins (Fig. 5).

Compartmentation of phenolic substrates and polyphenol oxidase (PPO) in plant cells.
The tissue localization of PPO differs depending on the plant and growth stage. In young apple fruit, PPO distributes in the whole fruit (Murata et al., 1995a),while in mature apple, PPO is mainly located around the core (Murata et al., 1993). Therefore, the core of the section turns brown to the greatest extent. On the other hand, in Japanese pear, PPO distributes in the whole fruit in both immature and mature fruits (Tsurutani et al., 2000).
When we first began studying the enzymatic browning of fruits and vegetables in the late 1980s, apple PPO had not been isolated as a pure protein. We prepared a plastid fraction from mature apple, and then the enzyme was released from the fraction. 5-CQA was used as a substrate for enzyme assays, although 4-methyl catechol was generally used. These improvements allowed us to successfully isolate PPO using about 40 kg of mature apples (Murata et al., 1992). Among polyphenols, 5-CQA was the optimal substrate and showed a smaller Km-value. The enzyme was stable at around pH 7, but the optimal pH was 4. When apple cells are broken, the pH of the juice approaches 4, at which the enzyme reaction progresses easily. At first, we examined the enzyme location in apple fruit immunohistochemically (Murata et al., 1993). The enzyme was mainly located around the core and just below the skin. There was little enzyme in the middle of the flesh. This location of PPO corresponded to the fact that the core of the fruit showed the greatest browning, followed by just below the skin. In immature apple fruit, the enzyme distributed uniformly, and the whole section turned brown. Next, we examined that the localization of PPO in apple cells using both cell fractionation and immunohistochemical methods (Murata et al., 1997). The enzyme was localized in plastids, which are cell organelles corresponding to chloroplasts in leaves. This result was supported by the fact that the genomic DNA of apple PPO had a transit peptide sequence to plastids or chloroplasts (Haruta et al., 1998). The sequence also showed the existence of the two Cu-binding domains.
The browning of apple, which is a typical example of the immediate type, depends on various factors, including the types and amounts of substrates or polyphenols, the amount and property of the active PPO, the presence or amount of oxygen, the pH and temperature of the reaction, the feasibility of the contact between substrates and PPO, and the existence and amounts of reducing agents or inhibitors of PPO. It is necessary to control these factors in the regulation of browning. Effective control methods exist, including heating, decrease of pH, and removal of oxygen and/or phenolics, to inactivate or reduce PPO activity. After PPO is inactivated by heating, the reaction does not proceed; however, as the reaction starts immediately after contact between the substrate and enzyme, other mitigation strategies are required to avoid the reaction before enzyme inactivation by heating. Further, some foods such as fresh fruits and vegetables are not suitable for heating from a quality characteristics standpoint. Therefore, various measures other than heating have been developed, and PPO inhibitors and reducing agents are in practical use (Table 2). Our group also showed that hinokitiol is a useful agent (Okumura et al., 2011). On the other hand, since the 1990s, a molecular biology method has been developed to repress PPO expression in plants (Martinez and Whitaker, 1995). At first, transgenic potato was prepared, in which the expression of PPO and browning were repressed (Bachem et al., 1994). A similar experiment was performed using commercial varieties (Coetzer et al., 2001). Our group also prepared transgenic apple shoots (Murata et al., 2000) and callus (Murata et al., 2001) where the expression of PPO and browning potency were repressed. These transgenic plants showing reduced browning potency have useful characteristics from a food processing standpoint.
| Type of inhibition | Example |
|---|---|
| Reducing agents | Ascorbic acid, K2SO3, Na2S2O5 |
| Quinone couplers | L-Cysteine, glutathione |
| Cu-chelator | NaCl, EDTA, tropolone, CO, citric acid |
| Substrate analog | Cinnamic acid, p-coumaric acid, ferulic acid, nobiletin |
| Others | PVPP, 4-hexylresorcinol, kojic acid, hinokitiol |
When an apple is crushed, the juice turns brown immediately. On the other hand, when Japanese pear is crushed, the juice shows minimal browning. However, when 5-CQA and/or catechins was added to Japanese pear juice at a similar concentration to that found in apple, it turned brown like apple juice (Tsurutani et al., 2000). This result indicates that the amounts of substrates or polyphenols are crucial to browning.
When lettuce leaves were crushed, the juice did not turn brown. However, when polyphenols were added to the juice, it turned brown immediately, similar to that observed for Japanese pear. On the other hand, cut lettuce leaves turned brown gradually during cold storage. These results suggest that the formation of polyphenols during storage is essential for browning. It is assumed that cutting induces the wound response of lettuce, which leads to de novo synthesis of polyphenols, which are successively oxidized by PPO to turn brown (Fig. 6; Hisaminato et al., 2001; Lopez-Galvez et al., 1996; Cantos et al., 2001). Polyphenols are synthesized from phenylalanine, which is a primary metabolite amino acid and is formed via the shikimic pathway. The key enzyme of polyphenol biosynthesis is phenylalanine ammonia-lyase (E.C. 1.14.18.1; PAL), which is at a branch point between the primary and secondary metabolites and catalyzes the formation of cinnamic acid from phenylalanine by oxidative deamination. Fig. 7 shows the mechanism of the PAL reaction (Ritter and Schulz, 2004; Cooke et al., 2009). The active site or catalytic component of the enzyme is 5-methylidene-3,5-dihydro-4H-imidazole-4-one (MIO), which is formed from Ala-Ser-Gly. Cinnamic acid is released from the adduct between phenylalanine and MIO through the elimination of ammonia.

Biosynthesis pathway of polyphenols and enzymatic browning.
CA, cinnamic acid; Cf-CoA, caffeoyl-CoA; 5-CQA, 5-caffeoylquinic acid; DHQ, 3-dehydroquinic acid; diCTA, dicaffeoyltartaric acid; EPSP, 5-enolpyruvylshikimatic acid-3-phosphate; EPSPS, EPSP synthase; PAL, phenylalanine ammonia-lyase; PPO, polyphenol oxidase; QA, quinic acid; S3P, shikimic acid-3-phosphate.

Active site (A) and reaction mechanism (B) of phenylalanine ammonia-lyase (PAL).
This figure is depicted based on literatures (Ritter and Schulz, 2004; Cooke et al., 2009). The active site (MIO) is formed from Ala-Ser-Gly non-enzymatically. The amino group of phenylalanine adds to MIO, and then cinnamic acid is formed from the adduct.
PAL is induced by wounding or cutting. Cinnamic acid is hydroxylated to form caffeic acid, which is combined with quinic and tartaric acids to form chlorogenic acid and dicaffeoyltartaric or chicoric acid. At first, we showed a positive relationship between the induced PAL activities and browning during cold storage of cut lettuce (Hisaminato et al., 2001). Next, the effect of inhibitors of polyphenol biosynthesis on the browning of cut lettuce was examined. Glyphosate, an inhibitor of 5-enolpyruvylshikimatic acid-3-phosphate synthase (EPSPS in Fig. 6) as an enzyme of the shikimic pathway, and 2-aminoindan-2-phosphine, an inhibitor of PAL, dose-dependently repressed the browning of cut lettuce during storage. These results support the importance of de novo synthesis of polyphenols for the browning of cut lettuce (Hisaminato et al., 2001), and that the development of practical methods for PAL inhibition or repression is crucial to control the browning of cut lettuce, leading to a novel means for distribution of cut vegetables.
Mild heat shock treatment is a practical method for regulating the browning of cut lettuce (Loaiza-Velarde et al., 1997; Salveit, 2000). After the cut lettuce immersed in warm water at 50 °C for 90 s was stored at 4°C, the browning, polyphenol content, and PAL activity were measured. The increases in PAL activity and polyphenol content, and browning were minimally observed at day 3 and day 6, while the control lettuce without mild heat treatment turned brown and both parameters increased (Murata et al., 2004). These results clearly showed that the mild heat shock treatment repressed PAL induction and de novo biosynthesis of polyphenol and that browning during cold storage was inhibited (Fig. 8). Lettuce samples treated with mild heat shock maintained their sensory quality, although longer storage induced tissue disruption and rapid bacterial growth (Murata et al., 2004).

Inhibitory effect of cinnamaldehyde and mild heat treatments against the browning of cut lettuce during cold storage. Cinnamaldehyde inhibited the induction of phenylalanine ammonia-lyase (PAL) at the stage of transcription (Tanaka et al., 2011), while mild heat treatment inhibited it at the stage of translation (Campos-Vargas et al., 2005). PPO, polyphenol oxidase.
Next, we screened for PAL inhibitors from food extracts and fermented broths of lactic acid bacteria. We found that cinnamon extracts inhibited the enzyme and the browning of cut lettuce. Cinnamaldehyde was isolated from the extract and identified as an active component (Fujita et al., 2006). Even after cinnamaldehyde-treated lettuce was washed with water, the color of the lettuce showed minimal change. This result suggested that cinnamaldehyde was not simply a PAL inhibitor. Then, we examined the effect of cinnamaldehyde treatment on the induction of PAL mRNA during cold storage of cut lettuce. The expression was repressed by cinnamaldehyde treatment and was at a similar level to mild heat treatment. Western blotting analysis indicated that the PAL protein level was significantly decreased. These results showed that cinnamaldehyde repressed PAL induction at the transcriptional level and regulated the browning (Fig. 8; Tanaka et al., 2011). To maintain the quality of cut lettuce, regulation of browning is crucial. Further development of practical approaches combined with MA packaging and temperature regulation for the control of browning is expected.
Mung bean sprouts are a popular sprout in eastern Asia including Japan, Korea, and China. When mung bean sprouts are refrigerator-stored, they turn brown gradually during cold storage. This browning appears to be a delayed-type of enzymatic browning. However, mung bean sprouts turn brown without cutting. We analyzed the browning in depth and the findings are summarized in Fig. 9 (Nishimura et al., 2012; Sameshima et al., 2016; Kogo et al., 2018). As observed in cut lettuce, PAL was induced and polyphenols were synthesized during cold storage. This appears to be a response to a physiological disorder. The major polyphenol in mung bean sprout is caffeoyltartronic acid. Active PPO, which was limitedly proteolyzed from the C-terminal end, was solubilized in the cytosol. As the cell membrane was partly injured, polyphenols in the vacuole leaked into the cytosol, which was successively oxidized by the enzyme.

Plausible schema of enzymatic browning of mung bean sprout during cold storage.
The top panel shows mung bean sprout before storage. An active polyphenol oxidase (PPO) is mainly present in cytosol, and phenolic compounds synthesized by phenylalanine ammonia-lyase (PAL) induction leak into the cytosol during cold storage, which causes enzymatic browning.
As shown in Fig. 10, PPO exists in various forms, which is dependent on the plant, growth stage, and physiological conditions. PPO mRNA is translated into a precursor PPO protein with a transit peptide to plastids or chloroplasts. In apple fruit, the mature protein transferred to the plastid was active (Murata et al., 1997). In Japanese pear fruit, the mature PPO protein was mostly solubilized in the cytosol and active, most of which was limitedly proteolyzed and remained active (Nishimura et al., 2003). In mung bean sprout, the mature form and the solubilized one in the cytosol were inactive, and the limitedly proteolyzed protein was active (Kogo et al., 2018). Wine PPO was also active in a limitedly proteolyzed form (Virador et al., 2010).

Multi-forms of polyphenol oxidase (PPO) proteins.
Precursor PPO having a transit peptide is transported to plastids or chloroplasts as a mature PPO protein, which is active or inactive. Inactive mature proteins are solubilized into the cytosol and proteolyzed to become active.
The chemistry of enzymatic browning products or brown pigments is not well understood. However, oxidative products of tea catechins have been intensively examined. In the production of black tea, various reaction products consisting of catechins such as theaflavins, theasinensins, and theacitrins are formed (Fig. 11; Tanaka and Matsuo, 2020). Among them, theaflavins and theasinensins are reddish-orange and yellow pigments, respectively. The chemical structures of thearubigins, which are polymers of catechins and major brown pigments, are still unclear. Some trimers and tetramers were identified in model solutions (Menet et al., 2004). These compounds are likely components of low-molecular-weight thearubigins.

Several oxidation products of tea catechins.
These structures are depicted referring to a literature (Tanaka and Matsuo, 2020).
5-CQA is one of the major polyphenols in various plant foods. The reactivity of chemically prepared 5-CQA quinone was examined (Murata et al., 2002). Isolated 5-CQA quinone by preparative HPLC showed an orange color, and then immediately converted to brown polymers. At the same time, about 10% of the quinone is reduced to 5-CQA. When cysteine was added to the solution of 5-CQA quinone, the color of the solution turned pale and cysteinyl-CQA was formed as the major reaction product (Murata et al., 2002).
When catechin was added to the quinone solution, the color became dense. This result corresponded to the fact that apples containing more catechins turned more intensively brown (Murata et al., 1995a). Apple PPO oxidized 5-CQA more rapidly than catechins. However, when 5-CQA and catechins co-existed, the decrease in catechins was promoted, suggesting that 5-CQA quinone oxidized catechins (Amaki et al., 2011).
Recently, the putative structures of dehydrodimers of 5-CQA were proposed as oxidative products of 5-CQA based on MS data (Castillo-Fraire et al., 2019; Fig. 12), although the structure indicates that they are colorless.

Putative structures of dehydrodimers of 5-caffeoylquinic acid (CQA).
A, 2,3-dihydro-1,4-benzodioxan type; B, 2,3-dihydrobenzofuran type; C, 1,2-dihydronaphtalene-type. Dotted enclosing lines show 5-CQA skeleton. These structures are depicted referring to a literature (Castillo-Fraire et al., 2019).
Enzymatic browning has a crucial impact on the quality of fruits and vegetables. The biochemical principle of enzymatic browning has been fairly well understood with the progress of enzymology and plant biochemistry. However, the chemistry of reaction products or brown pigments remains unclear. Practical and more effective measures to regulate browning will be developed with further understanding of the phenomena in respective foods.
Conflict of interest There are no conflicts of interest to declare.
caffeoylquinic acid
EPSPS5-enolpyruvylshikimatic acid-3-phosphate synthase
MIO5-methylidene-3,5-dihydro-4H-imidazole-4-one
PALphenylalanine ammonia-lyase
PPOpolyphenol oxidase