Abstract
Donkey meat is extremely popular in China owing to its high nutritional value and unique flavour. However, the physical and chemical properties of meat from the Dezhou black donkey have seldom been reported. This study aimed to compare differences in the physical characteristics, intramuscular fat (IMF) content, fatty acid profile, and antioxidant capacity of meat from Dezhou black donkey. The results showed that the L* and ΔE values of longissimus dorsi (LD) were significantly lower than those of gluteus maximus (GM) and biceps femoris (BF), while the b* value of LD was significantly higher than that of GM (p < 0.05). The shear force, hardness, adhesiveness, and elasticity values of LD and BF were significantly lower than those of GM (p < 0.05). The IMF content in LD was significantly higher than those in GM and BF (p < 0.05). The contents of fatty acids 15:0, 14:1, 18:2n-6, 20:4n-6, and polyunsaturated fatty acids in LD were significantly lower than those in GM and BF, while the 18:0, 18:1n-9, and monounsaturated fatty acid contents showed the opposite trend (p < 0.05). The superoxide dismutase activity of LD was significantly higher than those of GM and BF (p < 0.05), while the malonaldehyde content showed the opposite trend (p < 0.05). Overall, LD is an ideal edible part of Dezhou black donkey, owing to its higher IMF, tenderness, 18:1n-9, and antioxidant capacity levels, and lower 18:2n-6 and ΔE levels. This study provides novel insight into the development of donkey meat products.
Introduction
The donkey (Equus asinus) is a domestic animal of the equine family, which includes horses, zebras, and mules, and is an important livestock animal, providing leather, milk, and meat (Camillo et al., 2018; Seyiti and Kelimu, 2021). The phrase “Dragon meat in the sky, donkey meat on the ground” praises not only the deliciousness of donkey meat, but also its nutritional value and uniqueness. Previous studies have shown that donkey meat contains leaner meat, essential amino acids, unsaturated fatty acids (UFA), and hexanal (A grass fragrance mainly derived from the oxidative decomposition of UFA), but less total fat, cholesterol, and calories compared with pig, cow, and sheep meat (Polidori et al., 2009, 2015; Li et al., 2020a; You et al., 2008). Furthermore, donkey muscle fiber is more tender than that of other domesticated animal meats (Marino et al., 2015). Donkey meat is a traditional part of the diet in China. In the past, meat was normally obtained from old donkeys slaughtered at the end of their working life, leading to tough meat with bad sensorial attributes (Lorenzo et al., 2014). In recent years, meat production from young donkeys has improved the meat quality, resulting in donkey meat gradually becoming a niche food in the human diet (Lorenzo et al., 2014). Therefore, donkey meat consumption is becoming increasingly popular in China, especially in Hebei, Shandong, and Shanxi provinces (Seyiti et al., 2021). A number of traditional donkey meat snacks have emerged, such as Hejian's donkey pancake, Donga's donkey dumplings, Baoding's donkey meat roll in baked cake, Guangrao's cured donkey meat, Shangtang's salted donkey meat, Gaotang's donkey meat, and Nanjing's spiced donkey meat (Yuan, 2012).
Meat quality (such as cooking rate, water holding capacity, pH, colour, textural parameters, and protein, hydroxyproline, fat, and lactate contents) is affected by several factors, including animal breed, farm management, and animal nutrition (Listrat et al., 2016; Polidori et al., 2008, 2015). Intramuscular fat (IMF) is located in inner muscle bundles and fibres within the muscle bundles, and mostly consists of structural lipids, phospholipids, and triglycerides (Lo Fiego et al., 2010). Compared with other adipose tissues, IMF contains up to 20–30% phospholipids, which are rich in UFA, especially fatty acids 18:1, 18:2n-6, 18:3n-3, and 20:4n-6 (Li et al., 2021a). These properties give IMF special nutritional value. Simultaneously, IMF can positively influence sensory quality traits, such as tenderness, juiciness, taste, and flavour (Listrat et al., 2016; Bahelka et al., 2009). In contrast, oxidation is among the causes of quality deterioration in meat (Buckley et al., 1995). Lipid oxidation of meat is universal owing to the high levels of unsaturated lipids, such as polyunsaturated fatty acids (PUFAs), which are rich in double bonds and readily oxidise to produce malonaldehyde (MDA), resulting in off-flavour development, loss of nutrient value, and decreased shelf life (Falowo et al., 2014; Aslam et al., 2020). As this can be detrimental to meat quality, protecting IMF from lipid oxidation is important.
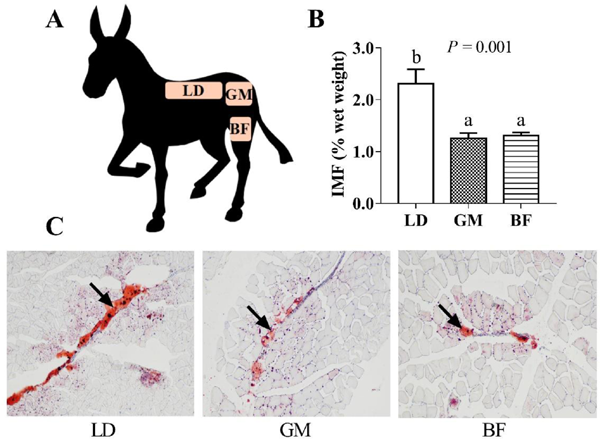
The Dezhou donkey is a large versatile breed, mainly divided into black and chestnut varieties, that has great potential for milk, skin, and meat production (Lai et al., 2021; Wang et al., 2020). Owing to their great adaptability, resistance to rough feeding, and strong disease resistance, the Dezhou donkey has been raised widely in China. A recent study showed that the body weights of Dezhou donkeys at ages of 0 (birth), 6 (weanling), 12, and 24 months were 31.36–32.06, 126.66–128.06, 180.28–180.48, and 218.31–242.27 kg, respectively (Li et al., 2020b). The physical and chemical characteristics of donkey muscles have been investigated at different ages (Polidori et al., 2015) and in different parts of the animal (Polidori and Vincenzetti, 2013). However, little research has been conducted on the meat quality and antioxidant capacity in the main food parts of donkey muscle, such as the longissimus dorsi (LD), gluteus maximus (GM), and biceps femoris (BF) (Fig. 1A). Therefore, the present study aimed to further systematically compare the physical characteristics, IMF content, fatty acid profiles, and antioxidant capacities of the main food parts of Dezhou donkey muscle. This work provides basic data for donkey meat slaughter, segmentation, and processing.
Materials and Methods
Experimental design All experimental procedures were approved by the Animal Care and Use Committee of Liaocheng University (No. LC2019-1). A total of 24 healthy Dezhou black donkeys (male, about 18 months and 150 ± 20 kg) were obtained from a local farmer in Liaocheng City (Shandong, China), and were randomly divided into six replicates, with four donkeys per replicate. All donkeys were fed the same diet, comprising 60% corn silage straw and 40% concentrated feed (corn meal/soybean meal ratio of 3:1) for four months, and were free to eat and drink during the experiment under the same conditions.
Sample collection At the end of the experiment, following a 12-h fast, the final body weight (200 ± 18.25 kg) of the donkeys was recorded. Six donkeys from each replicate were randomly collected and transported to a local slaughterhouse (Shandong Dong'e Tianlong Food Co., Ltd.). After slaughtering the donkeys, LD, GM, and BF samples were collected, immediately placed in liquid nitrogen, and stored at −80 °C for analysis of the fatty acid profile and antioxidant ability. Muscle samples were collected from every part of the donkey and fixed in 4% formaldehyde for oil red O staining. Furthermore, muscle samples (approx. 300 g) were transported to the laboratory for physical and chemical determination.
Evaluation of physical characteristics The cooked meat rate was measured by cooking in a water bath at 100 °C for 5 min, and calculated as a percentage based on the raw and cooked meat weight. The water holding capacity was measured by placing a meat sample in the middle of six layers of filter paper and pressing under a 1-kg weight for 5 min, and calculated as a percentage based on the weight before and after pressing. The muscle pH was measured using a pH meter (Mettler Toledo testo 205, Switzerland, Germany) equipped with an insertion glass electrode (1-cm insert). The L* (lightness), a* (redness), b* (yellowness), and ΔE (total colour difference) values of muscle were measured using a colourimeter (CR-10Plus, Minolta, Japan) with a 50-mm aperture and illuminant D65 daylight, repeated three times in three different locations. The textural properties of fresh donkey muscle samples, which were cut into cuboids of 2 × 2 × 2.5 cm, were measured using a texture analyser (BosinTech Instrument Technology Co., Ltd. Shanghai, China). Texture profile analysis was measured with a pressing speed of 1.0 mm/s and compressing to 60%.
IMF and fatty acid analysis Donkey IMF was extracted with petroleum ether (bp 30−60 °C) using a Soxhlet extractor (Fuchs lubricants Ltd. Shanghai, China), with the results expressed as a percentage of wet weight. Samples were fixed, and then placed in embedding agent, frozen at −80 °C, and processed into 10-µm sections using a cryosectioning machine (CM1900, Leica Corporation, Heidelberg, Germany). Oil Red O and hematoxylin staining were performed for 8 min and 90 s, respectively. Images were taken using a microscope (Olympus Corporation, Tokyo, Japan).
The fatty acid profiles of muscle were determined as reported in previous studies (Li et al., 2017). Lipids were extracted with chloroform/methanol (2:1, v/v) and converted into fatty acid methyl esters (FAMEs) using a mixture of KOH (0.50 mol/L), methanol, and boron trifluoride etherate. Gas chromatography (GC) using a hydrogen flame ionisation detector (GC, 7890B, Agilent Technologies, Palo Alto, CA, USA) equipped with a capillary column (60 m × 250 µm × 0.20 µm, CP7487, Agilent) was used to measure FAME profiles. N2 was used as the carrier gas at 1.0 mL/min, and the split/splitless ratio was 30:1. Air and H2 were used as combustion gas at 400 mL/min and 30 mL/min, respectively. The injector and detector temperatures were maintained at 250 and 300 °C, respectively. After sample injection (1 µL), the column oven temperature was maintained at 140 °C for 5 min, then increased from 140 to 220 °C at 4 °C/min, and finally held at 220 °C for 10 min. Fatty acids were identified and analysed by comparison with standards (CRM47885, Sigma-Aldrich LLC, MO, USA).
Determination of antioxidant ability A mixture of the sample and 0.9% physiological saline (1:9, mg/µL) was prepared on ice, and then ground using a high-flux tissue grinder (Xinzhi Biological Technology Co., Ltd. Ningbo, China) at 4 °C. The sample homogenate was centrifuged at 3 000 × g for 10 min, and the supernatant was taken for analysis of the antioxidant ability. The total antioxidant capacity (T-AOC), and catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GST-Px) activities, in addition to malondialdehyde (MDA) and total protein levels in muscle, were determined using specific kits (Jiancheng Bioengineering Institute, Nanjing, China).
Statistical analysis Data were analysed by one-way ANOVA and Tukey's test using Statistical Analysis system (SAS) v9.2 (SAS Institute Inc., Cary, NC, USA), and are expressed as the mean with standard error of the mean (SEM). Significant differences were set at p < 0.05.
Results and Discussion
Physical characteristic of muscle The physical characteristics of muscles are shown in Table 1. No statistical difference was observed in the cooked meat rate, water holding capacity, pH, and a* values of LD, GM, and BF (p > 0.05). The L* value of LD was significantly lower than those of GM and BF (p < 0.05), while the b* value of LD was significantly higher than that of GM. The ΔE values of GM and BF were significantly higher than that of LD, with the highest value of ΔE obtained in the BF (p < 0.05). These results indicated that the LD was the least dark and showed the lowest colour difference among the three muscles, in agreement a previous report on horse meat (Lorenzo et al., 2013). Compared with the glycolytic muscle fibers (fast-twitch fibers), the oxidative muscle fibers (slow-twitch fibers) have more capillaries, myoglobin, and hemoglobin, and the flesh colour is bright red and has a higher colour score (Lefaucheur et al., 2004). This was supported by the fact that the flesh colour scores of GM and BF with oxidative muscle fibers were higher than that of LD with a higher proportion of glycolytic muscle fibers (Chang et al., 2003). This further confirmed that meat colour depended on different physiological functions in relation to anatomical position.
Table 1.
Comparison of physical characteristic of muscles from different parts of Dezhou black donkey.
| Item |
LD |
GM |
BF |
SEM |
P valve |
| Edible quality |
|
|
|
|
|
| Cooked meat rate (%) |
59.65 |
59.06 |
59.51 |
0.33 |
0.768 |
| Water holding capacity (%) |
95.03 |
95.77 |
95.83 |
0.30 |
0.496 |
| pH |
5.55 |
5.59 |
5.56 |
0.02 |
0.521 |
| L* |
58.32a |
60.60b |
62.20b |
0.47 |
0.001 |
| a* |
13.72 |
12.68 |
13.70 |
0.33 |
0.354 |
| b* |
12.00b |
8.87a |
9.94ab |
0.52 |
0.031 |
| ΔE |
60.61a |
62.73b |
65.23c |
0.50 |
0.000 |
| Textural properties |
|
|
|
|
|
| Shear force (kgf) |
3.18a |
3.89b |
3.06a |
0.14 |
0.024 |
| Hardness (gf) |
1396.95a |
2350.40c |
1747.74b |
107.57 |
0.000 |
| Adhesiveness (gf-mm) |
5.45b |
4.56b |
1.56a |
0.39 |
0.000 |
| Chewiness (gf) |
310.96 |
318.55 |
266.81 |
17.37 |
0.964 |
| Gumminess (gf) |
619.05 |
723.01 |
607.45 |
36.59 |
0.862 |
| Elasticity (mm) |
0.46a |
0.52b |
0.45a |
0.01 |
0.011 |
| Cohesiveness |
0.45 |
0.46 |
0.49 |
0.01 |
0.452 |
| Resilience |
0.09 |
0.10 |
0.09 |
0.00 |
0.167 |
Values are presented as the mean and standard error of the mean (SEM, n = 6). Values with different letters in the same row indicate significant differences (p < 0.05). LD, longissimus dorsi; GM, gluteus maximus; BF, biceps femoris; L*, lightness; a*, redness; b*, yellowness; and ΔE, total colour difference.
The shear force, hardness, adhesiveness, and elasticity values of GM were significantly higher than those of LD and BF (p < 0.05). Furthermore, the adhesiveness values of LD and GM were significantly higher than that of BF (p < 0.05). However, the chewiness, gumminess, cohesiveness, and resilience values were not significantly different among the three muscles (p > 0.05). Evaluation of the shear force and hardness results showed that LD can be considered a tender meat. These results were consistent with previous studies, which reported that the tenderest muscle samples were LD and psoas major/minor, compared with BF and triceps brachii, which presented the highest values in horse foals (Lorenzo et al., 2013). Furthermore, previous studies have shown that the large difference in muscle tenderness in lamb and beef involves different histological characteristics, such as fascia, sarcomere length, muscle fibers types, collagen quality, and solubility (Boleman et al., 2004; Stolowski et al., 2006). BF belongs to the skeletal muscle series and is rich in fascia, which might lead to be a higher meat hardness value.
IMF content As shown in Fig. 1, the IMF contents of LD, GM, and BF in Dezhou black donkey were analysed (Fig. 1A), with that in LD found to be significantly higher than those in GM and BF (p < 0.05; Fig. 1B). These results were also shown by oil red O staining (Fig. 1C). This indicated that the IMF content was different in different muscles of Dezhou black donkey, in agreement with a previous study on muscles of donkey (Polidori and Vincenzetti, 2013; Li et al., 2021b). The IMF content is generally believed to be positively correlated with the proportion of slow oxidation-type muscle fibers, and negatively correlated with the proportion of fast glycolytic-type muscle fibers (Lee et al., 2010). In fact, there is no strict correlation between IMF content and oxidative metabolism of muscle fibers, such as pigs and bison having higher proportions of oxidative muscle fibers but low IMF contents (Hwang et al., 2010; Xiang, 2019). IMF variability depends on the difference in the number of intramuscular adipocytes between fiber bundles, while the correlation between the number of intramuscular adipocytes and muscle fiber types was poor (Damon et al., 2006). Furthermore, the IMF content in LD was up to 2.31% of the donkey, with similar results having been reported in horses (2.08%–2.22%) (Juarez et al., 2009) and sheep (2.0%–2.1%) (Grochowska et al., 2019). Furthermore, the IMF content in donkey muscle was significantly lower than that in cattle (Mirzaei et al., 2009) (4%) and pigs (3.02%) (Li et al., 2013). This was attributed to cattle and pigs having undergone long-term directional breeding, while donkeys have mainly been used as labour animals, with donkey meat quality requiring further improvement.
Muscle fatty acid profiles As shown in Table 2, fatty acids 16:0 (23.59%–26.39%), 18:1n-9 (22.00%–29.86%), and 18:2n-6 (20.14%–26.46%) were the most abundant saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), and PUFA, respectively, which was consistent with a previous study on the longissimus thoracis in donkeys (Polidori et al., 2020; Polidori et al., 2015). The MUFA was the most abundant fatty acid in LD, comprising up to 40.43% of the total fatty acid content, while the SFA was the most abundant fatty acid in GM and BF, comprising up to 33.71% and 33.02%, respectively. These findings indicated that the fatty acid profiles of different parts of the muscle were different. This was attributed to variability in the fatty acid profile of the animal caused by different locations (Polidori et al., 2013; Mi et al., 2019) and maybe also different physiological functions.
Table 2.
Fatty acid profiles of muscles from different parts of Dezhou black donkey.
| Item |
LD |
GM |
BF |
SEM |
P valve |
| 14:0 |
2.37b |
1.88a |
2.17ab |
0.08 |
0.034 |
| 15:0 |
0.16a |
0.20b |
0.20b |
0.01 |
0.031 |
| 16:0 |
26.39b |
23.59a |
23.95a |
0.47 |
0.017 |
| 18:0 |
5.34a |
6.86b |
6.16ab |
0.27 |
0.048 |
| SFA |
34.26 |
33.71 |
33.02 |
0.41 |
0.733 |
| 14:1 |
0.36a |
0.60b |
0.59b |
0.04 |
0.007 |
| 16:1 |
5.81 |
4.78 |
5.46 |
0.29 |
0.349 |
| 18:1n-9 |
29.86b |
22.00a |
24.32a |
1.05 |
0.001 |
| 18:1n-11 |
3.91 |
3.41 |
3.36 |
0.23 |
0.577 |
| MUFA |
40.43b |
30.07a |
34.26a |
1.37 |
0.001 |
| 18:2n-6 |
20.14a |
26.46b |
24.09b |
0.89 |
0.004 |
| 18:3n-3 |
0.97 |
0.91 |
0.94 |
0.05 |
0.934 |
| 20:4n-6 |
1.57a |
3.04b |
3.10b |
0.26 |
0.011 |
| PUFA |
23.68a |
31.95b |
29.32b |
1.18 |
0.007 |
Values are presented as the mean and standard error of the mean (SEM, n = 6). Values with different letters in the same row indicate significant differences (p < 0.05). LD, longissimus dorsi; GM, gluteus maximus; BF, biceps femoris; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; and PUFA, polyunsaturated fatty acid.
The SFA, 16:1, 18:1n-11, and 18:3n-3 contents were not significantly different among the three tissues (p > 0.05). The 14:0 content in LD was significantly higher (p < 0.05) than that in GM, while the 18:0 content showed the opposite trend. Furthermore, the 15:0, 14:1, 18:2n-6, 20:4n-6, and PUFA contents in LD were significantly lower than those in GM and BF (p < 0.05), while the 18:0, 18:1n-9, and MUFA contents showed the opposite trend (p < 0.05). These results indicated that muscles in each part of the donkey showed highly selective oxidation and utilisation of fatty acids, with GM and BF preferentially selecting SFA and MUFA for oxidative energy supply. This might be due to SFAs and MUFAs, such as 16:0, 18:1n-9, 20:1n-9, and 22:1n-11, in animal fats providing energy to the body more easily through mitochondrial β-oxidation (Sargent et al., 2003). In contrast, although PUFA can also undergo oxidation pathway decomposition, the participation of isomerase and epizyme is required (McKenzie et al., 1998; Tidwell et al., 1992). Furthermore, animals selectively use SFA and MUFA to provide energy for oxidation, mainly because PUFA is a biofilm component and biologically active substance, resulting in its greater important biological value compared with energy supply (Douglas et al., 1992). From a food nutrition perspective, LD is an ideal edible part, containing a higher 18:1n-9 content and lower 18:2n-6 content. This is further supported by 18:1n-9 playing a role in the prevention of breast cancer, colorectal cancer, and coronary artery disease (Wolk et al., 1998; Stoneham et al., 2000), while a high n-6 PUFA (especially 18:2n-6) content is associated with increased risk of disease, such as prostate cancer (Williams et al., 2011) and diabetes (Mezouar et al., 2016).
Antioxidant activity of muscles Meat oxidation is caused by an imbalance between pro-oxidants and antioxidants in the body, with T-AOC, CAT, SOD, and GSH-Px as the major antioxidant enzymes for removing reactive oxygen species (ROS) or peroxides produced during oxidation, which prevents lipid oxidation reactions and improves meat quality (Aslam et al., 2020; Ren et al., 2012; Duval et al., 2003). After animals are slaughtered, ROS in muscles increase rapidly, the speed of ROS scavenging and production cannot be balanced, and a large number of ROS attack cells and tissues, further aggravating muscle damage (Da Silva et al., 2018). The oxidation of lipid substances is universal in meat and is thought to be influenced by fatty acid profiles, especially the PUFA concentration (Guermouche et al., 2014; Li et al., 2020b). In the present study, the SOD activity in LD was significantly higher than those in GM and BF (p < 0.05), while the MDA content showed the opposite trend (p < 0.05, Table 3). Muscle with a stronger antioxidant capacity has been reported to have a higher water holding capacity, and more brightly coloured and tender meat quality, and the mechanisms involved might be muscle SOD inhibition of lipid oxidation and decreased MDA content from ROS elimination or reduction (Li et al., 2010). These findings indicated that LD had a high antioxidant capacity and excellent meat quality. Indeed, LD had a higher level of 18:1n-9 compared with other muscles. Fatty acid 18:1n-9 acts as an antioxidant and might cooperate with SOD to increase the antioxidant capacity (Lakshminarayana et al., 2009). In addition, ROS are highly reactive and attack PUFA in muscle, which can induce lipid oxidation to form unstable hydroperoxide, followed by rapid degradation to MDA (Tsikas, 2017). MDA is the final product of lipid peroxidation and a widely accepted biomarker of lipid peroxidation, with the occurrence of MDA closely related to PUFA levels (Falowo et al., 2014). The PUFA content in LD was significantly lower in the present study. This result was consistent with the report that excess PUFA in tissues can lead to increased lipid peroxidation and increased MDA production (Li et al., 2020c). This suggested that oxidation status is also a key factor in the nutritional value of meat.
Table 3.
Comparison of muscle antioxidant ability in different parts of Dezhou black donkey.
| Item |
LD |
GM |
BF |
SEM |
P valve |
| T-AOC (U·mg−1) |
3.12 |
2.96 |
3.02 |
0.02 |
0.582 |
| CAT (U·mg−1) |
2.04 |
2.43 |
2.17 |
0.11 |
0.145 |
| SOD (U·mg−1) |
269.34b |
244.18a |
236.61a |
1.33 |
0.044 |
| GSH-Px (U·mg−1) |
76.28 |
75.32 |
73.68 |
0.86 |
0.729 |
| MDA (nmol·mg−1) |
0.25a |
0.48b |
0.44b |
0.03 |
0.021 |
Values are presented as the mean and standard error of the mean (SEM, n = 6). Values with different letters in the same row indicate significant differences (p < 0.05). LD, longissimus dorsi; GM, gluteus maximus; BF, biceps femoris; T-AOC, total antioxidant capacity; CAT, catalase; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; and MDA, malonaldehyde.
Conclusions
In conclusion, meat quality depends on the anatomical location of the muscle. LD exhibits higher levels of IMF, tenderness, 18:1n-9 and antioxidant capacity, and lower levels of 18:2n-6 and ΔE, making it an ideal edible part of the donkey. This also shows that LD is suitable for making pies, dumplings, and hot pot, while GM and BF are suitable for making sauced meat. These results provide new information for the slaughter, segmentation, and processing of donkey meat.
Acknowledgements This work is supported by the Well-Bred Program of Shandong Province (2017LZGC020), the Scientific Research Fund of Liaocheng University (318052019), Taishan Leading Industry Talents-Agricultural Science of Shandong Province (LJNY201713), the Open Project of Shandong Collaborative Innovation Center for Donkey Industry Technology (3193308), Open Project of Liaocheng Universtiy Animal Husbandry Discipline (319312101-10), Innovation and Entrepreneurship Training Program for College Students (CXCY2021002 and 202110447017), and Shandong Province Modern Agricultural Technology System Donkey Industrial Innovation Team (SDAIT-27).
Ethics All experimental procedures were approved by the Animal Care and Use Committee of Liaocheng University (No. LC2019-1), and conducted in accordance with the National Institute of Health guideline for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Conflict of interest There are no conflicts of interest to declare.
References
- Aslam, S., Shukat, R., Khan, M.I., and Shahid, M. (2020). Effect of dietary supplementation of bioactive peptides on antioxidant potential of broiler breast meat and physicochemical characteristics of nuggets. Food Sci. Anim. Resour., 40, 55-73.
- Bahelka, I., Oravcová, M., Peškovičová, D., Tomka, J., Hanusová, E., Lahučký, R., and Demo, P. (2009). Comparison of accuracy of intramuscular fat prediction in live pigs using five different ultrasound intensity levels. Animal, 3, 1205-1211.
- Boleman, C.T., McKenna, D.R., Ramsey, W.S., Peel, R.K., and Savell, J.W. (2004). Influence of feeding vitamin D3 and aging on the tenderness of four lamb muscles. Meat Sci., 67, 185-190.
- Buckley, D., Morrissey, P.A., and Gray, J. (1995). Influence of dietary vitamin E on the oxidative stability of pig meat. J. Anim. Sci., 73, 3122-3130.
- Camillo, F., Rota, A., Biagini, L., Tesi, M., Fanelli, D. and Panzani, D. (2018). The current situation and trend of donkey industry in europe. J. Equine Vet. Sci., 65, 44-49.
- Chang, K.C., Da Costa, N., Blackley, R., Southwood, O., Evans, G., Plastow, G., Wood, J.D., and Richardson, R.I. (2003). Relationships of myosin heavy chain fibre types to meat quality traits in traditional and modern pigs. Meat Sci., 64, 93-103.
- Da Silva, W., Machado, Á.S., Souza, M.A., Mello-Carpes, P.B., and Carpes, F.P. (2018). Effect of green tea extract supplementation on exercise-induced delayed onset muscle soreness and muscular damage. Physiol. Behav., 194, 77-82.
- Damon, M., Louveau, I., Lefaucheur, L., Lebret, B., Vincent, A., Leroy, P., Sanchez, M.P., Herpin, P., and Gondret, F. (2006). Number of intramuscular adipocytes and fatty acid binding protein-4 content are significant indicators of intramuscular fat level in crossbred Large White x Duroc pigs. J. Anim. Sci., 84, 1083-1092.
- Douglas, R., Tocher, Gabriel, Mourente John, R., and Sargent. (1992). Metabolism of [1-14C]docosahexaenoate (22:6n-3), [1-14C]eicosapentaenoate (20:5n-3) and [1-14C]linolenate (18:3n-3) in brain cells from juvenile turbot Scophthalmus maximus. Lipids, 27, 494-499.
- Duval, C., Cantero, A., Auge, N., Mabile, L., Thiers, J., Negre-Salvayre, A., and Salvayre, R. (2003). Proliferation and wound healing of vascular cells trigger the generation of extracellular reactive oxygen species and LDL oxidation. Free Radical Bio. Med., 35, 1589-1598.
- Falowo, A.B., Fayemi, P.O., and Muchenje, V. (2014). Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res. Int., 64, 171-181.
- Grochowska, E., Borys, B., Lisiak, D., and Mroczkowski, S. (2019). Genotypic and allelic effects of the myostatin gene (MSTN) on carcass, meat quality, and biometric traits in Colored Polish Merino sheep. Meat Sci., 151, 4-17.
- Guermouche, B., Nassima, M., Bouanane, S., Merzouk, H., Merzouk, S.A., and Narce, M. (2014). Effect of dietary n-3 polyunsaturated fatty acids on oxidant/antioxidant status in macrosomic offspring of diabetic rats. Biomed Res. Int., 2014, 368107.
- Hwang, Y., Kim, G., Jeong, J., Hur, S., and Joo, S. (2010). The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci., 86, 456-461.
- Juarez, M., Polvillo, O., Gomez, M.D., Alcalde, M.J., Romero, F. and Valera, M. (2009). Breed effect on carcass and meat quality of foals slaughtered at 24 months of age. Meat Sci., 83, 224-228.
- Lai, Z., Wu, F., Li, M., Bai, F., Gao, Y., Yu, J., Li, H., Lei, C., and Dang, R. (2021). Tissue expression profile, polymorphism of IGF1 gene and its effect on body size traits of Dezhou donkey. Gene, 766, 145118.
- Lakshminarayana, R., Raju, M., Keshava Prakash, M.N., and Baskaran, V. (2009). Phospholipid, oleic acid micelles and dietary olive oil influence the lutein absorption and activity of antioxidant enzymes in rats. Lipids, 44, 799-806.
- Lefaucheur, L., Milan, D., Ecolan, P., and Callennec, C. (2004). Myosin heavy chain composition of different skeletal muscles in Large White and Meishan pigs. J. Anim. Sci., 82, 1931-1941.
- Lee, S.H., Joo, S.T., and Ryu, Y.C. (2010). Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci., 86, 166-170.
- Li, H., Zeng, Y., Wei, S., Chen, Q., Song, Y., Qian, Y., Dong, B., and Cui, Z. (2010). Changes of superoxide dismutase activity and malondialdehyde level in postmortem muscle and their association with meat quality in pigs. Acta Veterinaria et Zootechnica Sinica, 41, 257-261. (in Chinese with English abstract)
- Li, M.M., Zhai, S.S., Xie, Q., Tian, L., Li, X.C., Zhang, J.M., Ye, H., Zhu, Y.W., Yang, L., and Wang, W.C. (2017). Effects of dietary n-6:n-3 PUFA ratios on lipid levels and fatty acid profile of Cherry valley ducks at 15-42 days of age. J. Agr. Food Chem., 65, 9995-10002.
- Li, M.M., Zhang, M., Ma, Y.C., Ye, R.K., Wang, M., Chen, H.Y., Xie, D.Z., Dong, Y.W., Ning, L.J., You, C.H., Wang, S.Q., and Li, Y.Y. (2020c). Dietary supplementation with n-3 high unsaturated fatty acids decreases serum lipid levels and improves flesh quality in the marine teleost golden pompano Trachinotus ovatus. Aquaculture, 516, 734632.
- Li, M., Zhu, M., Chai, W., Wang, Y., Fan, D., Lv, M., Jiang, X., Liu, Y., Wei, Q., and Wang, C. (2021b). Determination of lipid profiles of Dezhou donkey meat using an LC-MS-based lipidomics method. J. Food Sci., 86, 4511-4521.
- Li, M., Zhu, M., Chai, W., Wang, Y., Song, Y., Liu, B., Cai, C., Song, Y., Sun, X., Xue, P., and Wang, C. (2021a). Determination of the heterogeneity of intramuscular fat and visceral adipose tissue from Dezhou donkey by lipidomics and transcriptomics profiling. Fron. Nutr, 8, 703.
- Li, X., Amadou, I., Zhou, G., Qian, L., Zhang, J., Wang, D., and Cheng, X. (2020a). Flavor components comparison between the neck meat of donkey, swine, bovine, and sheep. Food Sci. Anim. Resou., 40, 527-540.
- Li, Y., Feng, P., Li, H., Zhang, X., Ji, C., and Lu, X. (2020b). Study on growth curve fitting of Dezhou donkey. J. Henan Agr. Sci., 49, 143-148.
- Li, Y.S., Zhu, N.H., Niu, P.P., Shi, F.X., Hughes, C.L., Tian, G.X., and Huang, R.H. (2013). Effects of dietary chromium methionine on growth performance, carcass composition, meat colour and expression of the colour-related gene myoglobin of growing-finishing pigs. Asian-Australas J. Anim. Sci., 26, 1021-1029.
- Listrat, A., Lebret, B., Louveau, I., Astruc, T., Bonnet, M., Lefaucheur, L., Picard, B., and Bugeon, J. (2016). How muscle structure and composition influence meat and flesh quality. Sci. World J., 2016, 1-14.
- Lo Fiego, D., Paolo, M., and Piero, S. (2010). Lipid composition of covering and intramuscular fat in pigs at different slaughter age. Ital. J. Anim. Sci., 9, 200-205.
- Lorenzo, J.M., Pateiro, M. and Franco Ruiz, D. (2013). Influence of muscle type on physicochemical and sensory properties of foal meat. Meat Sci., 94, 77-83.
- Lorenzo, J.M., Sarriés, M.V., Tateo, A., Polidori, P., Franco, D., and Lanza, M. (2014). Carcass characteristics, meat quality and nutritional value of horsemeat: A review. Meat Sci., 96, 1478-1488.
- Marino, R., Albenzio, M., Della Malva, A., Muscio, A., and Sevi, A. (2015). Nutritional properties and consumer evaluation of donkey bresaola and salami: Comparison with conventional products. Meat Sci., 101, 19-24.
- McKenzie, D.J., Higgs, D.A., Dosanjh, B.S., Deacon, G., and Randall, D.J. (1998). Dietary fatty acid composition influences swimming performance in Atlantic salmon (Salmo salar) in seawater. Fish Physiol. Biochem., 19, 111-122.
- Mezouar, D., Merzouk, H., Merzouk, A.S., Merzouk, S.A., Belarbi, B., and Narce, M. (2016). In vitro effects of vitamins C and E, n-3 and n-6 PUFA and n-9 MUFA on placental cell function and redox status in type 1 diabetic pregnant women. Placenta, 42, 114-121.
- Mi, S., Shang, K., Li, X., Zhang, C., Liu, J., and Huang, D. (2019). Characterization and discrimination of selected China's domestic pork using an LC-MS-based lipidomics approach. Food Control, 100, 305-314.
- Mirzaei, H., Verbyla, A.P., Deland, M.P.B., and Pitchford, W.S. (2009). Describing variation in carcass quality traits of crossbred cattle. Pak. J. Bio. Sci., 12, 222-230.
- Polidori, P. and Vincenzetti, S. (2013). Meat quality in donkey foals. Ital. J. Food Sci., 25, 390-393.
- Polidori, P., Cavallucci, C., Beghelli, D., and Vincenzetti, S. (2009). Physical and chemical characteristics of donkey meat from Martina Franca breed. Meat Sci., 82, 469-471.
- Polidori, P., Pucciarelli, S., Ariani, A., Polzonetti, V., and Vincenzetti, S. (2015). A comparison of the carcass and meat quality of Martina Franca donkey foals aged 8 or 12 months. Meat Sci., 106, 6-10.
- Polidori, P., Vincenzetti, S., Cavallucci, C., and Beghelli, D. (2008). Quality of donkey meat and carcass characteristics. Meat Sci., 80, 1222-1224.
- Polidori, P., Vincenzetti, S., Pucciarelli, S., and Polzonetti, V. (2020). Comparison of carcass and meat quality obtained from mule and donkey. Animals, 10, 1620.
- Ren, W.K., Yin, Y.L., Yang, G., Li, T.J., and Wu, G.Y. (2012). Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids, 42, 2089-2094.
- Sargent, J.R., Tocher, D.R., and Bell, J.G. (2003). The lipids. Fish Nutr., 3, 181-257.
- Seyiti, S. and Kelimu, A. (2021). Donkey Industry in China: Current aspects, suggestions and future challenges. J. Equine Vet. Sci., 103642.
- Stolowski, G.D., Baird, B.E., Miller, R.K., Savell, J.W., Sams, A.R., Taylor, J.F., Sanders, J.O., and Smith, S.B. (2006). Factors influencing the variation in tenderness of seven major beef muscles from three Angus and Brahman breed crosses. Meat Sci., 73, 475-483.
- Stoneham, M., Goldacre, M., Seagroatt, V., and Gill, L. (2000). Olive oil, diet and colorectal cancer: An ecological study and a hypothesis. J. Epidemiol. Commun. H., 54, 756-760.
- Tidwell, J.H., Webster, C.D., and Clark, J.A. (1992). Effects of feeding, starvation, and refeeding on the fatty acid composition of channel catfish, ICtalurus punctatus, tissues. Comp. Biochem. Physio. A, 103, 365-368.
- Tsikas, D. (2017). Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem., 524, 13-30.
- Wang, C., Li, H., Guo, Y., Huang, J., Sun, Y., Min, J., Wang, J., Fang, X., Zhao, Z., Wang, S., Zhang, Y., Liu, Q., Jiang, Q., Wang, X., Guo, Y., Yang, C., Wang, Y., Zhuang, G., Fan, Y., and Zhong, J. (2020). Donkey genomes provide new insights into domestication and selection for coat color. Nat. Commun., 11, 6014.
- Williams, C.D., Whitley, B.M., Hoyo, C., Grant, D.J., Iraggi, J.D., Newman, K.A., Gerber, L., Taylor, L.A., McKeever, M.G., and Freedland, S.J. (2011). A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr. Res., 31, 1-8.
- Wolk, A., Bergstr M, R., Hunter, D., Willett, W., Ljung, H., Holmberg, L., Bergkvist, L., Bruce, A., and Adami, H.O. (1998). A prospective study of association of monounsaturated fat and other types of fat with risk of breast cancer. Arch. Intern. Med., 158, 41-45.
- Xiang, A.Q. (2019). The function and mechanism of IGFBP5 on fat deposition in intramuscular adipocytes and myocytes. Northwest A & F University, Xi'an. (in Chinese with English abstract)
- You, J., Luo, Y., Zhang, Y., and Zheng, Z. (2008). Nutrition composition of donkey meat and comparison with other livestock and poultry meat. Meat Res., 7, 20-22. (in Chinese with English abstract)
- Yuan A. (2012). Study on developing color and water-holding capacity of donkey meat during Pickling Process. Hebei: Hebei Agr. Univ. (in Chinese with English abstract)


