2022 Volume 28 Issue 4 Pages 307-315
2022 Volume 28 Issue 4 Pages 307-315
Acrylamide (AA) in foods is mainly generated from free asparagine through the Maillard reaction with reducing sugars. Mung bean sprouts are often eaten pan-fried (stir-fried or sauteed). The Food Safety Committee in Japan estimated that the intake of AA from pan-fried/sauteed sprouts was substantially high. Here we analyzed AA formation during pan-frying of mung bean sprouts. Raw sprouts contained about 780, 600, and 380 mg/100 g of fructose, glucose, and asparagine, respectively. The sprouts turned brown gradually during pan-frying, and the AA content increased with up to 9 min of heating; AA of 100 and 200 µg/100 g raw sample was reached after 3 and 9 min of heating, respectively. The sugar content decreased during pan-frying, although the asparagine content did not decrease significantly. When a homogenate of mung bean sprouts was incubated with asparaginase, the asparagine content and the AA generated by pan-frying were reduced. When the sprouts were pretreated at 37 °C for 3 h or 60 °C for 1 h, the AA formation by pan-frying was mitigated.
Acrylamide (AA), a genotoxic carcinogen, exists in various kinds of heated plant-derived foods. The compound is mainly generated in foods from free asparagine and sugars through the Maillard reaction (Fig. 1; Mottram et al., 2002; Stadler et al., 2002). AA can also be formed from 3-aminopropionamide, a degradation product of asparagine (Zyzak et al., 2003), and from acrolein and acrylic acid, especially in lipid-rich foods (Stadler and Scholz, 2004).

Acrylamide (AA) formation in heated foods and its genotoxicity.
AA is mainly formed from asparagine and reducing sugars in heated foods. Ingested AA is metabolized by CYP2E1, a member of the cytochrome P450 oxidase system, to glycidamide showing genotoxicity. This figure is depicted according to FSCJ (2016).
Risk assessments of AA in foods have been conducted in various countries and institutes including the U.S. Environmental Protection Agency (EPA; 2010), the Joint FAO/WHO Expert Committee on Food Additives (JECFA; 2006), and the European Food Safety Authority (EFSA; 2011). In 2016, the Food Safety Commission of Japan (FSCJ) also conducted a risk assessment on AA in foods generated through heating. The first estimate of the dietary AA intake in Japan was calculated as 0.158 µg/kg body weight (bw)/day, in which regular coffee (12 ng/kg bw/day), instant coffee (12 ng/kg bw/day), wheat flour snacks (10 ng/kg bw/day), potato chips (9.4 ng/kg bw/day), and sauteed potato (8.9 ng/kg bw/day) were the five highest contributors. These items occupied about 30% of the total intake. Further, data on heated vegetables such as bean sprouts, lotus root, and cabbage were added, and a revised estimate by FSCJ was performed. In this estimate, the dietary AA intake in Japan was 0.240 µg/kg bw/day and the intake from pan-fried or sauteed bean sprouts (66 ng/kg bw/day) was the largest (FSCJ, 2016).
In eastern countries such as Japan, Korea, and China, bean sprouts are a popular food, and among the various bean sprouts, mung bean sprouts are the most consumed in Japan. About 407 thousand tons of bean sprouts were produced in Japan in 2019 (Food Supply and Demand Sheet, Ministry of Agriculture, Forestry and Fisheries of Japan (i)), and mung bean sprouts account for about 90% of the sprouts (ii). Sprouts are always cooked or heated before eating. Pan-frying (stir-frying or sauteing) and boiling are two popular cooking methods for mung bean sprouts. The sprouts are often pan-fried or boiled at home with other vegetables and meat. As AA is generally formed at temperatures above 120 °C, although it can be formed at lower than 100 °C under drying conditions (Beclaski et al., 2003), pan-frying is more meaningful when considering AA formation and intake from mung bean sprouts. However, there is little data on the formation of AA in mung bean sprouts during heating (Takatsuki et al., 2004; FSCJ, 2016).
The purpose of this study is to clarify AA formation during pan-frying mung bean sprouts and to propose a mitigation method. Here we analyzed the substrate of AA, asparagine and reducing sugars, the formation of AA, and the relationship between browning and AA formation during pan-frying of sprouts. Further, we examined the effect of pretreatment of spouts on AA formation.
Materials Mung bean (Vigna radiata) sprout specimens were purchased from a retail shop in Tokyo between 2018 and 2020 and used for experiments without further storage. All chemicals used in this study were of analytical grade. Dansyl chloride and 1-13C-AA (99% atom% 13C) were purchased from Tokyo Chemical (Tokyo, Japan) and CDN isotopes (Pointe-Claire, QC, Canada), respectively. Asparaginase preparation (Acrylaway L) was supplied from Novozymes Japan (Tokyo).
Preparation of pan-fried mung bean sprouts A Teflon-coated pan (i.d. 24 cm; T-fal, Group SEB Japan, Tokyo) was heated to 250 °C using an induction or IH cooker (MR-20DE, Toshiba, Tokyo, Japan) set to maximum power. The surface temperature of the pan was checked by a digital thermometer (SN-350 Ver II, Netsuken, Tokyo, Japan). Mung bean sprouts (100 g) were put into the pan and stirred at the rate of twice per second with a spatula for 0, 3, 6, 9, and 12 min. Each sample was immediately weighed, before being left for about 10 min at room temperature and then homogenized with a mixer (SKR-M070-SF, TIGER, Monma, Japan) for 1 min.
Analysis of sugars Ethanol (50 mL) was added to the homogenate or paste of mung bean sprouts (10 g) and stirred for 5 min. After the suspension was filtered with filter paper (No.2, ADVANTEC, Tokyo, Japan), the filtrate was evaporated and the volume was fixed to 50 mL with a mixture of water and acetonitrile (50 : 50; v/v). The sugar concentration was determined by the external standard method using a high performance liquid chromatography (HPLC) system equipped with an evaporative light-scattering detector (ELSD). The HPLC conditions were as follows: column, HILICpak VG-50 4E (i.d. 4.6 mm × 250 mm, Showa Denko, Tokyo, Japan) equipped with the guard column HILICpak VG-50G 4A (i.d. 4.6 mm × 10 mm, Showa Denko); eluent, acetonitrile : methanol : water = 85 : 5 : 10 (v/v/v); flow rate, 1.0 mL/min; column temperature, 60 °C; detector, Alltech 3300 ELSD (Alltech, Grace, IL, USA). Fructose, glucose, and sucrose were detected at retention times of about 6.4, 8.2, and 12.3 min, respectively, under this condition. Contents were shown per 100 g of raw sample prior to pan-frying.
Analysis of asparagine Asparagine was extracted and analyzed according to the method described by Yokozeki et al. (2017) with some modifications. Twenty milliliters of 5% (w/v) trifluoroacetic acid aqueous solution was added to a homogenate of mung bean sprouts (1 g) and stirred for 20 min. After the suspension was filtered with filter paper, the filtrate (5 mL) was then loaded onto an OASIS HLB cartridge (Waters, Milford, MA, USA), which had been equilibrated with the trifluoroacetic acid solution prior to use. The cartridge was washed with the trifluoroacetic acid solution. The passed and washed fractions were combined, then the volume was fixed to 10 mL with the trifluoroacetic acid solution. The extracted asparagine was derivatized with dansyl chloride. An aliquot (1 mL) was mixed with 0.5 mL of 0.75 mol/L sodium carbonate aqueous solution and 1.0 mL of 1% (w/v) dansyl chloride-acetone solution, which was incubated at 45 °C for 30 min. n-Hexane (2 mL) was then added to the reaction solution and mixed well. After centrifugation at 1700 × g for 3 min, the aqueous layer was used for HPLC analysis. The HPLC conditions were as follows: system, Chromaster series (Hitachi, Tokyo, Japan); column, COSMOSIL C18-PAQ (i.d. 4.6 × 150 mm, 5 µm, Nacalai Tesque, Kyoto, Japan); eluent, solution A (0.01 mol/L ammonium acetate : acetonitrile = 95 : 5; v/v) and solution B (0.01 mol/L ammonium acetate : acetonitrile = 35 : 65; v/v), 0–100% B (v/v) for 24 min with a linear gradient; flow rate, 1.0 mL/min; column temperature, 40 °C; detection, 254 nm. The retention time of asparagine was about 14.5 min under this condition. Contents were shown per 100 g of raw sample prior to pan-frying.
Analysis of AA AA was extracted and analyzed with the internal standard method using a stable isotope of AA according to the OASIS manual (Waters Japan, Tokyo) and the method of Shimamura et al. (2017) with some modifications. A solution of 2 M NaCl (25 mL) and 12.5 µL of 100 mg/L 1-13C-AA was added to the paste of mung bean sprouts (5.0 g) and mixed well with a vortex mixer. After about 10 min, the suspension was centrifuged at 5 000 × g for 15 min and the obtained supernatant was filtered with filter paper. The filtrate (2 mL) was loaded onto an OASIS HLB cartridge (Waters), which had been equilibrated with 2 M NaCl solution prior to use. After washing with water, AA was eluted from the cartridge with 3 mL of methanol containing 1% formic acid. The eluent was then loaded onto an OASIS MCX cartridge (Waters), which had been equilibrated with methanol prior to use. The passed and eluted fractions from the cartridge with 0.5 mL of methanol were combined and evaporated with nitrogen. After being dissolved in 1.0 mL of water, the solution was applied to liquid chromatography-tandem mass spectrometry (LC/MS/MS) analysis. The HPLC conditions were as follows: column, Inertsil ODS-3 (i.d. 2.1 × 150 mm, 3 µm, GL Science, Tokyo, Japan); eluent, methanol : water : acetic acid = 0.4 : 99.5 : 0.1 (v/v/v); flow rate, 0.2 mL/min, column temperature, 30 °C. Tandem mass spectrometry was conducted as follows: instrument, Triple TOF 4600 (AB Sciex, Foster City, CA, USA); ion source, electrospray positive; spray voltage, 5 500 V; nebulizing gas, 50 psi; heating gas, 50 psi; curtain gas, 25 psi; source temperature, 500 °C. Acrylamide was detected at m/z 55.0 (12C- AA) and m/z 56.0 (13C- AA) in a product ion scan of m/z 72.0 (12C- AA) and of m/z 73.0 (13C- AA). For quantification, 50–2 000 ng 12C-AA/mL containing 100 ng 13C-AA/mL was used as standard solutions for calibration. Detection and quantification limits were about 8 µg/kg and 23 µg/kg, respectively. Contents were shown per 100 g of raw sample prior to pan-frying.
Measurement of the color of mung bean sprouts A homogenate of mung bean sprouts was placed in a plastic vessel (i.d. 4.5 × 1.2 cm), which was wrapped in a plastic bag (Uni-Pack B-4, Seisannipponnsha, Tokyo, Japan). The L*-, a*-, and b*-values of the homogenate surface covered with a plastic film were measured with a colorimeter (NF333, Nippon Denshoku, Tokyo, Japan; n = 8). At least five points per sample were measured. The ΔE-value, the color difference, was calculated using the following equation:
 |
Arbitrary pan-frying of mung bean sprouts We asked 5 students (21–23 years old) to pan-fry 100 g of mung bean sprouts with an arbitrary method without oil, seasonings, or other ingredients according to individual preferences of appearance.
Asparaginase treatment Asparaginase (Novozymes Japan, Tokyo; 0.1 mL, about 390 U) was added to 100 g of a homogenate of mung bean sprouts in a food-grade plastic bag with a zipper (Uni-Pack H-4, Seisannipponnsha), which was mixed well by hand for 30 s and left at 50 °C for 1 or 3 h. This homogenate was pan-fried for 3 min as described above.
Pretreatment of mung bean sprouts Mung bean sprouts (100 g) were packed in the plastic bag, which was incubated at 37 °C for 1 or 3 h (pretreatment at 37 °C). Mung bean sprouts (100 g) and 300 mL of hot water at 60 °C were together placed into the plastic bag with a zipper and then immersed in a water bath at 60 °C for 10 min or 1 h. After incubation, the water was removed with a strainer (pretreatment at 60 °C). Sugar and asparagine contents and formed AA after 3 min of pan-frying were measured as described above.
Bacterial counts Sterile saline (0.85% NaCl, w/v) containing 0.1% peptone (w/v; 90 mL, pH 7.0) was added to 10 g of a pan-fried sample in a sterile filter bag (Elmex, Tokyo, Japan), which was homogenized (MASTICATOR homogenizer, IUL, Barcelona, Spain) for 1 min. The suspension (1.0 mL) was mixed with heart infusion agar (Difco Laboratories, Detroit, MI, USA) or the diluted ones (0.1 mL) were spread on the agar and incubated at 37 °C for 24 h. The aerial bacterial number was expressed as a colony-forming unit (CFU) per gram sample.
Statistical analysis Statistical analyses were performed using Excel 2010 (Microsoft, Redmond, WA, USA) with the add-in software Statcel 4 (OMS, Tokorozawa, Japan). Differences among samples were analyzed by one-way ANOVA, followed by the Tukey-Kramer or Dunnett's multiple comparison test.
Decrease in weight and browning of mung bean sprouts during pan-frying First, the changes in weight and color of mung bean sprouts during pan-frying (stir-frying or sauteing) without cooking oil using a Teflon-coated pan were examined. We set the start temperature of the pan surface at 250 °C. Although cooking oil is usually used for pan-frying, oil was not used in this experiment to ensure as simple heating conditions as possible. Bean sprouts were stirred at the rate of twice per second to avoid burning. Fig. 2A shows the decrease in weight of mung bean sprouts. The weight decreased lineally during pan-frying. In this condition, the weight was about half of that originally after 9 min of heating, indicating that the solid content was approximately double that of the sample before heating. The core temperature of the mung bean sprouts was 66 ± 1 °C with 3 min of pan-frying. Fig. 2B shows the changes in the appearance of the mung bean sprouts, which gradually turned brown during pan-frying. The browning was estimated with the L*-, a*-, b*-, and ΔE-values of each sample (Fig. 3). The L*-value showing brightness decreased, while the b*-value showing yellow increased. The ΔE-value, the color difference from the color before heating, increased gradually. From the appearance, all samples seemed to be edible.

Decrease in weight (A) and changes in appearance (B) of mung bean sprouts during pan-frying or sauteing.
Mung bean sprouts (100 g) were heated on a pan preheated at 250 °C with stirring (n = 4).

Browning of mung bean sprouts during pan-frying.
Color changes of samples were shown by L*- (A), a*- (B), b*- (C), and Δ E- (D) values. Mung bean sprouts (100 g) were heated on a pan preheated at 250 °C with stirring. Different letters show significant differences (p < 0.05; n = 8).
Contents of asparagine and sugars and formation of AA during pan-frying AA is generated from free asparagine through the Maillard reaction with reducing sugars. Then, sugars and asparagine in mung bean sprouts were analyzed. Mung bean sprouts contained 375 ± 49 mg (mean ± standard deviation; n = 6) of asparagine/100 g fresh weight (FW). The content is substantially high among vegetables and beans. For example, potatoes contained about 570–1 400 µg/g FW (Ohara-Takada et al., 2005) or about 2 000–4 200 mg/kg FW (Amrein et al., 2003). Green coffee beans contained about 680 µg/g FW for robusta beans and about 360 µg/g FW for arabica beans (Murkovic and Derler, 2006). It is well-known that fried potato products and roasted coffee beans contain high amounts of AA (EFSA, 2011; FSCJ, 2016). The content of asparagine in mung bean sprout seemed to decline somewhat, about 25 mg/100 g after 12 min of panfrying on a raw sprout basis, however, the decrease was not significant (Fig. 4A).

Contents of asparagine (A) and sugars (B; fructose, glucose, and sucrose) and decrease in sugar (C) in mung bean sprouts and during pan-frying or sauteing.
Mung bean sprouts (100 g) were heated on a pan preheated at 250 °C with stirring. Each content was shown per 100 g of a preheated sample (n = 6). The decrease in sugar shows that in the sum of the averages of fructose, glucose, and sucrose contents.
The sprouts contained 776 ± 121, 601 ± 183, and 229 ± 188 mg of fructose, glucose, and sucrose/100 g FW, respectively. As shown in Fig. 4B, the contents of fructose and glucose seemed to decline, although not significantly. Fig. 4C shows the time course of the decrease in sugar, the sum of the average of each sugar content, during pan-frying. The decrease in sugar increased during pan-frying. After 12 min of heating, there was a decrease of about 230 mg/100 g sugar. Data from the analysis of model solutions containing glucose and lysine (Mikami et al., 2015) indicated that the decrease in about 120 mg/100 mL of glucose in the presence of lysine causes browning (absorbance of 0.5 at 400 nm). Thus, although asparagine and each reducing sugar were not significantly decreased during pan-frying, the browning occurred as shown in Figs. 2 and 3, indicating that the Maillard reaction and/or caramelization proceeded during pan-frying.
Next, the generation of AA was examined. As shown in Fig. 5A, AA was gradually formed during pan-frying. After 3 and 9 min of heating, the content reached about 100 and 200 µg/100 g of raw sprouts, respectively. Takatsuki et al. (2004) reported that mung bean sprouts oven-baked at 220 °C for 5 min contained about 550 ng/g raw sprouts (n = 1) or 55 µg/100 g. FSCJ (2016) described that AA content in bean sprouts was 752 ng/g FW, which was an average of pan-frying for 2 and 7 min. Our data reflects those data, although our values seem to be greater. Yuan et al. (2008) examined the decrease in asparagine and the formation of AA in a model solution containing fructose and asparagine. From the data, it was calculated that the decrease in ca. 40 mg/100 g of asparagine resulted in the formation of 100 µg/100 g AA. We could not significantly detect this level of decrease in asparagine here (Fig. 4B).

Acrylamide formation in mung bean sprouts during pan-frying (A) and a correlation between the Δ E-values and the acrylamide content (B).
Mung bean sprouts (100 g) were heated on a pan preheated at 250 °C with stirring. The AA content was shown per 100 g of a raw sample prior to pan-frying (n = 6). Different letters show significant differences (p < 0.05)
Then, a correlation between the Δ E-value and AA content during pan-frying was examined. The Δ E-value can be used as an indicator of browning and the Maillard reaction. As shown in Fig. 5B, a positive correlation (r2 = 0.949) was apparent. The more the sprouts turned brown, the greater the amount of AA was generated up to 9 min of heating. As shown in Fig. 5A, the AA content reached a maximum at 9 min of heating and seemed to plateau or subsequently decrease somewhat.
In general, high temperatures promote browning and the Maillard reaction. Frequent stirring should repress the rapid rise of the surface temperature of the sprouts, AA formation, and browning. It is necessary to examine the effect of stirring frequency and oil addition on AA formation and browning.
The shorter the duration of mung bean sprout heating, the less AA was generated. However, heating is essential for the food safety of mung bean sprouts. In our conditions, after 1.5 and 3 min of pan-frying, the core temperature reached 60 and 66 °C, respectively. Initial aerobic bacterial counts of retail mung bean sprouts were 2.3 ± 0.5 × 10 7 CFU/g (n = 8). After 1.5 and 3 min of pan-frying, the aerobic bacterial number was 290 (n = 4) and 18 CFU/g (n = 3), respectively. In our conditions, heating for 3 min resulted in more than 10−6 reduction of bacteria, and about 100 µg AA/100 g of raw sprouts corresponded to 125 µg AA/100 g pan-fried sprouts.
Cooking conditions seem to be dependent on individual preferences. As a preliminary experiment, we asked five students to pan-fry mung bean sprouts for consumption without seasonings and ingredients. As a result, the AA content varied among individuals and ranged from 2.7 to 92 µg/100 g of raw sprouts (29 ± 36 µg/100 g of raw sprouts). The decrease in the sample weight ranged from 11.9 to 26.3% (mean, 19.8%), corresponding to less than 3 min of heating in our experimental conditions. The color difference of these samples (ΔE-value, 10.8 ± 3.7) also corresponded to less than 3 min of heating in our experimental conditions. Examination of the effect of various cooking conditions including stirring, and the addition of oil and/or seasonings on the formation of AA in more detail is required. The use of oil during pan-frying might be a key to mitigating the formation of AA in pan-fried mung bean sprouts, as it prevents burning or a local rise of high temperatures. Viné et al. (2020) reported that the bottom surface temperature of heated potato placed on aluminum at 180 °C with oil was lower than that without oil and that the water loss of the heated potato with oil was less than that without oil.
Effect of asparaginase on AA formation in a homogenate of mung bean sprouts by pan-frying Asparagine is a precursor of AA, and although asparagine did not significantly decrease during pan-frying of mung bean sprouts, the effect of asparaginase on AA formation was examined using a homogenate of mung bean sprouts. As shown in Fig. 6, asparagine was decreased by the addition of asparaginase; after 1 and 3 h of incubation, it was reduced by less than 30 and 1% of the original content, respectively. Further, only 10% of AA was formed in the sample incubated with asparaginase for 1 h. AA was hardly detected in the sample incubated with asparaginase for 3 h. In a control sample without asparaginase and incubated for 3 h, asparagine content was almost the same as the control without incubation. These results clearly showed that asparagine was the major precursor of AA in mung bean sprouts. Although the reason why the significant decrease in asparagine during pan-frying was apparent in our experiments is unclear, it is necessary to point out the several thousand times difference in the AA and asparagine contents; the former is at a level of 100 µg/100 g, and the latter is at a level of some 100 mg/100 g. It was previously reported that asparaginase treatment is useful to mitigate AA formation in heated foods (Xu et al., 2016).

Effect of asparaginase on asparagine content and acrylamide formation in a homogenate of mung bean sprouts.
A homogenate added with asparaginase was incubated at 60 °C for 1 or 3 h, before being pan-fried for 3 min (n = 2). Asparagine (about 380 mg/100 g) and acrylamide (about 90 µg/100 g) contents in samples without asparaginase and incubation before and after pan-flying were set to 100%, respectively.
Effect of pretreatment of mung bean sprouts on the formation of AA by pan-frying As described above, the decrease of asparagine in mung bean sprouts leads to the repression of AA formation during pan-frying. This result suggests that a pretreatment capable of decreasing the contents of asparagine and reducing sugars would be efficient to mitigate AA formation. To identify such a method, various pretreatments were examined. Preliminarily, mung bean sprouts were left at 37 °C in a plastic bag overnight, and the results showed that this pretreatment reduced the formation of AA (data not shown). Then the effect of pretreatments at 37 °C for 1 and 3 h in a plastic bag or at 60 °C for 10 min and 1 h in warm water on asparagine and sugar contents and the formation of AA were examined. Fig. 7A shows that the pretreatments at 37 °C for 3 h and 60 °C for 1 h led to a significant reduction of AA formation. The contents of asparagine and sugars in the sprouts incubated in water at 60 °C for 1 h were significantly lower than the control (Figs. 7B and 7C), which explained the reduction in AA. The reason why the contents of AA in the sprouts incubated at 37 °C for 3 h was lower than the control despite the same levels of asparagine and sugars was unclear. In general, the surface temperature of the sprouts during pan-frying is higher than the inner temperature, since the inner part is rich in water, and the temperature is below the boiling point of water. The temperature at which AA formation begins is dependent on the moisture content of the food matrix. Gökmann et al. (2006) showed that about 70 and 2 700 ng AA/g were formed at the surface region of French fries, while the core region was free of AA, after frying for 9 min at 150 and 170 °C, respectively. These results suggest that AA formation mainly occurred at or near the surface of the sprouts. The contents of asparagine and/or reducing sugars near the surface of sprouts might be decreased by the treatment at 37 °C for 3 h, although the treatment did not reduce the total contents of these compounds.
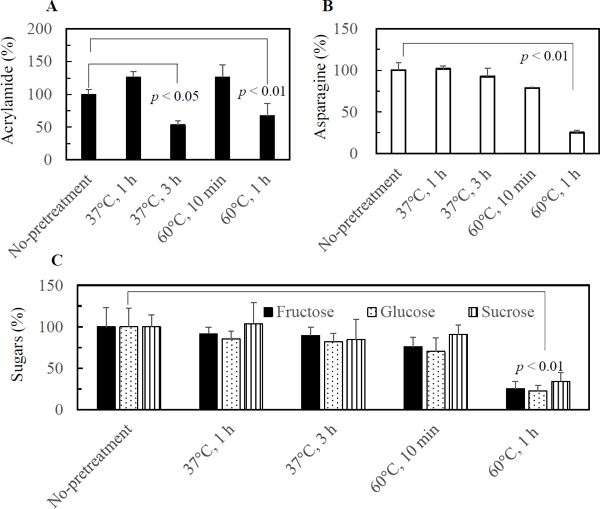
Effect of pretreatment of mung bean sprouts on acrylamide formation (A) and contents of asparagine (B) and sugars (C).
Mung bean sprout samples were preincubated at 37 °C for 1 or 3 h or at 60 °C for 10 min or 1 h, before being pan-fried for 3 min (n = 3). Contents of asparagine, fructose, glucose, and sucrose before pan-frying (about 360, 600, 440, and 130 mg/100 g) and content of acrylamide after pan-frying (about 0.15 mg/100 g) without pretreatment (No-pretreatment) were set to 100%, respectively.
In conclusion, the mung bean sprouts pan-fried/sauteed for 3 min, which showed a pale brown color and about 20% weight reduction, contained about 100 µg AA/100 g of raw sprouts. The AA formation was repressed by a reduction in asparagine content. Pretreatment by incubation at 37 °C for 3 h or 60 °C for 1 h mitigated AA formation during pan-frying. Reduction of asparagine in the surface area should be a useful mitigation method for AA formation. Further, it is necessary to examine the effect of stirring frequency and the addition of oil and other seasonings during pan-frying from the standpoint of home cooking.
Acknowledgements This study was supported by a Grant-in-Aid for Scientific Research (B) [grant number 17H01958] from the Japan Society for the Promotion of Science.
Conflict of interest There are no conflicts of interest to declare.
acrylamide
bwbody weight
EFSAEuropean Food Safety Authority
FSCJFood Safety Commission of Japan
FWfresh weight
HPLChigh performance liquid chromatography
JECFAJoint FAO/WHO Expert Committee on Food Additives
LC/MS/MSLiquid chromatography-tandem mass spectrometry
EPAU.S. Environmental Protection Agency