2022 年 28 巻 6 号 p. 467-478
2022 年 28 巻 6 号 p. 467-478
The traditional thermal processing of the fruit juices exhibited several disadvantages related to nutrients and quality loss. Thus, in order to get the full nutritional characteristics, there is a need to investigate the processing techniques which can maintain the quality of fruit juices. In this study the effect of high pressure carbon dioxide (HP-CO2) at different temperatures (20, 30, 40, 50, 60 and 70 ºC), an emerging juice processing treatment was investigated on the physiochemical properties, enzymatic activity and phenolic profile of peach, apple and pear juices. For comparison, the juices were also subjected to the thermal treatment at the same temperatures. Results showed that HP-CO2 caused significantly higher decline in pH, total soluble solids (TSS) and increase total color difference (ΔE) value then thermally treated juices. Because of having lower pH and other factors, comparing to thermal treatment, the HP-CO2 treated juices exhibited significantly lower relative enzymatic activity which caused lower relative browning degree of the juices. The phenolic compounds were slightly decline by HP-CO2 treatments. Generally, increase in temperature resulted in decrease in pH, TSS, enzymatic activity and increase in ΔE value. It is concluded that HP-CO2 juice processing technique can be used effectively for processing the fruit juices with quality attributes.
Fruit intake reduces the risk of several diseases especially oxidative stress related diseases such as cancer, aging?related disorders, cardiovascular and inflammation (Escudero-Lopez et al., 2016). The nutritious effects are due to the intake of certain biologically active compounds in the diet including phenolics, anthocyanins, tocopherols, polyphenols, carotenoids fibers, minerals, vitamins (Kongkachuichai et al., 2015; Liu, 2013). In many countries, fruit consumption is still below the recommended level, and the global burden of major diseases is due to low consumption (Mytton et al., 2014). Therefore, promoting fruit consumption is the main goal of food and nutrition policy (Rekhy and McConchie, 2014) Fruit consumption in the form of juices is a popular way to eat fruits and contribute to a healthy lifestyle with healthy diet (Wootton-Beard and Ryan, 2011). Among fruit juices, peach juice contains profuse nutrients, such as iron, carotenoids, proteins, vitamins and fiber as well as good taste (Spilimbergo and Ciola, 2010). Apple is another one of the most consumed fruits globally. Apple juice is considered a health promoting juice because of having diverse biologically active and nutritive compounds including organic acids and polyphenols (Kalinowska et al., 2014). Among temperate fruits, pear fruit ranks third after apple and grape1). Pear juices are the source of dietary fiber, vitamins, energy and minerals. The high amount of pear fiber is helpful in diabetes management and glucose metabolism. As compared to apple, pear provides more potassium and less sodium (Xie et al., 2007). In recent years, due to the peculiar pleasant taste of pear juice and the existence of functional food ingredients (such as phenolic compounds), people are increasingly interested in drinking pear juice. Phenolic compounds from pears can prevent the human body from suffering from many diseases (Zafra-Rojas et al., 2013).
The health promoting effects of fruit juices are usually related to food nutrients (Abid et al. 2014). Therefore, in the process of processing, the most important goal is to preserve food ingredients to obtain all the benefits provided by nature. Although, conventional thermal processing techniques ensure shelf life and safety of the fruit products but at the same time these methods caused nutritional, chemical and physical deterioration (Gomez et al., 2011). Traditional processing of juices can also lead to juice browning caused by oxidative enzymes which is linked to the high activity of peroxidase (POD) and polyphenol oxidase (PPO) because of quick degradation of polyphenols to quinones as a result more browning pigments are produced (Manzocco et al., 2017). From this perspective, the rapid inhibition of browning reaction has attracted widespread attention in the vegetable and fruit industry (Lee et al., 2016). Thermal treatment and vitamin C addition have generally been used to inactivate these oxidoreductases, but according to European Union (EU) regulations, manufacturers cannot label declarations about 100% juice and that may be an important disadvantage of high-quality juices. In all preservation methods, thermal treatment has obvious disadvantages regarding undesirable pigment formation, nutrient loss, quality deterioration and flavor changes (Marszałek et al., 2016). These concerns related to juice processing urged the scientists to develop the techniques which not only retain functional ingredients of food but also can improve their nutritive value (Bhat et al., 2011).
In recent past, the trend in food manufacturing has pointed to the adoption of novel processing technologies to meet the growing consumer demand for foods that have balanced nutrition and good taste. High-pressure carbon dioxide (HP-CO2) is a non-thermal technique that is beneficial because of its higher efficiency of enzyme inactivation and little effect on the sensory and nutritive values of food (Illera et al., 2018). In this method, highly pressurized CO2 (below 50 MPa) can be used for enzymes inactivation under mild operating conditions where thermal processing is not useful (Iqbal et al., 2018). CO2 is a less expensive, non-toxic and non-flammable gas, which can be easily removed by degassing and decompression during the processing of HP-CO2 treatment. Therefore, this technique is considered as cold pasteurization technology that uses CO2 for enzyme inactivation by modifying cell membranes, reducing pH and changing the molecular structure of enzymes (Murtaza et al., 2019). In this study, the effect of thermal and HP-CO2 treatment was investigated at different temperatures. The physiochemical, browning degree, enzymatic activity of PPO, POD analysis were done under the treatments. Furthermore, the total phenolic contents and phenolic profile was also investigated under the influence of thermal and HP-CO2 processed juices.
Sample preparation Fresh fruits of peach (Prunus persicae), apple (Malus domestica) and pear (Pyrus communis L.) were procured from fruit market in Luoyang, China in 2021. After washing, the fruits were peeled and cut into pieces followed by crushing in a juice extractor. For the removal of coarse particles and impurities the juices were filtered by using cheese cloth and centrifugation of the filtrates was done for five minutes at 4 000× g. The chemicals of HPLC grade or analytical grade were used in the experiment.
Juice processing For HP-CO2 processing, CO2 was allowed to enter the pressure vessel. Batch system apparatus was used to perform HP-CO2 processing. The batch system apparatus had two cylinders with 100 mL volume. Fruit juices at 30 mL were put in HP-CO2 processing sterile bottle for processing. For sanitizing HP-CO2 vessel ethanol was used for each experiment. After reaching the required temperatures of 20, 30, 40, 50, 60 and 70 °C, the bottles having fruit juices were subjected to chamber vessel where juices were processed under required temperature at 20 MPa for 20 min duration. Following HP-CO2 treatment, the sample vessel was depressurized gradually for 3–5 minutes and then the juice temperature is gradually reduced to 8–12 °C. The treatment time was not including the pressurization and depressurization time of the sample. The processed samples were then cooled quickly in the refrigerator and analyzed for different parameters (Manzocco et al., 2016).
For thermal processing the juices were processed for 20 minutes at temperatures 20, 30, 40, 50, 60, and 70 °C in a water bath having thermostat (Liu et al., 2018). The fruit juices were put in the test vessels at 30 mL/vessel and specific thermal treatment was applied in water bath and cooled down rapidly for analysis. The juice without any treatment was analyzed for control.
Browning degree and color analysis The fruit samples both treated and untreated were analyzed for browning degree ac-cording to previously reported method (Murtaza et al., 2019). Multiskan FC spectrophotometer (Thermo Scientific, Waltham, MA, USA) was used for measuring browning degree (BD) of the juices. First the juices were centrifuged for 20 minutes and 4 °C at 10 000× g and 100 µL of solution was added to the ELISA plate and using a simple kinetic method rapidly tested at λ = 420 nm. The color analysis of the juices was performed at 20 ± 1 °C (ambient temperature) by using chromometer (CR-400; Osaka, Japan). The a*, b* and L* values of the treated and control juices were measured which indicates green-ness/redness, yellowness and brightness of the treated juices respectively. The total color difference (ΔE) was calculated by using the equation 1:
 |
where ao, bo and Lo correspond to the greenness/redness, yellowness and brightness of the untreated juices respectively.
Physicochemical analysis The total soluble solids (TSS) were measured through digital Abbe Refraction meter (Shanghai Scientific Instrument Co., Shanghai, China). The pH of the juices was determined by using Thermo Orion pH meter-A211 (Thermo Scientific Inc., USA).
PPO and POD relative activity For measuring PPO and POD activity, 2 mL of each juice was mixed with 1% Triton X-100 and 1% polyvinylpolypyrrolidone for making crude enzyme (Murtaza et al., 2019). The samples were stored at 4°C for 1 h and then centrifuged for 20 min at 10,000 rpm. The resultant crude enzyme was processed for polyphenol oxidase and peroxidase (oxidoreductase enzyme) activity assay. The 50 µL of each crude enzyme was mixed with 200 µL catechol solution and phosphate buffer (pH 7.0; 0.05 M). By using spectrophotometric method, the extract absorbance was determined at 420 nm (Zhu et al., 2019). From the reaction curve, PPO activity (Abs/min) was considered as the first linear part of the slope (Murtaza et al., 2018). Relative PPO activity in percentage was measured through Eq. 2.
 |
Where At = activity of juice after thermal or HP-CO2 treatment and Ao = Activity of un-treated juice.
For the assessment of POD activity, the method used by Liu et al (2012) was employed with little modifications. Briefly, an aliquot of the supernatant (100 µL) was added to 150 µL of mixture solution (200 mM phosphate buffer, 5 mL guaiacol, and 10 mL hydrogen peroxide). The 100 µL of PBS was used as a control. Through spectrophotometric method the absorbance of the solution was measured at 470 nm wavelength by using simple kinetic method. Relative POD activity in percentage was measured through the same equation as for PPO.
Analysis of total polyphenol content Folin-Ciocalteu (FC) colorimetric method was used for the determination of total polyphenol content (TPC). The 1.25 mL of diluted supernatant was added to 1 mL of FC reagent and kept for 6 minutes which is followed by the addition of 1.8 mL of 10% Na2CO3 solution. Through spectrophotometric method, the mixture absorbance was measured at 765 nm. Gallic acid was kept as a standard. The results obtained were presented in milligrams of gallic acid equivalents (GAE) per liter of juice.
Analysis of phenolic compounds The procedure reported previously (Tsao et al., 2003) was used for HPLC analysis. Briefly, the 80 mL of juices were added to 400 mL of ethanol solution. After 4 hours the samples were centrifuged at 5 000× g for 15 min. By using rotary evaporator, the supernatant was evaporated at 45 °C. The phenolic compounds were extracted thrice by using 20 mL solvent of 20% ammonium sulfate and 2% metaphosphoric acid, and ethyl acetate. The extracts were combined and dried with rotary evaporator at 45 °C. The residue after dissolving in 5 mL of ethanol was filtered by 0.22 µm filter membrane and kept at −20 °C. By using HPLC the phenolic compounds were analyzed. Absorbance Detector (Waters 2478 Dual λ) was used to measure the absorbance at 280 nm. The water having 1% formic acid (A) and acetonitrile (B) were used as mobile phase. The flow rate of 1.0 mL/min and 20 µl injection volume were operated. HPLC-grade standards i.e. chlorogenic acid, catechin, rutin, epicatechin, 2-furanoic acid, caffeic acid, p-coumaroylquinic, phloretin, cyanidin, procyanidin, quercetin, arbutin and p-coumaric acid were obtained from Sigma Chemicals. The retention times of the peaks were compared with the standards for the identification. The phenolic contents concentration was presented as mg/L of the juice.
Statistical Analysis The experiments were done in triplicate and results are presented as mean ± standard deviation. Data were analyzed through Analysis of variance (ANOVA) using 95% level of significance. Analysis was done through statistic 8.1 software and means were com-pared by Tukey's test.
Physiochemical properties The physiochemical analysis of peach, apple and pear juices showed significant difference between thermal treated juices and HP-CO2 treated juices regarding pH, TSS and total color difference (Table 1, 2 and 3). The thermally treated juices showed slight decline in pH. The pH of peach, apple and pear juices was reduced from 3.91 to 3.75, 4.10 to 3.86 and 3.61 to 3.47 respectively. Comparing to thermal treatment, the HP-CO2 treatment reduced the pH of the juices with higher rate. The pH reduced from 3.91 to 3.35, 4.10 to 3.30 and 3.61 to 3.21 for HP-CO2 treated peach, apple and pear juices respectively. Similar results were obtained for TSS of the juices. Slight reduction was observed for thermal treatment while HP-CO2 caused significant reduction in TSS with higher rate. Results regarding total color difference indicated that HP-CO2 treated juices showed significantly higher ΔE then thermally treated juices. The highest ΔE 10.90 for peach, 13.60 for apple and 12.90 for pear were obtained for HP-CO2 treatment at 70 °C.
| Treatment | Temperatures | ||||||
|---|---|---|---|---|---|---|---|
| Control | 20 | 30 | 40 | 50 | 60 | 70 | |
| Peach | |||||||
| TP | 3.91±0.04 a | 3.90±0.03 a | 3.91±0.07 a | 3.87±0.03 b | 3.81±0.05 c | 3.78±0.08 cd | 3.75±0.04 de |
| HP-CO2 | 3.91±0.04 a | 3.86±0.06 b | 3.81±0.05 c | 3.66±0.04 f | 3.54±0.05 g | 3.41±0.07 h | 3.35±0.08 i |
| Apple | |||||||
| TP | 4.10±0.02 a | 4.06±0.05 ab | 4.01±0.07 bc | 3.97±0.04 bc | 3.91±0.03 d | 3.88±0.03 de | 3.86±0.06 e |
| HP-CO2 | 4.10±0.02 a | 3.97±0.07 bc | 3.81±0.04 f | 3.70±0.04 g | 3.52±0.07 h | 3.44±0.06 i | 3.30±0.07 j |
| Pear | |||||||
| TP | 3.61±0.07 a | 3.61±0.03 a | 3.60±0.08 a | 3.58±0.05 ab | 3.51±0.05 cd | 3.49±0.08 de | 3.47±0.06 e |
| HP-CO2 | 3.61±0.07 a | 3.55±0.03 bc | 3.49±0.08 d | 3.41±0.03 f | 3.33±0.06 g | 3.28±0.08 h | 3.21±0.05 i |
Each value is a mean of three replicates ± SD. Means (within same column or row separately for peach, apple and pear) sharing the same letter are not significantly different (p ≤ 0.05) as per Fisher's protected LSD test. TP = Thermal processing.
| Treatment | Temperatures | ||||||
|---|---|---|---|---|---|---|---|
| Control | 20 | 30 | 40 | 50 | 60 | 70 | |
| Peach | |||||||
| TP | 12.83±0.05 | 12.80±0.11 | 12.81±0.12 | 12.78±0.06 | 12.71±0.14 | 12.66±0.08 | 12.63±0.04 |
| HP-CO2 | 12.83±0.05 | 12.75±0.09 | 12.66±0.08 | 12.60±0.11 | 12.52±0.15 | 12.43±0.12 | 12.35±0.07 |
| Apple | |||||||
| TP | 12.93±0.05 | 12.91±0.04 | 12.90±0.06 | 12.87±0.06 | 12.85±0.08 | 12.80±0.13 | 12.78±0.12 |
| HP-CO2 | 12.93±0.05 | 12.82±0.03 | 12.75±0.11 | 12.59±0.12 | 12.31±0.13 | 12.20±0.09 | 12.12±0.09 |
| Pear | |||||||
| TP | 11.98±0.04 | 11.97±0.06 | 11.93±0.09 | 11.91±0.06 | 11.86±0.12 | 11.82±0.05 | 11.80±0.08 |
| HP-CO2 | 11.98±0.04 | 11.91±0.10 | 11.76±0.08 | 11.62±0.09 | 11.57±0.08 | 11.51±0.08 | 11.43±0.12 |
Each value is a mean of three replicates±SD. Means (within same column or row separately for peach, apple and pear) sharing the same letter are not significantly different (p ≤ 0.05) as per Fisher's protected LSD test. TP = Thermal processing.
| Treatment | Temperatures | ||||||
|---|---|---|---|---|---|---|---|
| Control | 20 | 30 | 40 | 50 | 60 | 70 | |
| Peach | |||||||
| TP | 6.75±0.23 | 6.95±0.18 | 7.45±0.32 | 7.80±0.31 | 8.13±0.20 | 8.60±0.32 | |
| HP-CO2 | 7.18±0.14 | 7.80±0.16 | 8.60±0.22 | 8.90±0.34 | 9.80±0.28 | 10.90±0.25 | |
| Apple | |||||||
| TP | 7.14±0.18 | 7.30±0.23 | 7.90±0.18 | 8.60±0.31 | 8.90±0.36 | 9.70±0.19 | |
| HP-CO2 | 7.80±0.17 | 8.50±0.14 | 8.90±0.28 | 9.80±0.28 | 11.50±0.34 | 13.60±0.23 | |
| Pear | |||||||
| TP | 6.10±0.17 | 6.40±0.18 | 7.40±0.28 | 7.80±0.23 | 8.45±0.34 | 9.20±0.12 | |
| HP-CO2 | 7.35±0.13 | 7.80±0.16 | 8.60±0.32 | 10.45±0.28 | 11.70±0.33 | 12.90±0.23 | |
Each value is a mean of three replicates ± SD. Means (within same column or row separately for peach, apple and pear) sharing the same letter are not significantly different (p ≤ 0.05) as per Fisher's protected LSD test. TP = Thermal processing.
Browning Degree The influence of thermal and HP-CO2 treatments on the browning degree (BD) value of three fruit juices at different temperatures is presented in Fig. 1. Results showed a clear significant difference between thermal and HP-CO2 treatments. Generally, browning degree of the thermally treated juices increased with increase in temperature while HP-CO2 treatments caused reduction in browning degree at a faster rate by increasing temperature. The un-treated peach, apple and pear juice showed a browning degree value of 0.78, 0.71 and 0.82 respectively. In case of peach, the thermally treated juice showed constant increase in browning degree with increase in temperature and reached up to 0.97 at temperature 60 °C and then reduced to 0.91 at 70 °C. The HP-CO2 treatment caused continuous significant reduction from the start and reached up to 0.31 browning degree value at temperature 70 °C. Similar trend was noticed for apple and pear juice where browning degree value of thermal treatments was increased from 0.71 to 0.90 for apple and 0.82 to 0.90 for peach at temperature 60 °C and then reduced slightly at 70 °C. The HP-CO2 treated apple and pear juices showed reduction from 0.71 to 0.35 and 0.82 to 0.30 at 70 °C.
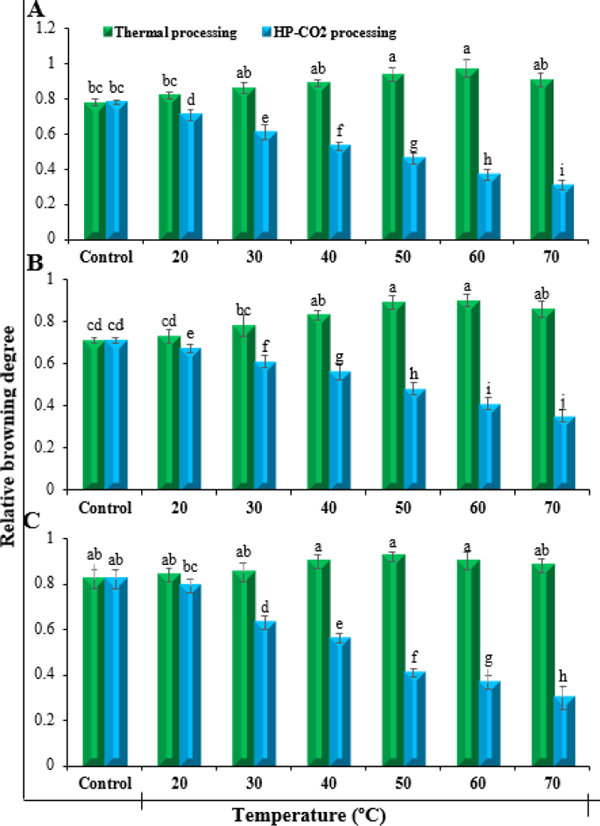
Relative browning degree of fruit juices under the influence of thermal and HP-CO2 processing at different temperatures. A: Peach, B: Apple and C: Pear. Each value is a mean of three replicates. Error bars represent standard deviations. Means sharing the same letter are not significantly different (p ≤ 0.05) as per Fisher's protected LSD test.
PPO Results regarding the effect of thermal and HP-CO2 treatments on the relative activity of PPO in peach apple and pear juices, evaluated at different temperatures showed significant differences (Fig. 2). For thermal treatments relative activity of PPO was increased initially up to 30 ºC but then decreased with increase in temperature. The HP-CO2 treatments however, caused reduction in relative activity of PPO from start to end. The relative activity of PPO in peach, apple and pear juices treated with thermal processing treatment was increased from 100 to 123%, 115% and 118% respectively at 30 ºC. At the temperature above 30 ºC, the PPO activity reduced up to 60%, 52% and 57% for peach apple and pear juices respectively at 70 ºC. A fast increase in the reduction of PPO activity was observed with increase in temperature for HP-CO2 treatments. The PPO activity was reduced from 100 to 10%, 7% and 4% for HP-CO2 treated peach, apple and pear juices respectively at 70 ºC.

Relative PPO activity of fruit juices under the influence of thermal and HP-CO2 processing at different temperatures. A: Peach, B: Apple and C: Pear. Each value is a mean of three replicates. Error bars represent standard deviations. Means sharing the same letter are not significantly different (p ≤ 0.05) as per Fisher's protected LSD test.
POD The relative activity of POD in peach, apple and pear juices under the influence of thermal and HP-CO2 processing at different temperature were evaluated. Significantly different results were observed for thermal and HP-CO2 treatments (Fig. 3). The TP treated peach and apple juices showed a slight increase in POD activity when temperature was raised up to 30 ºC at the start and then decreased with increase in temperature was observed while HP-CO2 treated pear juice had no increase in POD activity. The POD relative activity of TP treated juices 105% and 108% for peach, 104% and 106% for apple, 108% and 116% for pear was observed at temperature 20 and 30 ºC respectively. The final de-crease in POD activity of TP treated juices at temperature 70 ºC was reached to 78%, 74% and 77% for peach, apple and pear juice respectively. While on other hand, a faster and higher significant reduction rate was observed for HP-CO2 treatments where final de-crease in POD activity 25%, 30% and 32% was recorded for peach, apple and pear juices respectively.
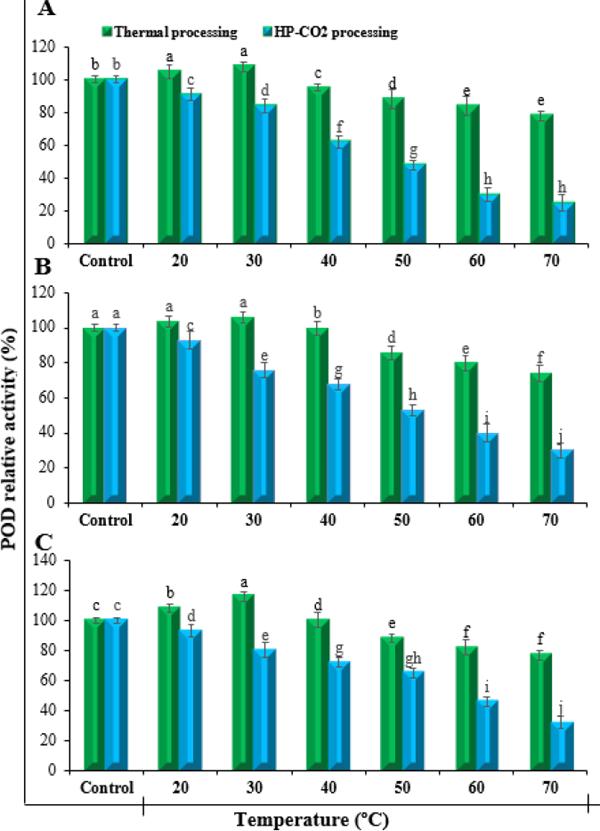
Relative POD activity of fruit juices under the influence of thermal and HP-CO2 processing at different temperatures. A: Peach, B: Apple and C: Pear. Each value is a mean of three replicates. Error bars represent standard deviations. Means sharing the same letter are not significantly different (p ≤ 0.05) as per Fisher's protected LSD test.
Total phenolic content Total phenolic contents of peach, apple and pear juices after thermal and HP-CO2 treatments at different temperatures were presented in Fig. 4. The amount of total phenol in peach, apple and pear juices significantly affected by thermal and HP-CO2 treatments. Generally, by increasing temperature, thermal treatments enhanced total phenolic content while HP-CO2 treatments reduced the total phenolic contents of the juices. The total phenolic concentrations in untreated control were 1 100, 1 000 and 1 230 mg GAE/L for peach, apple and pear juices respectively. The TP treatment enhanced total phenolic con-tent by increasing temperature from 20 to 70 ºC. The total phenolic contents 1 330, 1 135, 1 315 mg GAE/L were recorded for peach, apple and pear juices at temperature 70 ºC respectively. Unlike TP treatment, the HP-CO2 treatment resulted in a decrease in total phenolic content of the juices as the temperature was increased. At the final temperature 70 ºC the decrease in total phenolic content from 1 100 to 1 020, 1 000 to 830 and 1 230 to 1 145 mg GAE/L were observed for HP-CO2 treated peach, apple and pear juices respectively.

Total phenolic contents of fruit juices under the influence of thermal and HP-CO2 processing at different temperatures. A: Peach, B: Apple and C: Pear. Each value is a mean of three replicates. Error bars represent standard deviations. Means sharing the same letter are not significantly different (p ≤ 0.05) as per Fisher's protected LSD test.
Phenolic profile In peach juice the phenolic compounds chlorogenic acid, catechin, rutin, epicatechin, 2-furanoic acid, caffeic acid and p-coumaroylquinic acid were detected (Fig. 5). Apple juice was found to contain a variety of phenolic compounds such as rutin, phloretin, chlorogenic acid, cyanidin, procyanidin, quercetin, catechin, epicatechin and caffeic acid (Fig. 6). While in pear juice the phenolics compounds detected were arbutin, chloro-genic acid, rutin, catechin, epicatechin, caffeic acid and p-coumaric acid (Fig. 7). The phenolic compounds chlorogenic acid, catechin and rutin in peach juice, epicatechin, catechin, and chlorogenic acid in apple juice while chlorogenic acid and arbutin were found predominantly in pear juice. Significant changes were noticed regarding phenolic profile of the peach, apple and pear juices during the processing. In case of thermal treatments, the increase in temperature caused an increase in phenolic contents in all the three juices. For example, the initial concentration of chlorogenic acid, a common major phenolic compound, in untreated peach, apple and pear juices were 0.32, 0.40 and 0.34 mg/100 mL respectively. Its concentration increased and reached to 0.49, 0.47 and 0.41 mg/100 mL at 70 ºC in peach, apple and pear juice respectively. Almost, similar trend was observed for other major or minor phenolic contents. In contrast to thermal treatment, the HP-CO2 treated juices showed the reduction in the phenolic compounds of the juices with increase in temperature. The concentration of chlorogenic acid in peach, apple and pear juices treated with HP-CO2 was declined from 0.32 to 0.20, 0.40 to 0.26 and 0.34 to 0.23 mg/100 mL respectively at temperature 70 ºC.

Phenolic profile of peach juice under the influence of A: thermal and B: HP-CO2 processing at different temperatures.

Phenolic profile of apple juice under the influence of A: thermal and B: HP-CO2 processing at different temperatures.

Phenolic profile of pear juice under the influence of A: thermal and B: HP-CO2 processing at different temperatures.
The peach, apple and pear juices were investigated for their physiochemical characteristics, enzymatic activity and phenolic profile under the influence of thermal and HP-CO2 treatments at different temperatures. The significant differences were noted between thermal and HP-CO2 treated juices regarding physiochemical characteristics. Compared to thermal treatment the HP-CO2 treatment showed higher reduction in pH at the same temperature. This is because the dissolved CO2 distributes the bicarbonates and carbonates in the juice that lead to increase in acidity resulting in lower pH. These results are in line with previously reported studies in which carrot and quince juices were reported to have reduced pH value under HP-CO2 influence (Iqbal et al., 2018; Tsao et al., 2003; Liu et al., 2015).
Higher temperature under TP cannot reduce the enzymatic browning of the juices. Increase in temperature caused enhancement in browning degree of the thermally treated juice. It is evident from the results that temperature increase resulted in increased concentration of polyphenol which stimulated the PPO activity and enhanced juice browning. The HP-CO2 treatment of the juice at higher temperature caused reduction in enzyme activity which leads to decrease in the browning degree of the juice (Iqbal et al., 2018). As compared to TP treated juices and control group, the juices treated with HP-CO2 showed significantly lower BD. The reduction in juice browning by the application of HP-CO2 can be explained by structural destruction of the enzymes under CO2 at high pressure. In addition, by increasing the temperature under HP-CO2 treatment, the CO2 density is reduced, thereby improving the CO2 diffusivity, enhancing the molecular collision between enzymes and CO2, and lastly lowering the activity of enzymes which caused decrease in enzymatic browning of the juice (Illera et al., 2018). The HP-CO2 treatment may also have affected the reaction related to non-enzymatic browning in an environment having lower pH. Therefore, HP-CO2 treatment is directly involved in the inhibition of PPO activity and thereby decreasing the browning degree of the juices. It is reported that compared with TP treatment, the relative activity of PPO was lower with HP-CO2 treatment which proves that HP-CO2 is more effective in inhibiting enzyme inactivation (Illera et al., 2018). The fact that why is the browning degree lower than the control in this study is not fully clear. It can be speculated that the control sample that took some time after the juice has been made and has undergone browning, the enzyme was inactivated by HP-CO2 treatment at a stage when browning was not occurring, browning can be inhibited after the treatment. Results obtained in this study are in line with previously reported study, in which a complete inactivation of PPO enzymes in apple juices was reported for HP-CO2 application at 60 °C (Xu et al., 2011).
Results regarding PPO relative activity showed that increase in temperature caused an increase in PPO relative activity of TP treated juices, but when the temperature was higher than 30 °C, the relative activity of PPO decreased with increasing temperature. Several different isoforms of PPO such as latent, mature, immature and active exists in plants. Increase in PPO activity of the TP treated juices is may be because of latent PPO activation (Niu et al., 2010). Several vegetables and fruits such as apples, peaches, iceberg lettuce and grapes are reported to have different concentration of latent PPOs (Terefe et al., 2015). Several reports have shown that the latent PPO activation is the cause of enhanced PPO activity (Yemenicioǧlu et al., 1997; Buckow et al., 2009). Although the TP treatments at temperature higher than 30 °C caused reduction in PPO activity but at significantly lower rate than HP-CO2 treatement at the same temperature. The HP-CO2 treatement caused inhibition of PPO activity from the start and with faster rate by increasing temperature. The use of TP for enzyme inactivation have been reported to have negative effect on food quality (Marszałek et al., 2017). While on the other hand, many scientists reported that HP-CO2 treatment caused increased inhibition of PPO activity with increase in treatment time, pressure and temperature (Yu et al., 2013). This enzyme inhibitory effect of HP-CO2 can be justified by the fact that CO2 may dissolve in the solution causing the reduction in pH which ultimately lead to structural destruction of PPO resulted in enzyme inactivation (Buckow et al., 2009). Increase in juice browning can be attributed to higher PPO activity because of the polyphenol oxidation reactions. Thus inactivation of PPO is desirable that can be achieved by HP-CO2 application with much faster rate than the TP treatments at the same temperature.
In this study, the results showed more stability of POD than PPO enzyme. Compared with TP, the juices treated with HP-CO2 showed that POD activity decreased with in-creasing temperature. Consistent to PPO activity results, the faster POD inactivation is achieved by the application of HP-CO2. Findings of this study also confirm that under the same processing conditions different enzymes exhibited different stability level. The POD was active under HP-CO2 treatment at 70 °C and 20 MPa while the activity of PPO was completely inhibited under same treatment with same pressure and temperature. It is re-ported that comparing to PPO, POD is more stable to heat and can remain active at 70 °C (Quitão et al., 2008). This is because of the difference in the structure of PPO and POD (Marszałek et al., 2019). The POD is a larger complex molecule which requires more and long heat duration for its deactivation (Quitão-Teixeira et al., 2008). Structural alterations in the enzymes such as secondary and tertiary structures could also be one of the reason of their inactivation Zhou et al., 2009).
Results regarding phenolic contents of the juices are in line with previously reported findings for peach, apple and pear phenolic contents (Ferrentino and Spilimbergo, 2011; Gasperi et al., 2009; Will et al., 2008). Total phenolic contents increased gradually with increase in temperature of thermal treatment. This is because of the fact that most of the phenolic compounds in the juices are combined with polysaccharides and as a result of thermal treatment they can be released. Similar results were reported in a study where thermal treatment enhanced the phenolic content of the apricot nectar (Huang et al., 2013). In case of HP-CO2 treated juices, increase in temperature caused decrease in phenolic contents. This can be explained on the basis of hydrolysis of polyphenols by HP-CO2 treatment that caused reduction in phenolic contents in the environment having lower acidity (Marszałek et al., 2015). The detail mechanism of phenolic compound degradation by HP-CO2 is very complex and not known completely. It is reported that CO2 gas get dissolved in the juice and generate high reactive species such as hydrogen peroxide, superoxide and hydroxyl radical thereby induce acidic condition. The high reactive species are reported to have high reactivity with phenolic compounds. Researchers reported HP-CO2 modified total polyphenolic content significantly and increase in temperature of HP-CO2 treatment cause more degradation of phenolic compounds. Similarly, mulberry juice when treated with HP-CO2 for 10 min at 25 °C and 15 MPa showed reduction in the phenolic contents compared to untreated juice (Zou et al., 2016).
The concentration of major polyphenols such as catechin, chlorogenic acid, epicatechin, rutin, arbutin were increased under the effect of thermal processing at higher temperature. Previous study reported similar results in which the concentration of four phenolic compounds including (+)-catechin and (−)-epicatechin were increase under thermal processing at higher temperature (Huang et al., 2013). Phenolic compounds procyanidin B2 and epicatechin are the main reactants in the enzymatic browning of juices (Kim et al., 2017). Thus, thermal treated juices showed enhanced browning degree due to enhanced enzymatic activity. This is because of the increasing concentration of the different polyphenols. Under HP-CO2 treatment, juices showed decrease concentration of these polyphenols with increasing temperature. It was revealed previously in many studies that application of pressure and temperature significantly reduced the concentration of polyphenols in product processing (Wojdyło et al., 2008). These changes might be caused by the polyphenol hydrolysis because of enhanced acidity during pressurization, releasing enzymes and polyphenols that consequently react to form of polymeric brown pigments (Marszałek et al., 2018). Under the influence of HP-CO2, the reduction in polyphenol contents is because of the fact that high pressure could make a pressure gradient between the outside and inside of the polyphenol structure and could degrade the structure, which causes the rapid release of CO2 gas holding polyphenol, causing in the decrease of polyphenol contents (Liu et al., 2015).
The thermal processing and HP-CO2 processing treatments at different temperatures were investigated for their effect on quality attributes of peach, apple and pear juices. HP-CO2 processing juices were found to have good quality attributes than thermal treatments at the same temperature. Significantly higher reduction in pH, TSS, relative activity of PPO, POD and enzymatic browning were noticed for HP-CO2 treated juices. The con-centration of phenolic compounds was decline slightly in HP-CO2 treated juices. The thermal treatment at the same temperature caused slight reduction in pH, TSS and exhibited increased in relative activity of PPO, POD and browning of the juices. Findings of this study suggest that HP-CO2 treatment is more effective in maintaining the quality attributes of the juices then thermal processing and can be used as a potential processing technique for the processing of fruit juices.
Conflict of interest There are no conflicts of interest to declare.