2023 Volume 29 Issue 3 Pages 269-275
2023 Volume 29 Issue 3 Pages 269-275
Combined medium-high hydrostatic pressure (MHHP) and medium-high temperature (MHT) treatment is an effective process for liquid impregnation and food pasteurization. Although several fruit processing additives are known to inhibit polyphenol oxidase (PPO), the effect of additives on PPO activity after MHHP+MHT treatment has not been studied. This study describes the effect of citric acid and calcium chloride on PPO activity after MHHP+MHT treatment. The lyophilized powder of Fuji apples was subjected to MHHP+MHT treatment in a solution containing sucrose, citric acid, and calcium chloride, and residual PPO activity was measured. Combined citric acid and calcium chloride additive use reduced PPO activity more effectively than their individual use. Using this additive combination to decrease PPO residual activity in conjunction with MHHP+MHT proved to decrease apple compote browning homogeneously, which we believe will prove a useful method in preservation processes.
High hydrostatic pressure (HHP) treatment is regarded as a non- or low-thermal food process (Yamamoto, 2017). HHP involves the non-thermal inactivation of microorganisms present in food materials using hydrostatic pressures ranging 200–1 000 MPa. We studied the use of combined medium-high hydrostatic pressure (MHHP) and medium-high temperature (MHT) treatment for fruit compote preservation (Yamamoto et al., 2017). MHHP+MHT involves the use of hydrostatic pressures ranging 100–200 MPa with temperatures of around 55–75 °C (Yamamoto, 2017), resulting in effective liquid impregnation of foods (Vatankhah and Ramaswamy, 2019) and pasteurization (Morimatsu et al., 2019). MHHP+MHT is superior in terms of both equipment cost and pasteurization, especially for inactivation of spore-forming bacteria, compared to HHP use alone (Yamamoto, 2020).
Polyphenol oxidase (PPO) (E.C. 1.10.3.2) naturally occurs in fruits, vegetables, fungi, and animals (Mayer, 2006). This enzyme oxidizes polyphenols to o-quinones, forming brown polymers that cause undesirable color changes and food degradation (Yoruk and Marshall, 2003). While thermal treatment is the primary method for PPO inactivation, it requires either high temperatures or long treatment times (Ni Eidhin et al., 2006), potentially negatively impacting food quality. HHP requires approximately 1 600 MPa to inactivate PPO in mushrooms (Yi et al., 2015). In contrast, the PPO activity of pears treated with 200–600 MPa was 5 to 10-fold greater (Asaka and Hayashi, 1991). When thermal and high-pressure treatments are combined, Buckow reported that apple PPO remained active at pressures under 600 MPa (Buckow et al., 2009).
Various food additives are used in fruit or vegetable products to adjust characteristics such as pH, taste, and texture. Citric acid and calcium chloride are important additives for producing fruit compotes. Citric acid is generally used as an acidulant and to inhibit PPO (Queiroz et al., 2008). Calcium chloride is frequently used to maintain textural quality in fruit and vegetables (Garcia and Barrett, 2002) and is expected to have inhibition characteristics through chloride ion chelation effects (Yoruk and Marshall, 2003). However, there are few reports on the effects of these additives on PPO activity when combined with HHP. Techakanon and Barrett (2017) described no significant change in PPO activity in peaches after calcium chloride and calcium lactate treatment at 200 MPa. Plaza described a decrease in tomato puree PPO activity after treatment with the combination of high pressure, citric acid, and sodium chloride (Plaza et al., 2003). However, the effect of food additives in conjunction with MHHP+MHT treatment on PPO activity has not yet been analyzed. The present study explored the impact of calcium chloride and organic acids, including citric acid, in combination with MHHP+MHT treatment on Fuji apple PPO activity.
Chemicals and reagents The reagents D-sucrose, citric acid monohydrate, malic acid, calcium chloride anhydride, pyrocatechol (reagent grade, respectively), phytic acid (50 % aqueous solution), phthalate, pH standard solution and phosphate pH standard equimolar solution (JCSS grade) were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan).
Raw materials Fuji apples (Malus domestica cv. Fuji) were purchased from Japan Agricultural Cooperatives in Suzaka city, Nagano Prefecture, Japan. Apples were harvested in December 2021 and stored at 2 °C. Each apple weighed approximately 350 g. Apples were immediately lyophilized after purchase for use as described in the next section. Apple compotes were prepared with apples harvested in December 2022 and stored at 2 °C; these apples were the same size as those harvested in 2021.
MHHP+MHT treatment of lyophilized apple powder In this section, we evaluated the effect of citric acid and calcium chloride on PPO activity. Lyophilized Fuji apple powder was used due to the variation in PPO activity among individual raw fruits. The lyophilized powder (2 g) was weighed in a tube, to which 20 mL of 10 % (w/v) sucrose solution was added. The solutions contained 0.1–0.3 % (w/v) citric acid, 0.1 % (w/v) phytic acid, or 0.1 % (w/v) malic acid. One tube contained 0.1–0.3 % (w/v) calcium chloride. Untreated samples with 10 % sucrose solution were kept as controls. As the concentration of soluble solids is known to affect PPO activity in HHP (Kaushik et al., 2015), solutions in this experiment contained 10 % sucrose to adjust the soluble solids to approximately 20 % when mixed with the lyophilized powder. Generally, most pickled apples in syrup contain ∼20 % soluble solids. The tubes were shaken and the mixture was homogenized. Then, 5 mL of the mixture was collected in a polyethylene bag and vacuum packed at a degassing rate of > 99 %. These operations were performed with ice cooling. The packaged samples were then treated by MHHP+MHT (TFS2-50, Toyo Koatsu, Japan) (100 or 200 MPa, 65 °C, 15 min) or thermal treatment (0.1 MPa, 65 °C, 15 min) using a water bath. The treated and un-treated bags were then cooled in ice water and the contents were transferred to fresh 5 mL tubes followed by centrifugation (13 666×g, 15 min, 4 °C; CR21N, Eppendorf Himac Technologies, Japan). The supernatant was used for pH measurements and in enzyme assays as enzyme extracts (Fig. 1(a)).
Preparation of apple compote samples Apple compote samples were prepared as follows: Fuji apples were peeled to remove the skin and the core was removed, then the apples were initially cut into eight pieces, and then each piece was halved. Next, ten pieces were put in each plastic bag with half the apple weight of syrup. Syrup A consisted of 30 % (w/w) sucrose, 0.9 % (w/w) citric acid and 0.3 % (w/w) calcium chloride. Compared with syrup A, syrup B lacked calcium chloride and syrup C lacked citric acid. The bags were vacuum sealed at a > 99 % degassing rate then treated by MHHP+MHT (100 MPa, 65 °C, 30 min) or thermal treatment (0.1 MPa, 65 °C, 30 min). The treated bags were subsequently cooled in ice water. Five apple sections from each bag were used for color measurements. The remaining five apple sections were lyophilized and milled to a powder. The lyophilized raw apple powder was used as a control. The powder (0.4 g) was weighed in a 5 mL tube, and 4 mL of 0.1 M sodium phosphate buffer (pH 6.5) was then added. The tubes were centrifuged (13 666 × g, 15 min, 4 °C), and the supernatant was used for enzyme assays as enzyme extracts (Fig. 1(b)).
Enzyme assays Enzyme assays were performed according to Asaka and Hayashi (1991) with slight modifications. The substrate solution, 30 mM pyrocatechol, was prepared by dissolving the substrate in 0.2 M sodium phosphate buffer (pH 6.5). Then, 30 µL enzyme extraction solution and 180 µL substrate solution were mixed in the well of a 96-well microplate. Enzyme activity was measured by changes in absorbance at 410 nm at 30 °C every 5 s for a total duration of 90 s, using a multi detection microplate reader (Varioskan Flash, Thermo Fisher Scientific, Waltham, MA, USA) in triplicate. Enzyme activity was defined as a change of 1 unit of absorbance over the initial linear region per minute for 1 mL of enzyme extract. Residual activity was calculated as follows:
 |

Experimental flowcharts. (a) shows the “MHHP+MHT treatment with lyophilized apple powder”, (b) shows the “Preparation of apple compote samples”. (CA: citric acid, CC: calcium chloride).
Where A0 is the mean enzyme activity of the control samples, and A is the mean enzyme activity of the treated samples.
Color measurements MHHP+MHT treated samples were stored for 2 days in an incubator at 25 °C on a plastic petri dish covered by film wrap. Color measurements using the CIE L*a*b* system were obtained using a spectrophotometer (SE 7700, Nippon Denshoku Industries, Japan) at 0, 24, and 48 h. Instrument calibration used a white color standard, and three samples on a plastic petri dish were measured in reflection mode. The degree of browning was expressed as the total color differences (ΔE*), calculated as follows:
 |
Where L*, a*, and b* correspond to the color values of stored samples (24 h or 48 h), L0*, a0*, and b0* correspond to samples before storage (0 h).
pH measurements The extract pH was analyzed at 20 °C with a glass pH electrode (HM-26S, DKK-TOA, Japan), calibrated using phthalate pH standard solution (pH 4.01) and phosphate pH standard equimolar solution (pH 6.86).
Statistical analysis Results are shown as the mean ± standard deviation (n=3). Statistical analysis was conducted using the Tukey test in all experiments. The Tukey test was performed using Microsoft Excel 2016 (Microsoft, Redmond, WA, USA).
Effect of citric acid and calcium chloride on Fuji apple PPO activity Figure 2 shows the PPO residual activity of lyophilized Fuji apple powder in solutions of different citric acid and calcium chloride concentrations and then treated with MHHP+MHT or thermal treatment alone. In treatments with solutions without calcium chloride, PPO residual activity decreased with increasing citric acid concentration. This behavior is expected, as PPO stability is decreased at acidic pH (Murata et al., 1992). Surprisingly, there was an inverse relationship between MHHP+MHT treatment pressure and decrease in PPO activity. PPO activity partially recovered upon treatment with increasing pressure. Furthermore, this behavior was observed in the presence of citric acid with calcium chloride, albeit to a lesser extent. Importantly, the large PPO residual activity reduction compared to untreated samples indicates an enhanced inhibitory effect of calcium chloride in combination with citric acid. Some studies reported that calcium chloride (Gomes et al., 2014) and sodium chloride (Valelo and García-Carmona, 1998) conferred increased PPO inhibition at lower pHs. Our results support this observation.

PPO residual activity of the lyophilized powder of Fuji apples treated by MHHP+MHT (100 and 200 MPa) and thermal treatment alone (0.1 MPa) with different concentrations of citric acid and calcium chloride. Different letters indicate significant differences of each group of same treatment solutions (p < 0.05). Samples were exposed to 0.1–200 MPa, 65 °C, for 15 minutes, or untreated. The untreated sample with 10 % sucrose and no CA, CC was used as a control. Error bars represent standard deviation (n = 3). (u.t.: untreated, CA: citric acid, CC: calcium chloride).
Although calcium chloride decreased PPO residual activity at a concentration of 0.1 %, increasing the calcium chloride concentration only slightly decreased residual PPO activities in untreated and thermally treated samples. The inhibitory effect of calcium chloride without MHHP+MHT was not concentration-dependent. Furthermore, MHHP+MHT treated samples with 0.3 % calcium chloride solution showed higher residual PPO activity than MHHP+MHT treated samples with 0.1 and 0.2 % calcium chloride. This demonstrates that calcium ions suppress thermal PPO inactivation under MHHP. It may be that for lower concentrations of calcium chloride, PPO inhibition by chloride ions was greater than the suppression of PPO inactivation by calcium ions. However, at higher concentrations, the main influence was the suppression of PPO deactivation by calcium ions. Indeed, some enzymes, such as thermolysin, are reported to show thermal stability in the presence of low concentrations of calcium ions (Toma et al., 1991) and activation at high salt concentrations (Inouye et al., 1998). In addition, PPO activation by metal ions is also described (Akyilmaz et al., 2007; Langer et al., 2019). However, there are no reports of the recovery of PPO activity or thermal stability in the presence of calcium ions due to MHHP or HHP treatment. This unusual phenomenon requires further investigation.
Figure 3 shows the residual PPO activity of samples treated with MHHP+MHT or thermal treatment alone with several organic acids and 0.1 % calcium chloride. In the figure, the vertical series at points i, ii, iv, and vi correspond to citric acid concentrations of 0, 0.1, 0.2, and 0.3 %, respectively. The vertical series at iii and v correspond to concentrations of 0.1 % malic acid and 0.1 % phytic acid respectively. PPO activity decreased with decreasing pH, regardless of acid type. Although phytic acid was reported to have a greater PPO inhibitory effect than citric acid (Du et al., 2012), our data indicates that in the presence of calcium chloride, the decrease in PPO activity was primarily dependent on pH and independent of organic acid type. Furthermore, differences in residual activities between pressurized and non-pressurized samples were smaller at a low pH compared to higher pHs. These results indicate that calcium chloride acts as an inhibitor of PPO activity in MHHP+MHT, with increasing effectiveness at lower pHs.
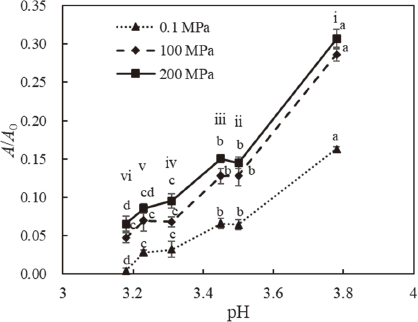
The effect of pH and organic acid type on PPO residual activity post MHHP+MHT (100 and 200 MPa) and thermal treatment alone (0.1 MPa). Different letters indicate significant differences of each treatment condition (p < 0.05). Numbers indicate treatment solution; i: no acid, ii: 0.1 % citric acid, iii: 0.1 % malic acid, iv: 0.2 % citric acid, v: 0.1 % phytic acid, and vi: 0.3 % citric acid. Each solution contains 10 % sucrose and 0.1 % calcium chloride, and each sample was exposed to 0.1–200 MPa, 65 °C, for 15 minutes. Error bars represent standard deviation (n = 3).
Suppression of apple compote browning by MHHP+MHT In this study, the combination of acid and calcium chloride effectively decreased PPO activity in lyophilized apples treated by MHHP+MHT. This treatment is expected to prevent browning during storage due to decreased PPO activity. In the previous section, the combination of 0.3 % citric acid and 0.1 % calcium chloride showed the greatest suppression of PPO activity. Therefore, syrup A, which was used in the preparation of apple compote samples, contained 30 % sucrose, 0.9 % citric acid, and 0.3 % calcium chloride. Mixing of the apple and syrup components results in an apple compote comprised of 20 % soluble solids, 0.3 % citric acid, and 0.1 % calcium chloride, according to our calculations. Figure 4 depicts the residual PPO activity of the apple compote in combination with the three syrups treated by MHHP+MHT and thermal treatment alone. Syrup A, composed of sugar, citric acid, and calcium chloride, most effectively decreased PPO residual activity, with only a slight difference in activity between MHHP+MHT and thermal treatment alone. The compote treated with syrup B (syrup A lacking calcium chloride) and C (syrup A lacking citric acid), particularly with MHHP+MHT treatment, possessed higher residual activities than treatment with syrup A. This tendency is in agreement with Figure 2. Importantly, the PPO residual activity of the compote after MHHP+MHT treatment decreased significantly in the presence of syrup A compared with syrup B or C, showing almost complete inactivation. This result indicates that the combination of citric acid and calcium chloride is effective in decreasing the PPO activity of apple compotes with MHHP+MHT treatment. Next, color changes during storage were examined.

PPO residual activities of apple compote treated with thermal (0.1 MPa) and MHHP+MHT (100 MPa) with syrups (syrup A, B, and C). Different letters indicate significant differences of each syrup of same treatment condition (p < 0.05). Syrup A consists of 30 % (w/w) sucrose, 0.9 % (w/w) citric acid and 0.3 % (w/w) calcium chloride. Syrup B lacks calcium chloride compared to syrup A, and syrup C lacks citric acid compared to syrup A. Error bars represent standard deviation (n = 5).
Figure 5 shows ΔE* of apple sections incubated for 24 and 48 h at 25 °C. The apple compote treated with syrup A possessed a lower ΔE* value for both treatments of MHHP+MHT (Fig. 5(a)) and thermal alone (Fig. 5(b)) than either syrup B or C. After 48 h storage, the apple compote treated with syrup A showed minimal color change. However, apple sections treated with syrups B and C showed significant browning after just 24 h. Though PPO showed re-activation after high-pressure treatment, our results demonstrate suppression of PPO activity in the presence of sugar, citric acid, and calcium chloride. These results show that the optimal additive combination is a high citric acid concentration with a low calcium chloride concentration in conjunction with MHHP+MHT treatment. In addition, after 48 h storage, apple sections treated with syrup A and MHHP+MHT had minimal ΔE* values (no significant difference) with a small standard deviation value when compared with thermal treatment alone. These results indicate that the homogeneous impregnation by the syrup could be attributed to the pressure treatment. It is presumed that in the thermal treatment alone, the syrup insufficiently impregnated some apple sections, resulting in a larger standard deviation in ΔE* values. Uniform product quality is an important factor in food processing, as sections with insufficient syrup impregnation are more prone to browning and decay, resulting in decreased consumer appeal.

Total color differences (ΔE*) of apple compotes incubated for 24 and 48 h at 25 °C after treatment with (a): MHHP+MHT (100 MPa, 65 °C, 30 min), and (b) only thermal (0.1 MPa, 65 °C, 30 min). Different letters indicate significant differences of each syrup of same storage time (p < 0.05). Error bars represent standard deviation (n = 5).
As described above, although MHHP+MHT is superior in contexts such as pasteurization and liquid impregnation, it has the drawback of continued PPO activity. To address this, the combination of citric acid and calcium chloride was used, and a large reduction in PPO activity for both MHHP+MHT and thermal treatments was observed. However, the use of thermal treatment alone is insufficient for long term storage. Indeed, in compotes with thermal treatment alone, more microorganisms survive than when treated by MHHP+MHT at the same treatment temperature (Yamamoto et al., 2017). Furthermore, the combination of syrup with MHHP+MHT treatment suppressed apple compote browning more homogeneously than with thermal treatment alone. When the compote is stored under cold conditions, browning is expected to be further suppressed. MHHP+MHT treatment with syrups using this combination of citric acid and calcium chloride is expected to produce compotes with suppressed browning and enhanced pasteurization compared to thermal treatment alone.
Acknowledgements This work was supported by JST Adaptable and Seamless Technology transfer Program through Target driven R&D (A STEP) Grant Number JPMJTM20NQ.
Conflict of interest There are no conflicts of interest to declare.