2023 年 29 巻 6 号 p. 481-488
2023 年 29 巻 6 号 p. 481-488
Lipid oxidation in food can result in the generation of off-odor compounds. We investigated the combined effects of α-tocopherol with rosmarinic acid and various antioxidants on the prevention and inhibition of off-odor formation. We added these combined compounds in a linoleic acid emulsion, followed by an oxidation reaction with 2′2-Azobis (2-methylpropionamidine) Dihydrochloride at 37 °C for 5 h. The hydroperoxide was measured by the ferric thiocyanate method, and the off-odor components were measured by gas chromatography. Median effect analysis was used to assess the combined effects. The results showed that the combinations of α-tocopherol with rosmarinic acid and caffeic acid exhibited a synergistic effect on inhibition of lipid peroxidation and some off-odor components, likely attributable to the catechol structure in these compounds. Interestingly, the combination of α-tocopherol and rosmarinic acid showed a potent synergistic effect on (E)-2-nonenal and (E)-2-octenal, indicating that the formation process of off-odor components may influence the combined effect.
Inhibiting lipid oxidation in food is essential for food quality control because such oxidation generates aldehydes with off-odors, decreasing food quality (Zhang et al., 2021; Zhang et al., 2015). The inhibitory effect of typical antioxidants, such as tocopherols, on the generation of hexanal, a significant odor compound generated by oxidation, has been reported (Ahn et al., 2002; Huang et al., 1994), and the efficacy of natural compounds such as quercetin and grape seed extract on its inhibition has also been demonstrated (Mielnik et al., 2006; Zhang et al., 2021). The antioxidant effects of herb-derived components were the focus of our study (Ohta et al., 2022). Extracts from herbs of the Perilla family, such as black peppermint, were effective in suppressing major off-odor components from linoleic acid. It was reported that this effect was mainly due to rosmarinic acid.
Recently, several studies have investigated the synergistic effects occurring between two antioxidants, especially between α-tocopherol and antioxidants, on efficient inhibition of the formation of hydroperoxides. Pedrielli et al. (2002) reported that the combined effects of α-tocopherol with epicatechin and quercetin on the peroxidation reaction of methyl linoleate were synergistic, with a more extended induction period observed than when α-tocopherol was used alone. Similarly, Yin et al. (2012) reported that α-tocopherol and catechins showed synergistic effects on the inhibition of hydroperoxide formation in sunflower oil-based emulsions, with a significantly increased induction period. In both cases, the synergistic effect between α-tocopherol and the antioxidants is considered to be caused by the regeneration of inactive α-tocopherol oxide by other antioxidants. Studies on antioxidants with the capacity to regenerate α-tocopherol have been reported where radicals were measured using electron spin resonance (ESR) (Iglesias et al., 2009). Moreover, synergistic effects produced by combining antioxidants have been observed in inhibition of the formation of hexanal. For instance, in a study using soybean oil emulsions, a combination of α-tocopherol and rosmarinic acid demonstrated a synergistic effect, increasing the induction period of hydroperoxide formation and significantly reducing the formation of hexanal (Panya et al., 2012). This synergistic effect was reported to be maximal under a condition of pH 7 (Kittipongpittaya et al., 2016). Although there are several reports on the inhibition of lipid oxidation through the synergistic effects of α-tocopherol and antioxidants, there is no standardized evaluation method, and the results vary among studies. There are also reports of an antagonistic effect when using a combination of α-tocopherol and rosmarinic acid, on inhibition of oxidation in a linoleic acid emulsion (Peyrat-Maillard et al., 2003).
As mentioned, one of the reasons for the discrepancies in the results of analysis of the synergistic effects of antioxidants may be attributable to the issues associated with the analysis methods themselves. Therefore, we investigated the application of median effect analysis, commonly used for analyzing the combined effects of drugs, to analyze the combined effects of antioxidants. In a previous study, we applied median effect analysis to investigate the synergistic effects of α-tocopherol and various antioxidants in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging system, and the results were published (Minami et al., 2021). Median effect analysis is a method proposed by Chou et al. (1984) to evaluate the combination of multiple compounds and is widely used for drug combination analysis in pharmacology because of its applicability to a wide variety of mechanistic reactions.
In this study, the synergistic effects of α-tocopherol and rosmarinic acid on the inhibition of the formation of hydroperoxides and 6 off-odor components from linoleic acid oxidation were investigated in detail using median effect analysis and compared with the effects of other antioxidants in combination.
Materials Linoleic acid (purity of > 90.0 %), Ammonium Thiocyanate, di-Potassium Hydrogen phosphate, Potassium dihydrogen phosphate, caffeic acid, and chlorogenic acid were purchased from Nacalai Tesque Inc. (Kyoto, Japan). Tween 40 and Iron (II) Chloride Tetrahydrate were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). 2′2-Azobis (2-methylpropionamidine) Dihydrochloride (AAPH), α-tocopherol, quercetin, luteolin, and ferulic acid were purchased from Tokyo Chemical Industry (Tokyo, Japan). Kaempferol was purchased from Sigma-Aldrich (Darmstadt, Germany). Rosmarinic acid was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All reagents were special grade. Ethanol was 99.5 %. For all experiments, ultrapure water was produced by PURIC-Z II (Organo Corporation, Tokyo, Japan).
Hydroperoxide analysis using the ferric thiocyanate method Linoleic acid emulsion (0.1 % v/w) was prepared based on our previous report (Ohta et al., 2022). To a vial, 10 mg of linoleic acid, 0.1 g of tween 40, and 10 mL of phosphate buffer (pH 7.4) were added, and it was subsequently vortexed for 10 s and sonicated for 10 min. Two hundred μL of antioxidants dissolved in ethanol and 2 mL of 100 mM AAPH solution was added to the emulsion. The concentration of the combined antioxidants was diluted to multiple concentrations after mixing based on the concentration at which 50% inhibition of hydroperoxide by each antioxidant was observed in a previous report (Ohta et al., 2022). The mol ratios of α-tocopherol to various antioxidants were in the range of 1:0.24–0.67. The control was replaced with 200 μL of ethanol. The sample solution was incubated at 37 °C for 5 h under shade using a water bath (LAB-THERMO SHAKER, TS-20, Advantec Toyo Kaisha, Ltd., Tokyo, Japan). After the incubation, 0.1 mL of sample solution, 0.1 mL of 30% ammonium thiocyanate, and 0.1 mL of 0.02 M iron (II) chloride in 3.5 % hydrochloric acid were added to 4.7 mL of 75 % ethanol. Absorbance at 500 nm of the mixture was measured after 3 min from the addition of iron (II) chloride in 3.5 % hydrochloric acid using a UV-visible spectrophotometer (UVmini-1240, Shimadzu, Kyoto, Japan). The inhibition rate of hydroperoxide of antioxidants was calculated using absorbance value by the following equation.
 |
Headspace solid-phase microextraction (SPME) After the incubation, the volatile components of the headspace area above the sample solution were collected using an autosampler (HS100, CTC Analytics AG, Zwingen, Switzerland) with SPME Fiber (50 / 30 μm DVB/CAR/PDMS SPME Fiber, Supelco, Bellefonte, PA, USA). The vials were heat-treated at 37 °C with agitation at 500 rpm for 30 min, after 5 min of preincubation.
Dual column high-performance gas chromatography (GC) analysis GC analysis was performed using a Heracles II electronic nose (Alpha MOS, Toulouse, France). The device had two capillary columns (MXT-5: polar, 10 m × 0.18 mm × 0.40 μm, Restec Corporation, Bellafonte, PA, USA; MXT-WAX: slightly polar, 10 m × 0.18 mm × 0.40 μm, Restec Corporation, Bellafonte, PA, USA) equipped in parallel, and two flame ionization detectors (FID) connected to each. The injected volume was 1.0 μL, with a rate of 50 μL/s, at 240 °C. Trapping was performed at 40 °C for 125 s with a split mode (10 mL/min). The oven temperature was set at 40 °C for 10 s, increasing at 1.5 °C/s to 40–250 °C, and held at 250 °C for 60 s. The carrier gas was H2 at a constant flow rate of 1.6 mL/min. The temperature of the two detectors was 260 °C. The results are the average of independent experiments conducted in triplicate. The inhibitory effect of the combined antioxidant on the off-odor formation was calculated using peak area by the following equation.
 |
Identification of volatile compounds Volatile compounds were identified by comparison of retention indices (RI) and AroChemBase (Ver. 6.0, Alpha MOS).
Analysis and determination of combined effects by median effect analysis The combined effects of α-tocopherol and various antioxidants were analyzed as previously reported (Minami et al., 2021). First, dose-effect curves for the inhibition of hydroperoxide or off-odor formation when each antioxidant was used alone and in combination were prepared. Then, a median effect plot was prepared based on the following equation.
 |
In Eq. 3, D is the dose, D m is the dose required for a 50% inhibition effect, which is equivalent to the median effect dose (IC50), fa is the inhibition rate at concentration D, fu is the noninhibition rate (fu = 1 − fa), and m is a coefficient representing the sigmoidal of the dose curve. The median effect plot plots log(D) on the horizontal axis and log(fa /fu) on the vertical axis, and the combination index (CI) is calculated from the m value in each line. The combination effect is determined from the CI. In this study, measurements were performed in triplicate and the mean was used. CIs were expressed as CI ± 2 standard deviation (SD), assuming a risk rate of approximately 5%. CI + 2SD < 1 indicated a synergistic effect, 1 < CI − 2SD indicated an antagonistic effect, and CI ± 2SD ≒ 1 indicated an additive effect. The CI plot shows the fa for each concentration level on the horizontal axis, and the behavior of the synergistic effect can be determined according to the concentration level. Median effect analysis was performed using commercially available software (CalcuSyn ver. 2.0, Hulinks Inc., Tokyo, Japan).
Statistical analysis Results are shown as mean ± SD (n = 3). Statistical analysis was performed by the Tukey-Kramer test using JMP Pro15 (Ver. 12.2.0, SAS Institute Japan, Inc., Tokyo, Japan).
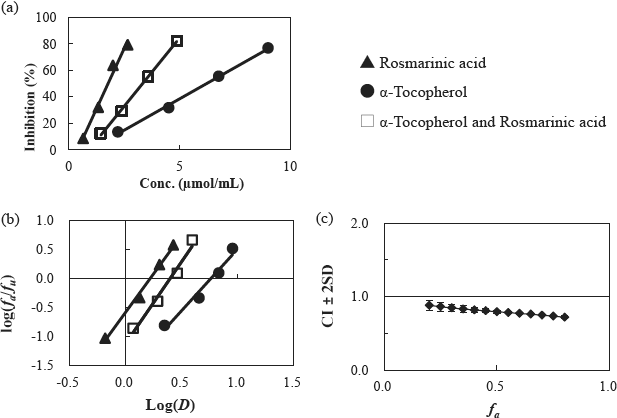
Combined effect analysis of α-tocopherol and rosmarinic acid on the inhibition of hydroperoxide generation. (a) the inhibition plot of hydroperoxide generation, (b) the median effect plot, and (c) the CI plot by the median effect analysis.
The combined effects of α-tocopherol and rosmarinic acid on the inhibition of hydroperoxide generation The synergistic effect of α-tocopherol and rosmarinic acid on the inhibition of hydroperoxide generation was examined (Fig. 1). Figure 1-a shows the concentration-inhibition curves of α-tocopherol and rosmarinic acid when added alone or in combination, and Fig. 1-b shows the median effect plot. As shown in Fig. 1-a, the inhibition curves are concentration-dependent alone and in combination. Linearity was confirmed in the measurement range plots alone and combination (Fig. 1-b). Fig. 1-c shows a CI plot analyzed based on the slope m of each line in Fig. 1-b. The CI plot showed a synergistic effect with CI + 2SD < 1 in the range of 0.2 ≤ fa ≤ 0.8. Although there is no standard for CI, based on previous studies (Minami et al., 2021), it was considered that the CI around 0.8 obtained in this experiment indicates a relatively strong synergistic effect. Since the high and low fa values were dependent on the concentration of antioxidants, it was confirmed that the synergistic effect was obtained for a wide range of concentrations under this experimental condition. As mentioned above, it has been reported that the combination of α-tocopherol and rosmarinic acid markedly prolongs the induction period and significantly inhibits hydroperoxide generation (Panya et al., 2012). The results of this study suggested that the combination of α-tocopherol and rosmarinic acid synergistically prolonged the induction period of hydroperoxide generation.
It was assumed that the synergistic effect of α-tocopherol and rosmarinic acid was due to the α-tocopherol regenerative reaction of rosmarinic acid. Although there is no report on the ability of rosmarinic acid to regenerate α-tocopherol, the regenerative effect of caffeic acid, an analogous compound to rosmarinic acid, has been confirmed, even though the reaction conditions differ (Iglesias et al., 2009). CI plots (synergistic effects) similar to those of rosmarinic acid were also observed for the combination of α-tocopherol and caffeic acid (data not shown). These results suggest that rosmarinic acid also has a synergistic effect on the regeneration of α-tocopherol.
The combined effects of α-tocopherol and antioxidants on the inhibition of hexanal formation The combined effect of α-tocopherol and rosmarinic acid on the inhibition of hexanal formation, the major off-odor component, was analyzed and the results are shown in Fig. 2. Fig. 2-a, Fig. 2-b, and Fig. 2-c show the concentration-inhibition curve, median effect plot, and CI plot, respectively. Concentration-dependent inhibition curves of hexanal formation were observed both alone or in combination (Fig. 2-a), and CI in Fig. 2-c was calculated based on the slope m of each line in Fig. 2-b. Since hexanal is a volatile component, some fluctuation was observed in the measured values. As shown in Fig. 2-c, the CI + 2SD exceeded 1 at an fa of around 0.8, which was determined as indicating an additive effect. On the other hand, a synergistic effect was confirmed with CI + 2SD < 1 in the range of 0.2 ≤ fa < 0.7. In particular, the CI in the range of 0.2 ≤ fa < 0.7 was less than 0.77, indicating a relatively strong synergistic effect. This result suggested that the synergistic inhibitory effect on hydroperoxide generation (Fig. 1-c) was strongly correlated with the synergistic inhibitory effect on hexanal formation.

Combined effect analysis of α-tocopherol and rosmarinic acid on the inhibition of hexanal formation. (a) the inhibition plot of hexanal formation (b) the median effect plot, and (c) the CI plot by the median effect analysis.
Fig. 3 shows the CI plot in combination with various antioxidants other than rosmarinic acid. The combined effects of α-tocopherol and caffeic acid also showed a synergistic effect on the inhibition of hexanal formation with CI > 1 for fa near 0.8, but CI + 2SD below 1 for 0.2 ≤ fa ≤ 0.65, similar to that of the combination with rosmarinic acid. (Fig. 2 and Fig. 3-a). Quercetin and kaempferol showed an additive effect at 0.55 < fa for quercetin and at any range for kaempferol, due to the fluctuation of CI (Fig. 3-d and Fig. 3-e). However, the CIs of α-tocopherol with quercetin and caffeic acid were less than 1 in the range of 0.2 ≤ fa ≤ 0.65, while the CIs of α-tocopherol with kaempferol were less than 1 in the range of 0.2 ≤ fa ≤ 0.8 as well as with rosmarinic acid, confirming the presence of a certain combined effect. The CI of α-tocopherol and luteolin was less than 1 in all ranges, indicating a synergistic effect (Fig. 3-f). On the other hand, the CIs of α-tocopherol with chlorogenic acid (Fig. 3-b) and with ferulic acid (Fig. 3-c) were both approximately 1, indicating that both showed little interaction with α-tocopherol. The synergistic effects of caffeic acid and quercetin were due to their α-tocopherol regenerative potential, as well as rosmarinic acid (Pedrielli et al., 2002: Iglesias et al., 2009), possibly reflecting the synergistic effect exerted on prolonging the induction period of hydroperoxide generation. Furthermore, the three antioxidants, except for kaempferol, have a catechol structure. The reaction rate of catechins was measured by stop-flow spectrophotometry, and the antioxidants with catechol and pyrogallol rings had a particularly high α-tocopherol regeneration capacity (Mukai et al., 2005), suggesting that these antioxidants led to a synergistic effect.

CI plot of α-tocopherol and antioxidants for hexanal formation. (a) caffeic acid, (b) chlorogenic acid, (c) ferulic acid, (d) quercetin, (e) kaempferol, and (f) luteolin.
The combined effects of α-tocopherol and antioxidants on the inhibition of off-odor formation The combined effects of α-tocopherol with rosmarinic acid or other antioxidants on a total of six off-odor components, hexanal, (E)-2-heptenal, (E)-2-octenal, (E)-2-nonenal, (E,E)-2,4-nonadienal, and (E,E)-2,4-decadienal, were then compared. The CI at fa = 0.5 for each antioxidant combination is shown in Table 1. These six components were selected with reference to a previous report (Ohta et al., 2022), and a–c in the table indicate significant differences among the antioxidants and w–z indicate significant differences among the different off-odor components.
| Antioxidants | Hexanal | (E)-2-heptenal | (E)-2-octenal | (E)-2-nonenal | (E-E)-2,4-nonadienal | (E,E)-2,4-decadienal | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rosmarinic acid | wxy 0.72 ± 0.21 a | Syn | wxy 0.79 ± 0.07 b | Syn | xy 0.63 ± 0.20 ab | Syn | y 0.48 ± 0.10 bc | Syn | wx 1.03 ± 0.42 a | Add | w 1.16 ± 0.09 a | Ant |
| Caffeic acid | x 0.79 ± 0.02 a | Syn | w 0.98 ± 0.05 a | Add | x 0.79 ± 0.02 a | Syn | z 0.32 ± 0.01 c | Syn | y 0.50 ± 0.08 b | Syn | w 0.93 ± 0.08 b | Add |
| Chlorogenic acid | w 0.99 ± 0.20 a | Add | w 0.94 ± 0.13 a | Add | w 0.98 ± 0.27 a | Add | w 1.10 ± 0.30 a | Add | w l.04 ± 0.17 a | Add | w 1.28 ± 0.09 a | Ant |
| Ferulic acid | wx 1.01 ± 0.28 a | Add | wx 0.93 ± 0.07 ab | Add | wx 0.94 ± 0.21 a | Add | x 0.74 ± 0.17 b | Syn | w 1.08 ± 0.08 a | Add | wx 0.92 ± 0.06 b | Syn |
| Quercetin | wxy 0.74 ± 0.27 a | Add | wx 0.87 ± 0.13 ab | Syn | wx 0.88 ± 0.23 a | Add | w 1.11 ± 0.05 a | Ant | y 0.44 ± 0.09 b | Syn | xy 0.66 ± 0.15c | Syn |
| Kaempferol | x 0.80 ± 0.27 a | Add | x 0.78 ± 0.07 b | Syn | x 0.73 ± 0.13 a | Syn | w 1.12 ± 0.10 a | Ant | y 0.34 ± 0.02 b | Syn | x 0.73 ± 0.07 bc | Syn |
| Luteolin | x 0.65 ± 0.20 a | Syn | w 0.92 ± 0.08 ab | Syn | y 0.38 ± 0.15 b | Syn | xy 0.59 ± 0.10 bc | Syn | xy 0.55 ± 0.03 b | Syn | x 0.65 ± 0.18 c | Syn |
*Synergy (Syn): mean ± 2SD<1. addition (Add): mean ± 2SD ≒ 1, antagonism (Ant): mean ± 2SD>l
*The letters a–c show the significant difference among antioxidants, w–z show the significant difference among off-odors.
The combination of α-tocopherol and rosmarinic acid showed synergistic effects on the formation inhibition of (E)-2-heptenal, (E)-2-octenal, and (E)-2-nonenal, as well as on hexanal. In particular, the CI for (E)-2-octenal and (E)-2-nonenal were significantly low (0.63 and 0.48, respectively), confirming a strong synergistic effect. On the other hand, no synergistic effect was observed for (E,E)-2,4-nonadienal and (E,E)-2,4-decadienal, indicating that the synergistic effect of α-tocopherol and rosmarinic acid was not observed for all odor components. This difference was possibly attributable to the difference in the formation pathway of off-odor components. (E)-2-octenal and (E)-2-nonenal are degradation products of L-9-hydroperoxide (Zhang et al., 2015). L-9-hydroperoxide is formed during the radical oxidation of linoleic acid (Kato et al., 2022), and this study uses AAPH. Therefore, (E)-2-octenal and (E)-2-nonenal were assumed to be more likely to show synergistic effects.
Luteolin had synergistic effects with α-tocopherol on all off-odor components, and was effective against (E,E)-2,4-nonadienal and (E,E)-2,4-decadienal, whereas rosmarinic acid showed no such effectiveness against the above compounds. Similar to the results for rosmarinic acid, caffeic acid, quercetin, and kaempferol all showed synergistic effects on several off-odor components, but the trends partially differed for each antioxidant. The combination of α-tocopherol and caffeic acid had synergistic inhibitory effects on the formation of hexanal, (E)-2-octenal, (E)-2-nonenal, and (E,E)-2,4-nonadienal. Whereas the combination of α-tocopherol and quercetin or kaempferol showed synergistic effects on (E)-2-heptenal, (E,E)-2,4-nonadienal, and (E,E)-2,4-decadienal, and antagonistic effects on (E)-2-nonenal. On the other hand, when combined with α-tocopherol, chlorogenic acid showed antagonistic effects for (E,E)-2,4-decadienal, and ferulic acid showed synergistic effect for (E)-2-nonenal and (E,E)-2,4-decadienal, but only additive effects on most off-odor components.
The principal component analysis (PCA) based on the CI of combined effects of α-tocopherol and antioxidants To clarify the combined effect of each antioxidant with α-tocopherol on the 6 off-odor components, principal component analysis was attempted using the CI (fa = 0.5) of each off-odor component in Table 1 as a parameter. Fig. 4-a shows the score plot from the principal component analysis and Fig. 4-b shows the loading plot; the cumulative contribution of PC1 and PC2 was 73.3 %. From Fig. 4-a, antioxidants in combination with α-tocopherol were distributed in four major groups. The luteolin alone group in the positive direction of PC1 had the largest value, while the chlorogenic acid and ferulic acid group in the negative direction had the smallest value. The other four antioxidants in combination with α-tocopherol were placed in intermediate positions in PC1. The rosmarinic acid and caffeic acid groups and the quercetin and kaempferol group were found to be distributed separately above and below PC2, respectively. From the loading plot in Fig. 4-b, all of the 6 off-odor components were located in the negative direction of PC1, indicating that the groups located in the positive direction of PC1 were more synergistic with α-tocopherol with respect to their inhibitory effect on off-odor components. All groups except for the chlorogenic acid and ferulic acid group were in the positive direction of PC1, and these antioxidants had a common intramolecular catechol structure, except for kaempferol. It has been reported that compounds with a catechol structure have a high regenerative capacity for α-tocopherol (Mukai et al., 2005). It has also been considered that combination with an antioxidant compound having a catechol structure is effective not only on hexanal but also on other off-odor components.
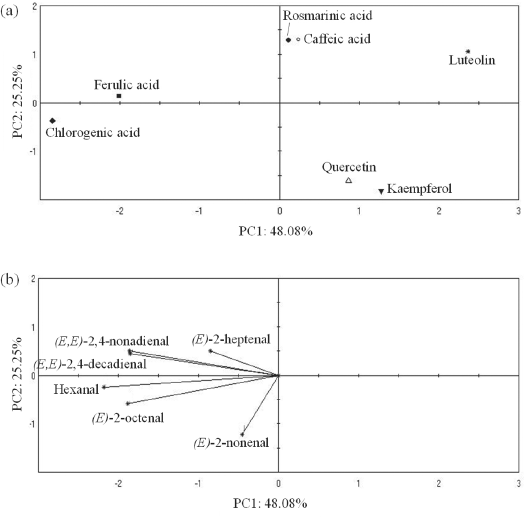
The principal component analysis of the CI (fa = 0.5) for off-odor components. (a) PCA, (b) Loading plot.
*The cumulative contribution of PC1 and PC2 was 73.3 %.
Focusing on PC2, the rosmarinic acid and caffeic acid group located in the positive direction, and the quercetin and kaempferol group located in the negative direction showed characteristic behavior toward the individual off-odor components. The rosmarinic acid and caffeic acid group in the positive direction of PC2 was located opposite to the (E)-2-nonenal and (E)-2-octenal vectors, confirming that they are likely to develop synergistic effects with α-tocopherol with respect to these two components. The group of quercetin and kaempferol was positioned opposite to (E)-2-heptenal, suggesting that they are likely to have a synergistic effect on the inhibition of (E)-2-heptenal formation. (E)-2-octenal and (E)-2-nonenal are degradation products of L-9-hydroperoxide, and (E)-2-heptenal is a product of L-12-hydroperoxide (Zhang et al., 2015). It was considered that the pathway of formation of the off-odor components affected the combined effect.
However, few reports directly examined the combined effect between α-tocopherol and various antioxidants in emulsions, and the combined effect varies depending on the pH of the emulsion and the emulsifier used (Barouh et al., 2021). Further study is needed to determine the detailed mechanisms.
The combined effects of α-tocopherol and rosmarinic acid, and various antioxidants, on inhibition of lipid peroxidation and formation of off-odor components were investigated using linoleic acid emulsions and determined by median effect analysis. Our results showed that the combination of α-tocopherol and rosmarinic acid had a synergistic effect on the inhibition of lipid peroxidation. Caffeic acid showed a similar trend to rosmarinic acid. On the other hand, effects on the inhibition of off-odor component formation differed from effects on the inhibition of lipid peroxidation, showing that the combined effect differs depending on the off-odor components in question. The synergistic effects observed on the off-odor components may reflect the combined effect on the inhibition of hydroperoxide generation and may also be affected by the formation processes of the off-odor components. This was also observed in the combinations of α-tocopherol and other antioxidants, and it was also shown that the synergistic effect on the formation inhibition of off-odor components was particularly strong with antioxidants having a catechol structure. Thus, it is important to select antioxidants with consideration of the target component.
Acknowledgements This work was supported by JSPS KAKENHI Grant Number JP 16K00823.
Conflict of interest There are no conflicts of interest to declare.