2023 Volume 29 Issue 6 Pages 509-519
2023 Volume 29 Issue 6 Pages 509-519
As part of our studies on the prevention of lifestyle-related diseases by processed foods, we evaluated the xanthine oxidase (XO) inhibitory activity of barley tea. The XO inhibitory activity of the tea (a hot-water extract of roasted barley) was not as potent as that of another roasted beverage, coffee. However, the activity of an ethyl acetate-soluble substance in the extract was significantly stronger than that in coffee. Purification of the substance revealed that pyrogallol was the most potent compound. After establishing a quantification method for pyrogallol in barley tea, we analyzed correlations between pyrogallol content, roasted grain color, and XO inhibitory capacity of barley tea. The findings suggest that the XO inhibitory activity of barley tea is related to the roasting degree and chroma b* of barley grains through the content of pyrogallol, although other active compounds may be present in barley tea.
Humankind has utilized thermal food processing and cooking since ancient times, offering many benefits, including the inactivation of foodborne pathogens, improved digestibility, and enhanced sensory quality. Thermal treatments convert the original constituents of food into other materials. This conversion implies that the original functions of the constituents are altered to new functions during processing. Roasting is a thermal processing method executed under relatively high temperature conditions (around 200 °C) (Barham, 2001). This process is often applied to cereal grains to enhance sensory quality (von Boekel et al., 2010). In the Oriental region, barley grains (Hordeum vulgare) are roasted at temperatures above 200 °C and extracted using boiling water to produce traditional barley tea (Mugi-Cha in Japan). Although the health benefits of barley tea have recently garnered interest as a caffeine-free and healthy tea (Fernandes et al., 2018/19; Xiong, et al., 2020), the constituents with the greatest health benefits remain unknown owing to their complex nature derived from high-temperature roasting reactions (Sharma et al., 2022; Shahidi et al., 2021).
Gout is a well-known disease that causes arthritis and impacts human quality of life. Although gout has been referred to as “a disease of kings” in the past, a number of epidemiological studies suggested that the prevalence and incidence of gout have increased in the last few decades (Hak and Choi, 2008; Hakoda and Kasagi, 2020). This increase may be attributed to the recent changes in lifestyle and dietary factors. Xanthine oxidase (XO), the final enzyme involved in human purine catabolism, catalyzes the oxidation of hypoxanthine and xanthine. These reactions produce uric acid, accompanied by reactive oxygen species, such as superoxide and hydrogen peroxide, which contributed to inflammation, metabolic disorders, aging, hypertension, and carcinogenesis (Kang and Nakagawa, 2005). Notably, the onset of gout is caused by an over-accumulation of plasma uric acid. Thus, inhibition of XO overactivity is vital for preventing not only gout but also reactive oxygen-related diseases.
Recently, we discovered the XO inhibitory activity of barley tea. Barley products have also been reported to reduce uric acid formation both in vitro and in vivo (Lee et al., 2010; Hokazono, et al., 2010). In this study, we identified an XO inhibitor in barley tea and estimated its contribution to the XO inhibitory capacity of the tea using commercially available roasted barley grains for tea preparation.
Materials and instruments Roasted barley grains (Product name: Gold Mugi-Cha, the raw grains of which was harvested in western Japan) were provided by Moriuchi Syokuryou (Sakai, Japan). They were used for constituent analysis and large-scale purification. The water used for the extraction of roasted barley grains was the spring water (hardness 20 mg/L, pH 7) provided by Suntory (Osaka, Japan), and XO and allopurinol were obtained from Sigma-Aldrich (Tokyo, Japan). Toyopearl HW-40F resin for size-exclusion chromatography was obtained from Tosoh (Tokyo, Japan), and other reagents and solvents [extra-pure or high-performance liquid chromatography (HPLC) grades] were obtained from Nacalai Tesque (Kyoto, Japan) or Kanto Chemicals (Tokyo, Japan). Nuclear magnetic resonance (NMR) spectra were recorded using a JNM-ECZ400S spectrometer (400 MHz; JEOL, Tokyo, Japan), and mass spectra (MS) were recorded on a JMS-T100 spectrometer (JEOL) using direct analysis in real-time (DART) with the time-of-flight measurement mode. The elemental compositions of the compounds were calculated by high-resolution mass spectrometry (HR-MS) data using the ChemCalc web application (Patiny and Borel, 2013). Analytical HPLC was performed using an LC-20AD liquid chromatography pump (Shimadzu, Kyoto, Japan) equipped with an SPD-M20A diode array detector (Shimadzu). Data were analyzed using the LC-solution v.1.25 software (Shimadzu). Preparative HPLC was performed using an LC-20AR recycling system (Shimadzu) equipped with an SPD-20A UV detector (Shimadzu).
Nine types of roasted barley grains for barley tea preparation were purchased online. The grains consisted of three types of six-row hulled barley grains (Marubishi., Okayama, Japan; Ogawa Sangyo, Tokyo, Japan; and Iseso, Tokyo, Japan), three types of six-row hull-less barley grains (Arutosu, Fujieda, Japan, and Moriuchi Syokuryou, Sakai, Japan), and three types of two-row hulled barley grains (OSH-Odani, Kochi, Japan, and Kajishouten, Higashiosaka, Japan). Unroasted barley grain (six-row hull-less barley grain) was provided by Moriuchi Syokuryou.
Measurement of XO inhibitory activity The XO inhibition assay was performed according to a previously reported protocol with slight modifications (Honda and Masuda, 2016). Briefly, a reaction medium consisting of 100 μL of 100 μmol/L xanthine in 12.5 mmol/L phosphate buffer containing 0.1 mmol/L ethylenediaminetetraacetate (pH 7.4), 10 μL of appropriate concentration of test sample in DMSO, and 70 μL of the same buffer was preincubated at 37°C for 5 min. To this solution, 20 μL of 0.032 units/mL XO buffer solution was added. After incubation at 37 °C for 10 min, 3 % HClO4(aq.) (25 μL) was added to quench the reaction. An aliquot of the solution (20 μL) was injected into the HPLC column (Mightysil RP-18 GP Aqua column, Kanto Chemical, Tokyo, Japan; 250 × 4.6 mm i.d., 5 μm) to quantify the uric acid under the following conditions: flow rate, 1.0 mL/min; solvent, 2.5:97.5 (v/v) methanol–0.1 % phosphoric acid in water; detection wavelength, 290 nm; temperature, 35°C. The percentage inhibition was calculated according to the following equation: Inhibition (%) = 100 × [(peak area of uric acid in control experiment) – (peak area of uric acid in sample experiment)] / (peak area of uric acid in control experiment).
Preparation and solvent partitioning of barley tea Boiling water (300 mL) was added to the roasted barley grains (50 g: roasted six-row hull-less barley grains from Moriuchi Syokuryou), and the mixture was boiled for 5 min. After standing for 2 h, the mixture was filtered using filter paper (No. 2; Advantec, Tokyo, Japan) to obtain a hot-water extract of roasted barley grains (hereafter referred to as barley tea). The extract (15 mL) was partitioned with ethyl acetate (15 mL) to separate the ethyl acetate-soluble and water-soluble components. Evaporation of the ethyl acetate-soluble part, the water-soluble part, and 15 mL of the hot-water extract yielded oily substances (14 mg, 1.2 g, and 1.0 g, respectively).
HPLC analysis of the ethyl acetate-soluble part of barley tea The ethyl acetate-soluble substance (16 mg) of barley tea was prepared according to the methods employed in the previous experiment and dissolved in methanol (0.5 mL). The solution was passed through a solid phase extraction cartridge (Strata C18-E, 100 mg, Phenomenex, Torrance, CA, USA), and the cartridge was eluted with 0.5 mL of methanol, followed by 0.5 mL (×2) of CH3CN to give a solution (8 mg/mL) of the ethyl acetate-soluble substance. This solution (10 μL) was then subjected to HPLC analysis under the following conditions: detection wavelength, 280 nm; column, Cosmosil 5C18-AR-II (250 × 4.6 mm i.d., 5 μm, Nacalai Tesque); solvents: A, 0.1 % phosphoric acid in H2O and B, CH3CN; flow rate: 1 mL/min.; gradient conditions, linear gradient from 5 % solvent B (0 min), 30 % solvent B (40 min), 100 % solvent B (100–110 min), and 5 % solvent B (110–120 min).
Large-scale purification and isolation of peak compounds Roasted barley grains (7 kg) were powdered using a blender (WB-1, Osaka Chemical, Osaka, Japan) and soaked in ethyl acetate (12 L) for one week at room temperature with occasional stirring. The powder was then filtered and soaked again in a sample volume of ethyl acetate for one day. After filtration, the filtrates were combined and evaporated to dryness to obtain the ethyl acetate extract (118 g). The ethyl acetate extract (30 g) was subjected to size-exclusion chromatography and separated into 12 fractions [Column, Toyopearl HW-40F, i.d. 4 × 0.8 m; eluent, ethyl acetate-methanol 4:1 (v/v); fractionated volume, approx. 200 mL] yielding: Fr. 1, 0.02 g; Fr. 2, 0.04 g; Fr. 3, 17 g; Fr. 4, 8 g; Fr. 5, 2 g, Fr. 6, 0.6 g; Fr. 7, 0.3 g; Fr. 8, 0.4 g; Fr. 9, 0.2 g, Fr. 10, 0.1 g; Fr. 11, 0.1 g; Fr. 12, 0.07 g. The XO inhibitory activity of these fractions was measured, and the more active fractions 6–10 were purified further using repeated HPLC, as shown in Fig. 1. Finally, nine compounds 1–9 were obtained and used for structural and corresponding HPLC peak identification (yield: 5 mg for 1, 2 mg for 2, 11 mg for 3, 6 mg for 4, 25 mg for 5, 4 mg for 6, 4 mg for 7, 10 mg for 8, and 2 mg for 9).

Purification Scheme of Constituents of the Ethyl Acetate Extract of Roasted Barley Grains.
Acetylation of compound 8 For structural elucidation, acetylation of compound 8 (1 mg) was performed using acetic anhydride (0.5 mL) and pyridine (0.5 mL) at 25 °C for 2 h. The mixture was evaporated in vacuo and purified by silica gel thin layer chromatography (eluent: 1 % CH3OH in CH2Cl2) to yield acetate 8a (1 mg).
Analytical data for the structures of compounds 1, 2, 3, 4, 5, 7, 8, 8a, and 9
Preparation of barley tea from commercially available roasted barley grains and quantitative analysis of pyrogallol in the tea Commercially available roasted barley grains were ground using a mortar and pestle. The obtained powder was separated through three sieves (mesh size from top to bottom: 2.8, 1.18, and 0.5 mm). The 0.5–1.18 mm powder (6.66 g) was added to a 50 mL screw-top test tube (NX-50, Maruem, Osaka, Japan) containing 40 mL of boiling water, the screw cap was tightly closed, and the mixture was heated in boiling water for 5 min. The mixture was then allowed to stand for 2 h at 25°C and then filtered through filter paper (Advantec, No. 2, 150 mm) to prepare the barley tea. From the resulting barley tea, 5 mL was collected and placed in a 15 mL polypropylene tube, followed by the addition of 5 mL of ethyl acetate and vigorous stirring for 5 min. The tube was centrifuged at 450 × g for 3 min and the ethyl acetate layer was collected. The same procedure was repeated twice more for the remaining aqueous layer, and the resulting ethyl acetate layers were combined and evaporated to dryness. The obtained ethyl acetate-soluble substance was dissolved in 500 μL methanol, 20 μL of which was used to analyze pyrogallol (retention time: 34 min) under the following HPLC conditions: detection wavelength, 266 nm; analytical column system, columns connected from a Cosmosil 3C18-PAQ (250 × 4.6 mm i.d., Nacalai Tesque) to a Cosmosil 5C18-PAQ (250 × 4.6 mm i.d., Nacalai Tesque); solvent, CH3CN–0.1 % phosphoric acid in H2O [2.5:97.5 (v/v)], column temperature, 35 °C; flow rate, 0.5 mL/min.
L*a*b* measurements of roasted barley grains The Commission Internationale de l'Eclairage (CIE) L*a*b* values of roasted barley grains were measured using a V-750 spectrophotometer (Jasco, Hachioji, Japan) attached with an integrating sphere (ISV-900, Jasco). Ground roasted barley grains were placed in a PSH-002 powder cell (Jasco) and measured at 380–780 nm by the specular component exclusion method (Illuminants, D65; Observers, 2°; and the white standard provided by Jasco), and the data were analyzed using Jasco spectra manager (v. 2) with the color assessment program VWCD-960 (Jasco) to obtain the values for the L*a*b* color space. Measurements were performed in triplicate, and the values were expressed as the average of the three data points.
Measurement of the browning degree of barley tea The browning degree of barley tea was evaluated using the absorbance (ABS) value at 420 nm. The absorbance at 420 nm was measured using a UV-1900i UV-VIS spectrometer (Shimadzu) in a 1-cm glass cuvette using appropriately diluted barley tea. The browning degree was expressed as the calculated ABS value (observed ABS × dilution ratio).
Statistical analysis Correlation analysis and Dunnett's test were carried out using BellCurve for Excel (v. 4.04, Social Survey Research Information, Tokyo, Japan), with p < 0.05 being considered significant.
| Roasted Barley Extraction Sample | Concentration (mg/mL) | Inhibition % (mean ± SE) | IC50 (mg/mL) |
|---|---|---|---|
| Hot-Water extract | 0.6 | 14.3 ± 1.0 | 4.6 |
| Ethyl Acetate-Soluble Substance | 0.3 | 89.5 ± 0.2 | 0.04 |
| Water-Soluble Substance | 0.3 | −1.7 ± 1.5 | − |
Activity of coffee (roasted): 18 % inhibition (0.6 mg/mL) for hot-water ext. and 59–65 % inhibition (0.3 mg/mL) for ethyl acetate-soluble substance (Honda et al. 2014; Honda and Masuda, 2016).
XO inhibitory activity of barley tea and major constituent analysis We elucidated the XO inhibitory activity of roasted beverages for the control of gout, a lifestyle-related disease that is markedly influenced by dietary habits. In familiar East Asian roasted beverages [roasted green tea (Camellia sinensis leaves), roasted barley tea (Hordeum vulgare grains), roasted corn tea (Zea mays grains), roasted coffee (Coffea arabica beans), etc.], the non-polar fraction of roasted barley tea (Mugi-Cha in Japan) showed remarkably strong XO inhibitory activity. Table 1 shows the XO inhibitory activity of the hot-water extract of roasted barley grains (barley tea) and its ethyl acetate-soluble substance in terms of percentage inhibition and the activity of coffee, which has been reported to prevent gout (Choi et al., 2007), as reference data. Although the activity of the hot-water extract of roasted barley is not stronger than that of coffee, the activity of the ethyl acetate-soluble substance in the barley tea was found to be stronger [half-maximal inhibitory concentration (IC50), 0.04 mg/mL] than that in coffee (IC50, approx. 0.06–0.07 mg/mL; Honda and Masuda, 2016).
The components of the active ethyl acetate-soluble substance in barley tea were analyzed by HPLC in the gradient mode using octadecylsilyl silica gel (ODS) columns, and the data are shown in Fig. 2. The HPLC data show that the ethyl acetate-soluble substance contains very complex components and does not form completely separate peaks over the entire range of the retention time up to 75 min. Nevertheless, several relatively large peaks were observed up to a retention time of 20 min, so we decided to purify the compounds corresponding to the peaks (hereafter, peak compounds) to identify their chemical structures and estimate their XO inhibitory activity.

HPLC Analytical Data of the Ethyl Acetate-Soluble Substance of Barley Tea and Peak Identification of Purified Compounds 1–9.
Prior to the extraction of a large amount of roasted barley grains, we examined the extraction method and found that the same peak compounds in the above-mentioned analysis could be obtained by direct extraction of powdered roasted barley grains with ethyl acetate. Therefore, we attempted to purify the peak compounds from the ethyl acetate extract of finely powdered roasted barley grains (7 kg). The extract showed numerous broad peaks in the HPLC data, which we considered to be attributable to complex and polymeric materials based on our previous findings of roasting reactants (Shindo et al., 2023). Therefore, we attempted to remove these materials by size-exclusion chromatography. The activities of 12 fractions obtained by the chromatography were then measured, with the data shown in Fig. 1. The fractions containing lower molecular weight components with slower elution rates showed stronger activity than fractions that eluted faster. To obtain the peak compounds, further purification was performed using active fractions 6–10, as shown in Fig. 1. Finally, nine compounds were purified, and their corresponding peaks were identified in the HPLC data (see expanded data in Fig. 2) by comparing their retention times under the same analytical conditions.
The HR-MS data of compound 1 revealed its molecular formula to be C4H4N2O2 (m/z 113.0356 [M+H]+). The 1H NMR data of 1 showed only two olefinic signals (δ 7.31 and δ 5.53) with a 7.8-Hz coupling constant. These data, in addition to the UV absorption maxima at 256 nm, indicated that 1 was the nucleic acid base, uracil. Compound 3 had a similar UV spectrum (λmax 263 nm) to that of 1. The 1H NMR data of 3 showed one aromatic proton signal at 7.13 ppm and a signal (δ 1.75) due to a methyl group adjacent to a double bond. From these data and the molecular formula (C5H6N2O2), which was predicted from HR-MS data (m/z 125.0330 [M-H]−), 3 was determined to be the nucleic acid base, thymine. The identity of both nucleic acid bases was confirmed using commercially available samples. Compounds 2 and 9 were both molecules containing one nitrogen, which was revealed from their HR-MS data (m/z 96.0449 [M+H]+ for C5H6NO and m/z 112.0394 [M+H]+ for C5H6NO2, respectively). The 1H NMR data of 2 showed four aromatic signals, two of which were relatively low-field-shifted to 7.90 and 7.75 ppm. The UV spectrum of 2 showed characteristic absorption maxima at 315, 276, and 247 nm. Based on these data, 2 was determined to be 3-hydroxypyridine, which was confirmed by previously reported data (Vögeli and Philipsborn, 1973; Korobeinicheva et al., 1990). The 1H-NMR data of 9 showed three aromatic proton signals, one of which was high-field-shifted to 6.06 ppm, and their coupling constants were relatively small (3.7, 2.7, and 1.4 Hz), indicating the presence of a 5-membered aromatic ring such as pyrrole or furan with an electron-withdrawing group at the 2-position. These data and the molecular formula (C5H5NO2) clarified that 9 is pyrrole-2-carboxylic acid (Shimokawa et al., 1970). Compound 5 was identified as C6H6O3 from HR-MS data, with signals due to an aldehyde group (δ 9.42) and an isolated methylene group (δ 4.50) observed in the 1H NMR spectra. These data, in addition to the observed two aromatic signals (δ 7.29 and δ 6.47) in the 1H NMR data of 5, indicated that 5 was 5-hydroxymethylfurfural, which was confirmed using a commercially available sample. The HR-MS of compounds 4 and 7 indicated the molecular formulas to be C6H6O3 and C6H6O2, respectively. The 1H NMR data of 4 and 7 showed only high-field-shifted aromatic signals at 6.38 (1H) and 6.21 (2H) ppm for 4, and 6.86 (2H) and 6.79 (2H) ppm for 7. From these data, 4 was identified as pyrogallol and 7 as catechol. The molecular formula of compound 8 was deduced as C9H8O3 from its HR-MS data (m/z 165.0542 [M+H]+). The 1H NMR data of 8 revealed two aromatic signals with ortho coupling (J = 8.2 Hz in 8a) and two methylene signals with an AA′BB′ coupling pattern. The 13C NMR data of 8 indicated the presence of a ketone from the signal at 209.3 ppm. Long-range carbon-proton correlations were observed between C1 and H2, C1 and H7, and C5 and H6 in the HMBC spectrum of 8. From these data, the structure of 8 should be as shown in Fig. 3. The presence of two phenolic groups was confirmed by the 1H-NMR data obtained for the diacetate of 8 (8a) [acetyl proton signals: δ 2.34 (3H, s) and 2.31(3H, s)]. This compound has been identified as 4,5-dihydroxyindan-1-one, a fungal metabolite reported by Paz et al. (2011). Peak compound 6 showed unclear data in MS and very broad signals in the 1H NMR data of 6. Therefore, the chemical structure of 6 remains unclear (Fig. 3).

Identified Chemical Structures of Peak Compounds 1–5 and 7–9.
1, Uracil; 2, 3-Hydroxypyridine; 3, Thymine; 4, Pyrogallol; 5, 5-Hydroxymethylfurfural; 7, Catechol; 8, 4,5-Dihydroxyindan-1-one; 9, Pyrrole-2-carboxylic acid.
Among the eight identified compounds, the XO inhibitory activities of compounds 1, 4, 5, and 7 have been reported previously. Uracil (1) and catechol (7) were reported as soy source inhibitors by Li et al. (2016). 5-Hydroxymethyl-2-furfural (5) was shown to be the main inhibitor of red-koji vinegar by Lin et al. (2012). We previously found that pyrogallol (4) is an inhibitor of roasted coffee beans (Honda and Masuda, 2016). In this investigation, the XO inhibitory activity of the peak compounds 1–9 was examined using purified or commercially available samples; Fig. 4 shows the inhibitory activities at a concentration of 50 μg/mL as the mean value of percentage inhibition ± SE. Under the conditions employed, compounds 1, 2, 3, 7, 8, and 9, which included the reported inhibitors 1 and 7, did not exhibit any activity. Compounds 5 and 6 showed weak activity, although compound 5 was reported as a potent inhibitor in vinegars (Lin et al. 2012). Only compound 4 (pyrogallol) clearly demonstrated a much stronger activity (approx. 100 % inhibition) than the other compounds. Therefore, pyrogallol may contribute significantly to the XO-inhibitory potential of barley tea. The potent XO inhibitory activity of pyrogallol has been reported previously (Honda and Masuda, 2016; Habib et al., 2023). Furthermore, Honda et al. (2017) suggested that this activity is derived from purpurogallin, which is formed by the oxidative dimerization of pyrogallol under physiological pH conditions (pH 7.4). The XO inhibitory activity of purpurogallin has been reported and its mechanism has recently been discussed using molecular docking simulations (Sheu et al, 1998; Honda et al., 2017; Wang et al, 2021).

XO Inhibitory Activity of Peak Compounds 1–9 (50 μg/mL).
Data are expressed as the means ± SE (n = 3).
* Statistically significant difference was observed against control data.
Development of a quantitative analytical method for pyrogallol in barley tea using HPLC Pyrogallol is a simple polyphenolic compound that widely distributed in natural and industrial products (Gupta et al., 2021). Various quantitative analytical methods for small amounts of pyrogallol have been reported using gas chromatography-mass spectrometry (Tor et al., 1996), flow-injection coupled with chemiluminescent detection (Sun et al., 2000), and liquid chromatography-tandem mass spectrometry (Dutschke et al., 2021). In this study, a simple HPLC quantification method specific for pyrogallol in barley tea was developed. During the investigation, we found that pyrogallol was fully extractable by ethyl acetate; however, compared to other ethyl acetate-soluble constituents, pyrogallol showed a lower affinity for ODS columns its elution rate was faster. Therefore, roasted barley tea was first extracted with ethyl acetate to obtain all of the pyrogallol and to exclude most of the other polar constituents that interfere with the selective detection of pyrogallol. Next, we examined the optimal HPLC conditions for the selective detection of pyrogallol. Our investigation allowed us to detect pyrogallol as a single peak through a pair of linked two ODS columns [COSMOSIL 3C18-PAQ (250 × 4.6 mm i.d.) + COSMOSIL 5C18-PAQ (250 × 4.6 mm i.d.)] eluted with CH3CN-0.1 % phosphoric acid in H2O, as shown in Fig. 5. The purity of the pyrogallol peak was confirmed through the total peak purity method implemented in the LC-solution software package (Shimadzu), which detects any impurities in the observed peaks based on peak shape at each wavelength. Without ethyl acetate extraction, the pyrogallol peak overlapped with the peaks of other components. According to the validation method reported by Gampe et al. (2022), the values of the calibration curve and recovery, including the accuracy and precision information, for this method were obtained as summarized in Table 2. The data show that this method meets the validity guidelines for analytical methods proposed by the Ministry of Agriculture, Forestry, and Fisheries of Japan (1989).
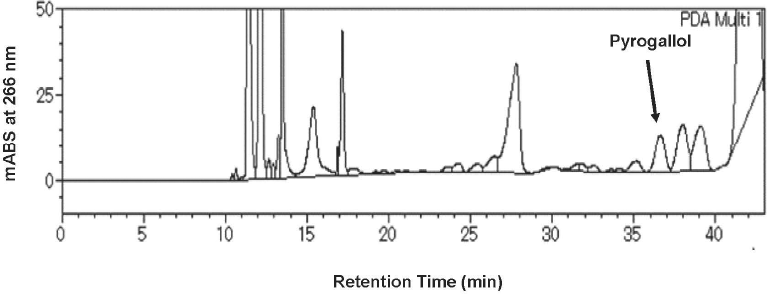
HPLC Analytical Profile of Pyrogallol in Barley Tea Used for Its Quantitative Analysis.
| Calibration Curves | Recovery Results (accuracy & precision information) | |||
|---|---|---|---|---|
| Linearity | 0.08 – 15.86 nmol | Spiked Pyrogallol | Recovery (%) | RSD (%) |
| Range | (nmol)2) | (Mean±SD) | ||
| Equation | y = 62 790×–18,7111) | 5.06 | 85 ± 15 % | 7.0 |
| R2 | 0.996 | 4.21 | 99 ± 7 % | 7.0 |
| LOQ | 0.04 nmol | 2.18 | 101 ± 10 % | 5.6 |
Correlation analysis of pyrogallol concentration, dilution rate calculated for 50 % inhibition, and browning degree of barley tea, roasting degree and chromaticity (a*, b*) of roasted barley grains Barley can be classified into four main types, and barley tea in Japan is produced primarily from three of these types of barley grains (six-row hulled barley, six-row hull-less barley, and two-row hulled barley). In this study, we obtained nine kinds of commercially available roasted barley grains (three types of barley × three roasted products) that are used to make barley tea and captured their color information through CIE L*a*b* measurements. These values are indicative of the roasting conditions of the grains. The L* value (Lightness), which is directly related to the roasting intensity, was converted to the roasting degree using the following equation: roasting degree (%) = 100 × [(L* value of unroasted barley grain) – (L* value of roasted barley grain)] / [(L* value of unroasted barley grain) – (L* value of darkest roasted grain)]. The L* value of the unroasted barley grains was 77.8, and that of the darkest roasted grains (i.e., 18.3) was adopted from the observed maximum value of roasted barley grain (Duh et al., 2001) (Table 3).
| Grain | Tea | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of barley | Roasted barley products | Roasting degree (%) 1) | a* | b* | Browing degree2) of tea | Concentration of ethyl acetate-soluble substance (g/L) | IC50 of ethyl acetate-soluble substance (g/L) | Dilution rate of tea calculated to inhibit xanthine oxidase by 50 %3) | Pyrogallol concentration4) in tea (nmol/L) |
| 6-row | A | 78.1 | 7.63 | 14.01 | 14.2 | 1.09 | 12×10−3 | 91 | 31.6 (2.2) |
| hull- | B | 76.4 | 7.21 | 12.80 | 22.6 | 0.56 | 9×10−3 | 62 | 33.8 (0.8) |
| less | C | 84.6 | 7.18 | 11.50 | 23.8 | 0.82 | 7×10−3 | 117 | 30.8 (1.1) |
| 6-row | D | 70.4 | 7.65 | 16.25 | 18.7 | 0.99 | 27×10−3 | 37 | 26.5 (1.3) |
| hulled | E | 56.3 | 6.09 | 16.78 | 17.4 | 0.75 | 11×10−3 | 68 | 26.3 (2.2) |
| F | 76.2 | 5.50 | 10.69 | 22.4 | 1.01 | 23×10−3 | 44 | 28.0 (0.5) | |
| 2-row | G | 63.1 | 7.85 | 18.06 | 17.3 | 0.96 | 20×10−3 | 48 | 23.4 (0.9) |
| hulled | H | 62.5 | 6.89 | 17.52 | 15.2 | 0.77 | 26×10−3 | 30 | 22.9 (0.8) |
| I | 73.4 | 7.56 | 14.59 | 25.4 | 1.04 | 32×10−3 | 33 | 23.6 (0.9) | |
The roasted grains were powdered and extracted with boiling water using the method as described in the experimental section. This brewing method, while stronger than the commonly used preparation methods in Japan, is more convenient for component analysis (Kajimoto, 2000; Todoriki et al., 2013). The browning degree of brewed barley tea was measured based on the absorbance at 420 nm (Table 3).
The tea was then extracted three times with ethyl acetate, and the ethyl acetate layer was evaporated and dried to obtain an ethyl acetate-soluble substance. The XO inhibitory activity of the substance was measured, and its IC50 was determined. Next, the theoretical dilution rate of tea required to exert 50 % inhibition was calculated as follows: dilution ratio = concentration of ethyl acetate-soluble substance in tea (g/L) / IC50 value of the substance (g/L). This dilution rate was used as an indicator of the inhibitory ability of the tea. The activity of the liquid tea itself is not strong, and it is difficult to determine directly its exact inhibitory activity. Therefore, this dilution rate was calculated from the activity (IC50) of the ethyl acetate-soluble substance, which could be determined accurately, and its amount (concentration) in the tea. Thus, the obtained higher value indicates greater XO inhibitory capacity of the tea. Finally, the pyrogallol concentration in barley tea was measured using the HPLC method described above (Table 3).
The results of the correlation analysis of the properties of barley grain and tea are summarized in Table 4. Table 4 shows that strong correlations were observed between the roasting degree of grains and the pyrogallol concentration in tea (r = 0.70), between the b* value and the pyrogallol concentration (r = −0.71), between the pyrogallol concentration and the dilution rate of tea that inhibits XO activity by 50 % (r = 0.69), and between the roasting degree and the b* value (r = −0.85). Slightly stronger correlations were also observed between the roasting degree and the dilution rate (r = 0.53), between the b* value and the dilution rate (r = −0.46), between the roasting degree and the browning degree (r = 0.53), and between the b* value and the browning degree. As mentioned above, we identified pyrogallol as the predominant XO inhibitor among the complex constituents of barley tea. The concentration of pyrogallol in tea showed a strong correlation with the calculated dilution rate, which was an indicator of the XO inhibitory activity of barley tea. The XO inhibitory function of barley teas commonly sold in Japan was attributed mainly to the pyrogallol content in the present study. However, the results of the correlation analysis (r is less than 1) for pyrogallol amount-activity capacity (estimated by dilution rate) and the difficulties associated with analyzing the roasted food constituents, which are typically very complex after high-temperature treatment, indicate that other XO inhibitory compounds might still be considered. Although pyrogallol has been identified as an XO inhibitor in roasted coffee (Honda and Masuda, 2016), other thermal reaction products from raw coffee constituents are predicted to contribute to the inhibitory activity (Honda et al., 2014; Fukuyama et al., 2018, Masuda et al., 2019). Müller et al. (2006) suggested that pyrogallol in roast coffee was derived from chlorogenic acid. Chlorogenic acid is the main phenolic constituent of raw coffee beans (2.61–3.39 % of the dry weight of C. arabica beans, Ky et al., 2001), and its thermal degradation yields pyrogallol during coffee roasting. It should be noted that the chlorogenic acid content of barley (0.01–0.02 % of dry barley grains; Yu et al., 2001) is much lower than that of coffee, therefore, pyrogallol in barley tea may not be derived from chlorogenic acid. Exploring its origin in future studies may therefore be promising. By identifying the raw material for pyrogallol formation, a roasting method that produces pyrogallol efficiently can be developed. As a result, it may be possible to add the food function of xanthine oxidase inhibition, and therefore gout prevention, by roasting foods containing the substances. Although the correlations between the inhibitory capacity of the tea and roasting intensity, as well as the chroma value b*, were not very high, each of which may be indirectly involved through pyrogallol concentration, measuring the roasting degree or b* of barley grains may allow us to predict a higher XO inhibitory capacity of barley tea at the present stage and, furthermore, to predict its gout-preventive function.
| Correlation coefficient (r) | Roasting degree of grain | a* of grain | b* of grain | Browning degree of tea | Dilution rate of tea calculated to inhibit xanthine oxidase by 50 % | Pyrogallol concentration in tea (nmol/L) |
|---|---|---|---|---|---|---|
| Roasting degree | 0.16 | −0.85 | 0.53 | 0.53 | 0.70† | |
| a* | 0.38 | −0.12 | 0.06 | −0.03 | ||
| b* | −0.63 | −0.46 | −0.71† | |||
| Browing degree | 0.04 | 0.19 | ||||
| Dilution rate | 0.69† |
Acknowledgements Grants from the Council for Promotion of Barley Foods of Japan (Oomugi Syokuhin Kyougikai) and JSPS (KAKENHI Grant Number 23H00912) are gratefully acknowledged. We also thank Moriuchi Syokuryou Inc. for providing us with the raw materials for the roasted barley product.
Conflict of interest The authors declare no conflict of interest associated with this manuscript.