2015 年 84 巻 2 号 p. 172-177
2015 年 84 巻 2 号 p. 172-177
2,4-Pyridinedicarboxylic acid (2,4-PDCA) was shown to prolong the vase life of cut flowers of spray-type ‘Light Pink Barbara’ (LPB) carnation, mainly due to the reduced ethylene production caused by inhibition of 1-aminocyclopropane-1-carboxylate oxidase in the flowers. In addition, 2,4-PDCA has been suggested to accelerate flower opening in the flowers (Satoh et al., 2014). In the present study, we successfully developed a procedure to evaluate the activity of chemicals to accelerate flower (bud) opening by determining the shortened time (in days) to flower opening. Using this procedure, we could show the activities of several PDCA analogs to accelerate flower opening, in addition to their already-known activity to extend the vase life in cut flowers of ‘LPB’ carnation. Judging from their effectiveness in the acceleration of flower opening and extension of vase life, 2,3-PDCA and 2,4-PDCA were thought to be suitable agents for treatment of the flowers. The present study confirmed that PDCAs accelerate flower opening and retard senescence, which increase the number of open flowers, resulting in extension of the vase life of cut flowers of ‘LPB’ carnation.
During the senescence of carnation flowers, a climacteric increase in ethylene production occurs, and the evolved ethylene induces in-rolling of petals, resulting in wilting of whole flowers (Satoh, 2011). The effect of ethylene on flower senescence can be diminished by treating flowers with inhibitors of ethylene biosynthesis or action. Satoh et al. (2014) have discussed several examples of such chemicals, which have been tested in trials or used practically in the flower industry. Treatment with these inhibitors prolongs the vase life of cut carnation flowers as well as other ethylene-sensitive ornamental flowers.
Vlad et al. (2010) reported that 2,4-pyridinedicarboxylic acid (2,4-PDCA) inhibited ethylene production in detached flowers of ‘White Sim’ carnation, which belongs to the standard category of carnation flowers, and delayed senescence of the flowers. The latter action was interesting practically; however, flower senescence was delayed in only 40%, but not in the remaining 60%, after treatment with the chemical. They hypothesized that 2,4-PDCA probably inhibited 1-aminocyclopropane-1-carboxylate (ACC) oxidase by competing with ascorbate, a co-substrate of the enzyme action. Then, Fragkostefanakis et al. (2013) showed that 2,4-PDCA inhibited the in vitro activity of ACC oxidase prepared from tomato pericarp tissues. This observation supported the hypothesis that 2,4-PDCA inhibits ACC oxidase action by competing with ascorbate in carnation flowers and extends their vase life.
Satoh et al. (2014) confirmed that 2,4-PDCA inhibited ACC oxidase action using a recombinant enzyme produced in Escherichia coli cells from the carnation ACC oxidase gene (DcACO1 cDNA). They also confirmed the strange behavior of 2,4-PDCA’s action in extending the vase life of detached single flowers of carnation by observing that 2,4-PDCA prolonged the vase life of some of the flowers but not of the remaining ones. Moreover, they showed that PDCA treatment significantly prolonged the vase life of cut ‘Light Pink Barbara’ and ‘Mule’ carnation flowers, both of which belong to the spray category of carnation flowers, in which the effect of PDCA on the vase life of flowers was determined by observing the number of open flowers, that is, the percentage of open flowers to the total number of initial flower buds (Satoh et al., 2005). It is noteworthy that the increase in the number of fully open and non-senescent flowers might be caused by both acceleration of flower (bud) opening and delayed senescence of fully open flowers (Satoh et al., 2014), although solid proof for such acceleration was not obtained at that time. Therefore, it remains to be elucidated whether the extension by 2,4-PDCA of the vase life of cut spray-type carnation flowers was caused by its possible dual action. It is important to determine whether analogs of 2,4-PDCA also have such activity in order to understand the mechanism of its action.
In the present study, we aimed to demonstrate the acceleration of flower opening by 2,4-PDCA in cut spray-type carnation flowers, and to compare its action with those of its structural analogs, such as 2,3-PDCA and 2,5-PDCA (Fig. 1), in order to obtain further information on 2,4-PDCA’s action.

Structures of pyridinedicarboxylic acid (PDCA) analogs and 2-oxoglutaric acid (2-OxoGA). Drawings of the chemical structures were taken from HP of Sigma-Aldrich Co. LLC.
Dianthus caryophyllus L. ‘Light Pink Barbara’ (LPB), which belongs to the spray type of carnation flowers, was used. Flowers at the usual commercial stage of flowering when the first flower out of six to eight flower buds on a stem was partially open, were harvested with 80-cm-long stems at the nursery of a commercial grower in Miyagi Prefecture, Japan. The flowers were not treated with any flower preservatives, such as STS, after harvest (in April and May 2014). The harvested flowers were transported without supply of water to Kyoto Prefectural Institute of Agricultural Biotechnology in Kyoto Prefecture the day after harvest. After arrival, they were placed in plastic buckets with their cut stem end in tap water under continuous light from white fluorescent lamps (14 μmol·m−2·s−1 PPFD) at 23°C and 40–60% relative humidity. The flowers were used for experiments several hours after their arrival.
Determination of the vase life and time to flower opening of carnation flowers treated with PDCA analogsSatoh et al. (2014) determined the vase life of spray-type carnation flowers, as affected by treatment with 2,4-PDCA, by observing the change in the percentage of open flowers to the total number of initial flower buds. Therefore, we conducted the present experiment by treating carnation flowers according to the appropriate method with necessary modifications. The test solutions were distilled water (control) and 1 mM and 2 mM PDCA analogs as follows: 2,3-, 2,4-, 2,5-, 2,6-, 3,4-, and 3,5-PDCA (Fig. 1). All analogs of PDCA were products of Wako Pure Chemical Industries, Ltd., Osaka, Japan. The vase life of cut flowers in days was determined by the number of days during which the percentage of fully open and non-senescent flowers was 40% or more. The time to opening of the flowers was determined as the number of days from the start of the experiment until the time when the percentage of fully open and non-senescent flowers reached 40%. When stems of the flowers at opening stage (Os) 6–senescing stage (Ss) 2 broke, those flowers were regarded as having reached the end of their vase lives. The definitions of opening stage (Os) and senescing stage (Ss) of carnation flowers were the same as described by Harada et al. (2010) and Morita et al. (2011), respectively. Data are shown as the means of 3 replicates, each consisting of 25 buds.
Statistical analysesStatistical analyses were carried out by Steel’s or Dunnett’s multiple range test using an online statistical analysis program, MEPHAS (http://www.gen-info.osaka-u.ac.jp/testdocs/tomocom/, June 7, 2014).
Figure 2 shows changes in the percentage of fully open and non-senescent flowers for cut ‘LPB’ flowers treated with different concentrations of 2,4-PDCA; this figure was adapted from Figure 4 of Satoh et al. (2014). The vase life was significantly lengthened by treatment with 2,4-PDCA, attaining 53, 111, and 135% increases at 0.3, 1, and 2 mM 2,4-PDCA, respectively, compared with the control (Satoh et al., 2014).

Evaluation of flower opening by determining the time to flower opening in cut flowers of ‘Light Pink Barbara’ (LPB) carnation treated continuously with different concentrations of 2,4-PDCA. This figure was adapted from the original data that appeared as Figure 4 in Satoh et al. (2014). The percentage of fully open flowers (Os 6–Ss 2) was calculated from the proportion of those flowers to the total number of initial flower buds (25 buds per 5 flowers). Each point is the mean of 3 replicates, each with 5 flowers. The vase life of the cut flowers in days was defined as the duration when the percentage of fully open and non-wilted flowers was 40% or more. The time to flower opening was defined as the time in days from the start of the experiment until the percentage of open flowers reached 40%. (1) time to flower opening; (2) vase life. ●, Control (0 mM 2,4-PDCA); ○, 0.3 mM 2,4-PDCA; □, 1 mM 2,4-PDCA; △, 2 mM 2,4-PDCA.
The data shown in Figure 2 suggest that 2,4-PDCA treatment tended to accelerate flower (bud) opening compared with that in the untreated control. Therefore, we defined ‘the time to flower opening’ as a measure of flower opening, as described in Materials and Methods. When we determined the time to opening of the flowers treated with different concentrations of 2,4-PDCA, it was 4.4 days for the control, 4.3 days for 0.3 mM, 3.3 days for 1 mM, and 3.8 days for 2 mM (Fig. 2). These observations suggested that 2,4-PDCA treatment tended to accelerate flower bud opening, although only the treatment with 1 mM 2,4-PDCA was significantly different from the control by Steel’s multiple range test (P < 0.05). We considered that this procedure for determining “the time to flower opening” is useful to evaluate the acceleration of flower opening as affected by treatment with PDCA analogs.
Acceleration of flower opening and extension of vase life in cut ‘LPB’ flowers by treatment with PDCA analogsFigure 3 shows the changes in the percentage of open flowers in cut ‘LPB’ flowers treated continuously with or without PDCA analogs. The experiments were conducted separately with the combinations of 0 (control) and 1 mM PDCA analogs or 0 (control) and 2 mM PDCA analogs. In these experiments, the mean percentage of fully open and non-senescent flowers was obtained from 3 replicates, as described in Materials and Methods.
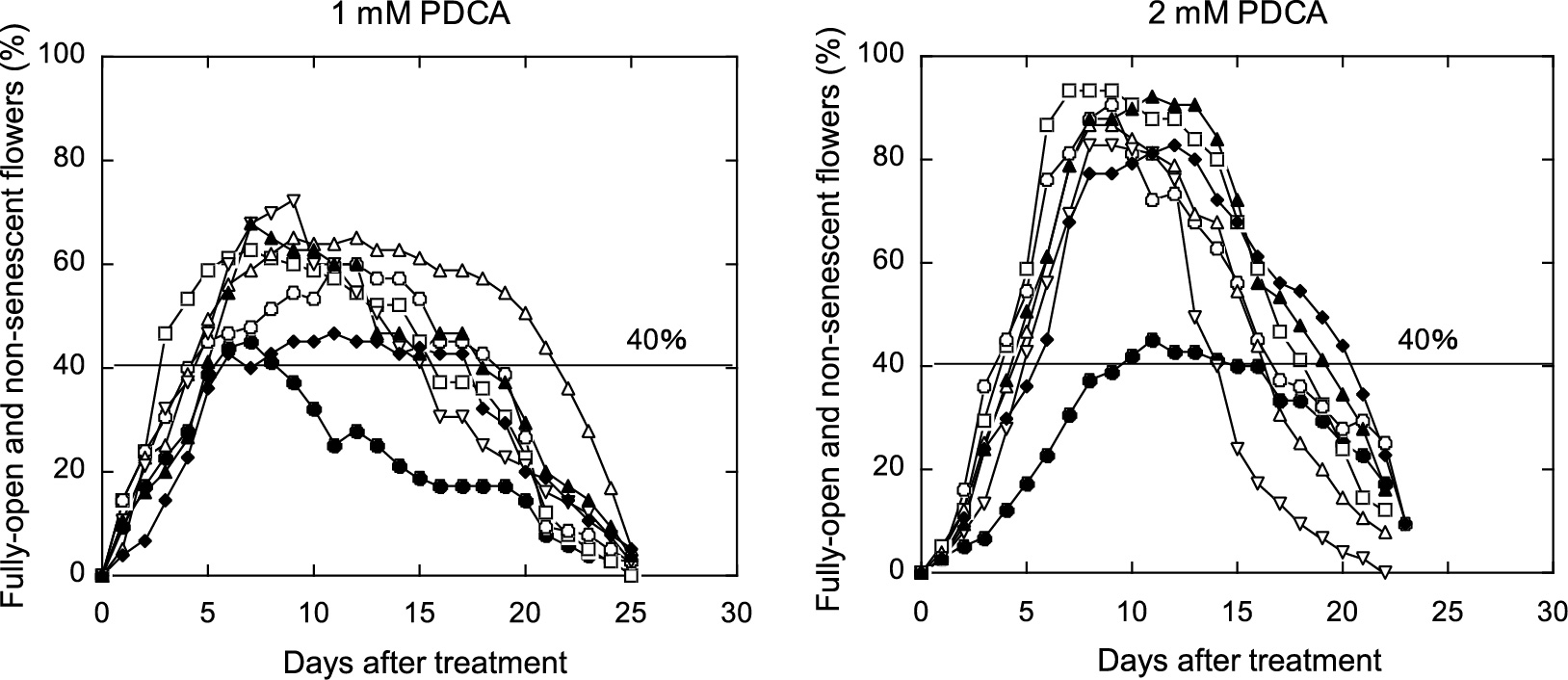
Changes in the percentage of fully open and non-senescent flowers for cut ‘LPB’ flowers treated continuously with or without PDCA analogs at 1 mM or 2 mM. Procedures for determination of the percentage of fully open and non-senescent flowers and the data presentation are the same as described in the legend to Figure 2. ●, Control; □, 2,3-PDCA; ○, 2,4-PDCA; △, 2,5-PDCA; ▽, 2,6-PDCA; ◆, 3,4-PDCA; ▲, 3,5-PDCA.
In the control flowers, the number of fully open and non-senescent flowers increased gradually and peaked on day 7 in the experiment with 1 mM PDCA analogs, and on day 11 in the experiment with 2 mM PDCA analogs. Figure 4 shows flower opening and senescing profiles of the control and flowers treated with 2,3-, 2,4-, 2,5-, and 3,4-PDCAs at 2 mM, which were chosen as typical specimens out of 3 replicates 0, 3, and 10 days after the start of the experiment. On day 3, open pink flowers were seen more among the flowers treated with PDCAs, especially with 2,3- and 2,4-PDCA, than in the control. These observations indicated that treatment with PDCAs accelerated flower opening compared with that in the untreated control flowers, and the acceleration effect was greatest with 2,3-PDCA among the PDCAs. On day 10, the control flowers remained as a mixture of buds, open flowers, and senesced flowers. The senesced flowers showed in-rolling and wilting of petals, typical symptoms of senescence in response to ethylene. On the other hand, almost all of the flowers treated with PDCAs were fully open with non-wilted and turgid petals. At the later stage, the PDCA-treated flowers withered with browning at the petal margins, as well as with jumbled, but turgid, fading petals, as reported previously (Satoh et al., 2014), or lost their vase lives by the breaking of flower stems, as described below.

Flower-opening and -senescing profiles of cut ‘LPB’ flowers treated without (control) or with 2,3-, 2,4-, 2,5-, and 3,4-PDCA at 2 mM. Typical profiles for each treatment out of 3 replicates were chosen for 0, 3, and 10 days after the start of the experiment shown in Figure 3 and photographed. (See online article for color versions of the figures.)
Table 1 summarizes the effects of various PDCA analogs on the time to flower opening and the vase life of cut ‘LPB’ flowers. In the experiment with 1 mM PDCA analogs, the control (0 mM) flowers reached the stage of flower opening (40%) on day 5.5. Only the treatment with 2,3-PDCA significantly shortened the time to flower opening, in other words, stimulated flower opening. The number of days for the time to flower opening was the shortest at 3.3 days for 2,3-PDCA, followed by 2,4-PDCA and 2,6-PDCA (both, 4.3 days), showing that the latter two chemicals also tended to shorten the time to flower opening.
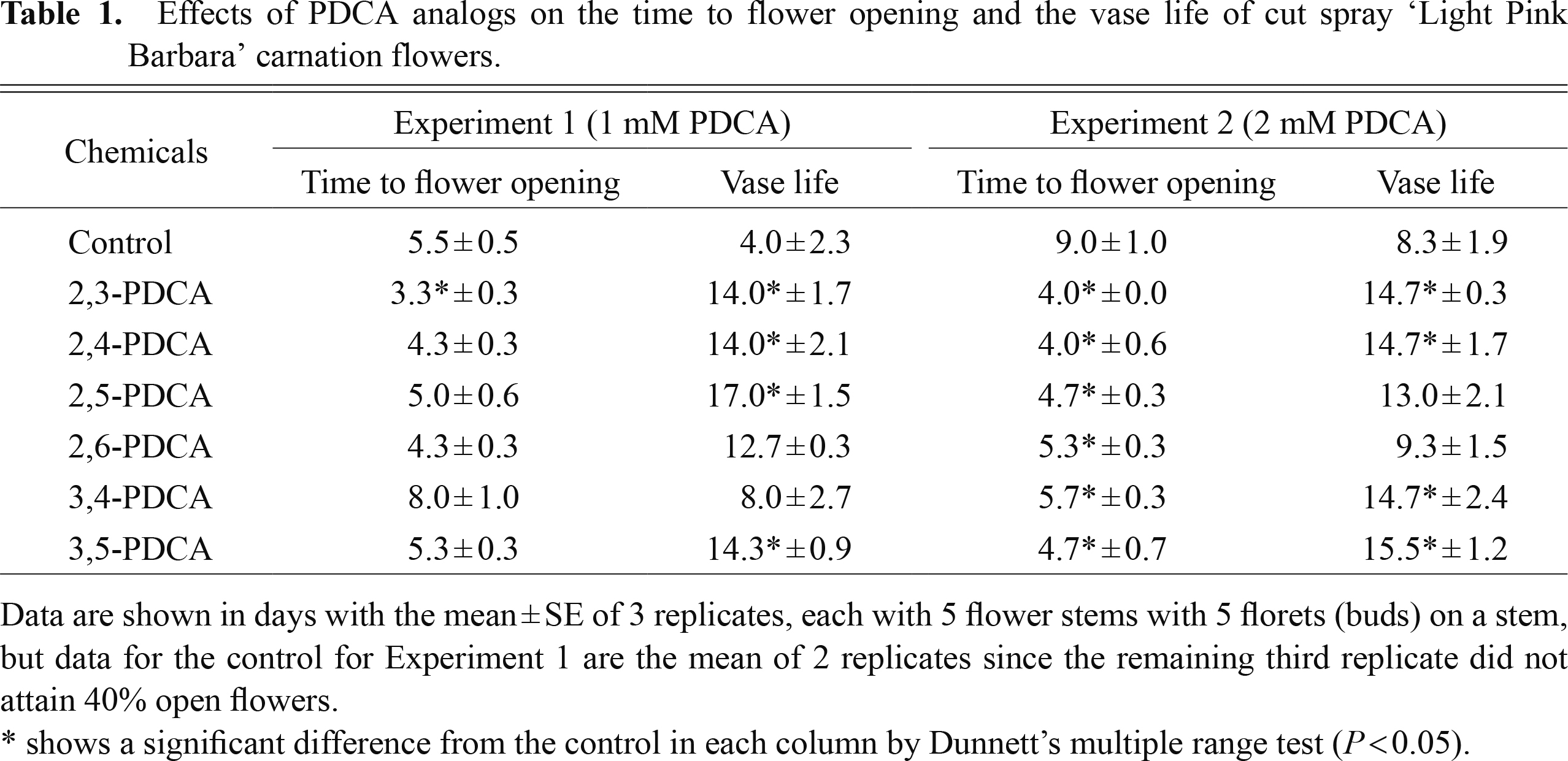
Effects of PDCA analogs on the time to flower opening and the vase life of cut spray ‘Light Pink Barbara’ carnation flowers.
In the experiment with 2 mM PDCA analogs, the time to flower opening was 9.0 days for the control flowers, and treatment with all of the PDCA analogs shortened significantly the time to flower opening. The order among PDCA analogs in terms of their action was similar to that found in the experiment with 1 mM PDCA analogs; 2,3- and 2,4-PDCAs were most effective and resulted in a time to flower opening of 4.0 days.
All of the PDCA analogs, except 2,6-PDCA and 3,4-PDCA, at 1 mM significantly lengthened the vase life over the control (4.0 days); the vase life was longest with 2,5-PDCA (17.0 days), followed by 3,5-PDCA (14.3 days) and 2,3-PDCA and 2,4-PDCA (both, 14.0 days). At 2 mM, four of the PDCA analogs (2,3-, 2,4-, 3,4-, and 3,5-PDCA) significantly lengthened the vase life compared with that of the control (8.3 days); the vase lives varied from 14.7 days to 15.5 days.
We found that the stems of some flowers abruptly broke at the later stage of the display period, even when their flowers (florets) were fully open and non-senescent (Table 2). There were no or few broken stems in the control flowers. The number of broken stems varied from 7.7 (2,4-PDCA) to 14.3 (2,6-PDCA) with 1 mM PDCA analogs, and from 6.3 (3,5-PDCA) to 22.0 (2,5-PDCA). The numbers of broken stems were large with 2,5-PDCA and 2,6-PDCA; 22.0 and 18.3, respectively.
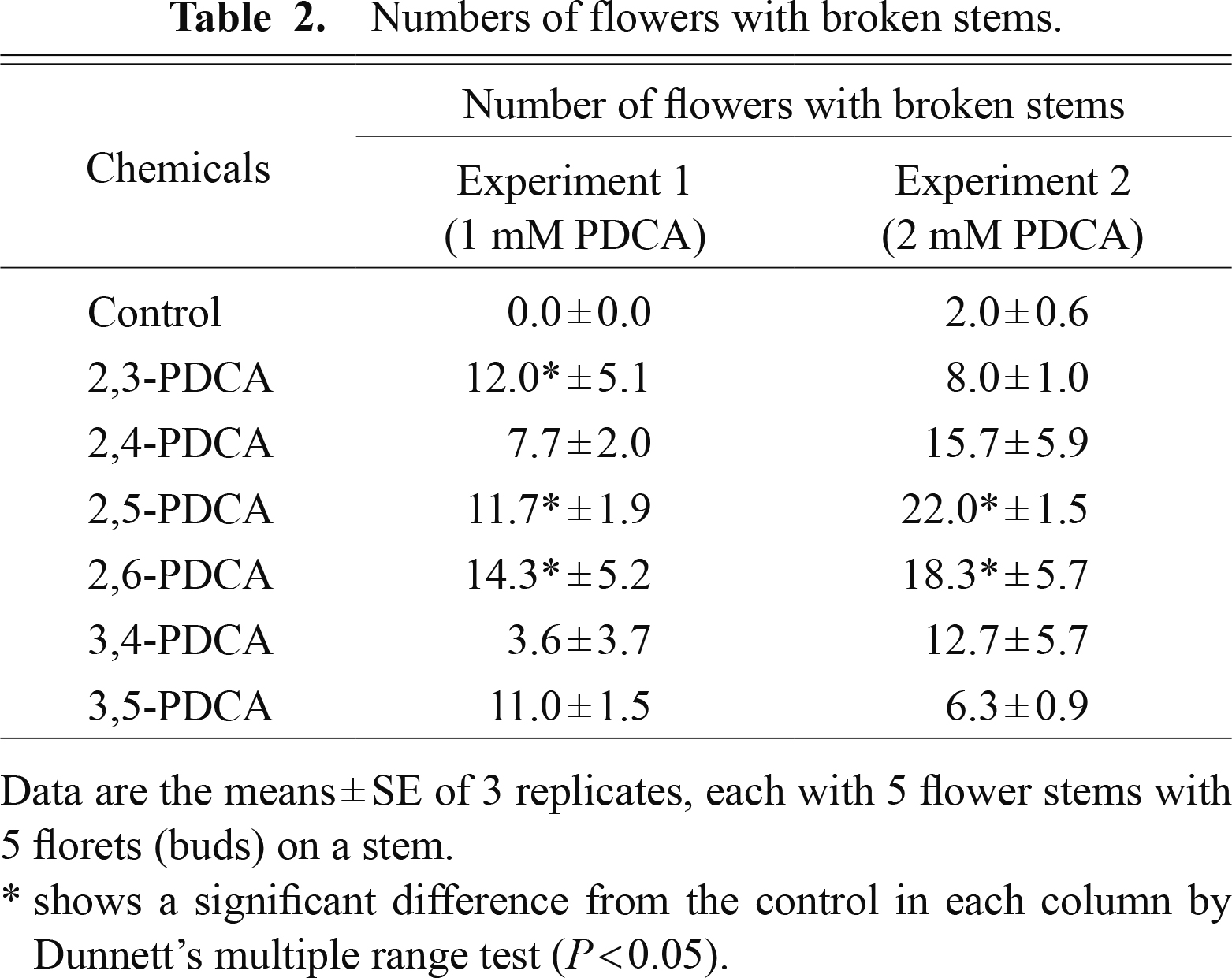
Numbers of flowers with broken stems.
In the present study, the maximum values of the percentage of fully open flowers of the untreated control, which slightly surpassed 40%, were much lower than those obtained previously with ‘LPB’ flowers, for example, 66–88% (Satoh et al., 2014) and 100% (Satoh et al., 2005). The low maximum percentage of open flowers in the control flowers was found in cut spray-type ‘Mule’ carnation flowers, and it was thought likely to be caused by low vigor (Satoh et al., 2014). The low maximum percentage of open flowers in the present study might have resulted from differences in the vigor to open among flower samples, which were cultivated and harvested in different years and seasons, but the precise cause of the difference was not investigated further.
We showed that PDCA analogs, including 2,4-PDCA, extended the vase life of ‘LPB’ flowers. The activity for extending the vase life was markedly high with 2,3-PDCA and 2,4-PDCA among the PDCA analogs (Table 1; Fig. 3). We also demonstrated that PDCA analogs accelerated flower (bud) opening, which was proven by shortening of the time to flower opening (Table 1; Fig. 3). The activity of PDCA analogs in accelerating flower (bud) opening was higher with 2,3-PDCA and 2,4-PDCA than with other PDCA analogs. PDCA analogs caused breaking of the stem of fully open and non-senescent flowers. This deleterious effect was found with all of the PDCA analogs to different degrees, but was markedly strong with 2,5-PDCA and 2,6-PDCA (Table 2). Although PDCA analogs had this detrimental effect of stem breaking, it did not seem to nullify their effects on extending the vase life, since stem breakage usually occurred at the late stage of the flower display period when the extension of the vase life had already been substantially attained.
Satoh et al. (2014) proved that 2,4-PDCA inhibited the in vitro action of ACC oxidase, by acting as a probable analog of ascorbate, a co-substrate of ACC oxidase action. They also postulated that the extension of vase life by 2,4-PDCA treatment in fully open ‘LPB’ carnation flowers resulted mainly from the inhibition of ethylene production in the flowers by the chemical. This notion is probably applicable to PDCA analogs, other than 2,4-PDCA, for their mechanism of action to prolong the vase life of the flowers. It will be necessary in the future to test each chemical in terms of whether it can actually inhibit the action of ACC oxidase.
Saks et al. (1992) reported that paclobutrazol, an inhibitor of gibberellin (GA) biosynthesis, prevented carnation buds from opening fully, suggesting the involvement of GA in the process of flower opening. Satoh et al. (2014) hypothesized on the association of GA with the promoting action of 2,4-PDCA on flower (bud) opening in cut spray-type carnation flowers. This hypothesis arose from the notion that 2,4-PDCA is a structural analog of 2-oxoglutarate (2-OxoGA) and, by competing with 2-OxoGA, 2,4-PDCA probably inhibits 2-OxoGA-dependent dioxygenases (Vlad et al., 2010; Flower Senescence and P4Hs, from HP of Laboratory of Horticultural Genetics and Biotechnology, Mediterranean Agronomic Institute of Chania, Greece, http://www.maich.gr/en/research/horticultural_genetics_and_biotechnology, September 13, 2014), which include those responsible for GA biosynthesis and metabolism, such as GA 3β-dioxygenase (GA 3β-hydroxylase), GA-44 dioxygenase, and GA 2β-dioxygenase (GA 2β-hydroxylase) (Hedden and Kamiya, 1997; Lange et al., 1994a, b; Smith and MacMillan, 1984, 1986).
This hypothesis for the mechanism of action of 2,4-PDCA might be applicable to PDCA analogs other than 2,4-PDCA. However, in the present study, 2,3-PDCA and 3,4-PDCA acted as effectively as 2,4-PDCA in accelerating flower opening and extending the vase life of ‘LPB’ flowers (Table 2; Fig. 4). In a strict sense, 2,3-PDCA and 3,4-PDCA are not structural analogs of 2-OxoGA, which is a linear dicarboxylic acid (Fig. 1). Therefore, the precise mechanism of action of PDCA analogs remains to be elucidated, and such investigation will include determination of the GA content and the expression of genes for GA biosynthesis and action in carnation flowers treated with PDCA analogs.
It is well known that sugars, such as sucrose, glucose, and fructose, promote flower opening and retard senescence in cut flowers of carnations (Borochov and Mayak, 1984; Mayak and Dilley, 1976; Mayak and Kofranek, 1976; Satoh et al., 2005, 2013). The concentration of sugars used for the treatment of flowers is usually from 1 to 10% (w/v), corresponding to about 29 to 290 mM in the case of sucrose. GA3 was reported to promote flower opening and retard senescence in cut flowers of standard-type ‘White Sim’ carnation. The former action was not directly demonstrated but deduced from the findings described above (Saks et al., 1992). PDCAs act at 1 or 2 mM, concentrations that are far lower than those of sugars, in terms of prolonging vase life by accelerating flower opening and retarding senescence in cut flowers of spray-type ‘LPB’ carnations (Table 1; Fig. 2; Satoh et al., 2014). The functional concentrations of PDCAs are far lower than those of sugars, and their use may prevent bacterial and fungal infection at the stem ends of cut flowers in vase solutions, which are observed very often with sugars.
The present study demonstrated that PDCAs, including 2,4-PDCA, have activity to accelerate flower opening, which was suggested in a previous study (Satoh et al., 2014), in addition to retarding senescence, resulting in extension of the vase life in cut flowers of ‘LPB’ carnation. From the present results, we could anticipate that, if cut flowers of spray-type carnation were treated with PDCAs just after harvest of the flowers, the flowers would exhibit accelerated flower opening during shipping and marketing, and arrive at consumers at a more advanced stage of flower opening (fully open stage), and eventually the consumers would enjoy the fully open flowers for a longer period of time than for untreated ones. PDCAs are thought to have merit as flower care chemicals that condition cut flowers for optimal quality. On the basis of the above-described effectiveness for retarding senescence, accelerating flower opening, and a less detrimental effect on stem breaking, we suggest that 2,3-PDCA and 2,4-PDCA will make more suitable agents for the treatment of cut flowers, as shown with ‘LPB’ carnation in the present study. We are planning to examine whether PDCAs are practically applicable to cut flowers of spray-type carnation cultivars other than ‘LPB’ in our next experiment. This issue will also be examined, irrespective of the involvement of ethylene in the process of flower senescence, with other species of ornamentals, of which flowers are used as spray-type flowers, such as Eustoma, Gypsophila, and Alstroemeria flowers and spray-type Chrysanthemum.