2015 年 14 巻 2 号 p. 36-42
2015 年 14 巻 2 号 p. 36-42
After the accident of Fukushima's nuclear power plant, soil contamination was caused by radioactive cesium. In this study, the new cesium-adsorbent: Cs1-xNaxMnII(CN)3 Prussian blue analogue was theoretically designed to solve the problem. In Prussian blue (iron cyano-compound), both water defect and iron vacancy are closely related to cesium-adsorption. On the other hand, in Cs1-xNaxMnII(CN)3, the cesium-adsorption mechanism is completely different. Hybrid Kohn-Sham DFT calculations were performed to investigate the cesium-adsorption mechanism. It was concluded that more stable and efficient cesium-adsorption can be realized by the ion exchange between counter cations (between sodium and cesium ions), under applied voltage.
On 11 March 2011, there was the huge earthquake in Fukushima, Japan. Hydrogen explosion occurred at Fukushima's nuclear power plant, due to a loss of electric source. As the Japanese government reported, radioactive materials such as 134Cs, 137Cs and 131I were emitted into the atmosphere [1]. As the result, soil contamination by radioactive cesium remains as the present unsolved problem [2]. It is because the half-life of radioactive cesium is much longer than radioactive iodine (134Cs: 2 years, 137Cs: 30 years, 131I: 8 days). Previously, the fallout of radioactive cesium occurred at the accident of Chernobyl's nuclear power plant. Soil contamination was also observed in many European countries [3,4,5,6,7,8,9].
Cesium exists as a stable cation in water, due to a large electronic affinity [10]. Geological works suggested that cesium tends to be much adsorbed in soil, in comparison with other alkaline metals such as Na+ and K+ [11,12,13]. It is necessary to collect cesium by the use of adsorbents, after eluting the contaminated soil into water or acid solution such as nitric acid. Prussian blue (iron cyano-compound) has been expected as cesium adsorbent [14,15]. It is because it has been used for medicine to eliminate radioactive cesium from the human body [16].
Very recently, Ishizaki et al. suggested that cesium is adsorbed into iron vacancy, in Prussian blue: FeIII4/3FeII1/3(CN)3·xH2O (x = 10/3–5) [17]. Cyano-ligand vacancy is accompanied by iron vacancy, due to charge compensation. It is considered that cesium can be adsorbed into iron vacancy, and some water defects are coordinate to iron. However, as positively charged cesium cannot migrate into the inside of the cube, this cesium-adsorption is limited on the surface.
The nano size-crystallizing of Prussian blue is applicable to enhance the cesium-adsorptivity, as the larger surface area can be given. In fact, Kawamoto et al. demonstrated the higher cesium-adsorptivity in nano size Prussian blue [18]. The existence of many iron vacancies on the surface theoretically enhances the cesium-adsorptivity. However, as the vicinity of iron vacancy shrinks or is reoriented, the cubic structure cannot be kept there. In addition, as water defect coordinate to iron can migrate easily, it is considered that it prevents the cesium-adsorption. Hence, the more stable and efficient cesium-adsorption cannot be expected in Prussian blue.
The purpose of this study is to propose the more stable and efficient cesium-adsorbent, in comparison with Prussian blue. Here, the new Prussian blue analogue: Cs1-xNaxMnII(CN)3 is considered as the best cesium-adsorbent. The more stable and efficient cesium-adsorption mechanism can be expected, because of the stable crystal structure without manganese and cyano-ligand vacancies and ion-conduction between cesium and sodium ions under applied voltage.
The calculations presented here are performed using the BHHLYP hybrid Kohn–Sham density functional theory (DFT) method [19], which properly reproduces electronic structures of transition metal compounds [20]. In BHHLYP theory, the total exchange and correlation energies are expressed by 50% HF exchange, 50% Becke exchange and LYP correlation energies. The Tatewaki-Huzinaga MINI [21] basis is used for manganese, sodium and cesium, combined with the 6-31G (d) basis for carbon and nitrogen. All calculations are performed with the GAMESS program [22]. The molecular orbitals (MOs) are plotted using MOLEKEL 4.3 [23].
2.2 Computational modelFigure 1 depicts the cubic crystal structure of CsMnII(CN)3 at room temperature. Cesium exists at the cubic centre, and there exists no water defect and manganese vacancy. Note that CsMnII(CN)3 is often expressed as Cs2MnII[MnII(CN)6]. One hexa-coordinate MnII is surrounded by six carbon atoms, and the other one is surrounded by six nitrogen atoms. Miller et al. reported that the MnII spin configuration is t2 g3eg2, and Mn-C, Mn-N and C–N distances are 1.93Å, 2.19Å and 1.18Å, respectively [24]. When sodium ions are doped at some cesium sites, Cs1-xNaxMnII(CN)3 can keep a cubic structure. As shown in Figure 2, CsMnII8(CN)12 and NaMnII8(CN)12 models were constructed, to examine the ion exchange between cesium and sodium ions.

The cubic crystal structure of CsMnII(CN)3 at room temperature. The red, green, blue and yellow dots denote manganese, carbon, nitrogen and cesium, respectively.
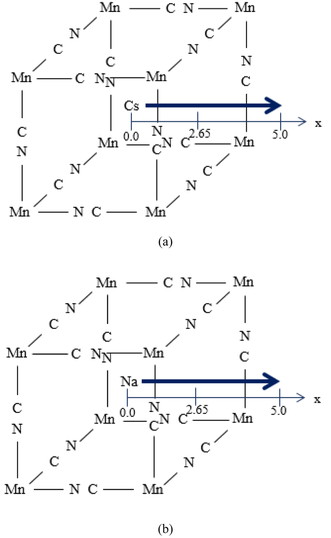
The cluster models for Cs1-xNaxMnII(CN)3:(a) CsMnII8(CN)12, (b) NaMnII8(CN)12 models. Cesium or sodium ion migrates along x axis, following an arrow.
Molecular orbital (MO) analysis is very useful to examine chemical bonding formation. Onishi chemical bonding rule [20] can be applicable to judge chemical bonding character (covalency or ionicity) related to conductive ion (See Figure 3).For example, in Cs1-xNaxMnII(CN)3, the chemical bonding character on cesium (sodium) can be judged, following the next scheme.

The schematic picture on Onishi chemical bonding rule.
1. In MOs including outer shell electrons of cesium (sodium), check whether the orbital overlap between cesium (sodium) and others exists or not.
2. With orbital overlap, the bonding character of cesium (sodium) is covalent. Without orbital overlap, it is ionic.
In 4.2, chemical bonding analysis related to cesium and sodi um ions in Cs1-xNaxMnII(CN)3 is illustrated.
In this study, Cs1-xNaxMnII(CN)3 Purssian blue analogue, is considered as candidate material [25]. Note that the cesium-adsorption mechanism is completely different. (See Figure 4) It is because counter cation such as sodium ion at the cubic centre has an important role in the cesium-adsorption. Water and manganese defects can be eliminated, when the formal charge of all manganeses is +2 [24,25,26,27]. Hence, it can be expected that sodium ion is exchanged for cesium ion, through sodium ion-conduction under applied voltage. Hybrid Kohn-Sham DFT calculations were performed to investigate the cesium-adsorption mechanism in Cs1-xNaxMnII(CN)3, from the viewpoint of ion-conduction.

The cesium-adsorption mechanism.
Figures 5 (a) and (b) show the potential energy curves for CsMnII8(CN)12 and NaMnII8(CN)12 models, displacing cesium and sodium ions along x axis (x = 0.0 ~ 2.65Å), respectively.

The potential energy curves for (a) CsMnII8(CN)12 and (b) NaMnII8(CN)12 models, displacing cesium and sodium ions along x axis (x = 0.0 ~ 2.65Å) in CsMnII8(CN)12 and NaMnII8(CN)12 models, respectively.
In the CsMnII8(CN)12 model, the minimum total energy was given at the centre. The activation energy for cesium ion-conduction, which can be estimated from the total energy difference between the cubic centre (x = 0.0Å) and bottleneck (x = 2.65Å), was 4.14 eV. It is concluded that cesium cannot migrate from the cubic centre. It means that cesium ion is kept fixed in the cube. It is due to the steric repulsion between cesium ion and others. The ionic radius of cesium is 1.81Å [28].
On the other hand, in NaMnII8(CN)12 model, the minimum total energy was given at 1.8Å, and the maximum total energy was given at the cubic centre. The activation energy for sodium ion-conduction, which can be estimated from the total energy difference between the minimum and cubic centre, was 0.19 eV. It is much smaller than our designed sodium ion conductor such as NaxAl1-x/3(CN)3 (0.71 eV) [29]. It is concluded that sodium ion-conduction easily occurs. It is because the steric repulsion between sodium ion and others becomes smaller, due to the smaller sodium ionic radius (1.16Å) [28].
4.2 Chemical bonding formations related to cesium and sodium ionsFigure 6 depicts the selected MOs related to the outer shell of sodium ion (2s and 2p orbitals) for NaMnII8(CN)12 model. There is no orbital overlap between sodium 2s and 2p orbitals, and others. From Onishi chemical bonding rule, it is found that sodium ion forms ionic bonding with cyano-ligand, during sodium ion-conduction.

The molecular orbitals (MOs) related to the outer shell of sodium ion (2s and 2p orbitals) for NaMnII8(CN)12 model: (a) the cubic centre (x = 0.0Å), (b) minimum (x = 1.8Å), and (c) bottleneck (x = 2.65Å).
Figure 7 depicts the selected MOs related to the outer shell of cesium ion (5s and 5p orbitals) for CsMnII8(CN)12 model. At the centre, there are slight orbital overlaps between cesium 5p orbital and cyano-ligand in MO139α, MO139β, MO140α and MO140β. From Onishi chemical bonding rule, it is found that cesium ion forms a weak covalent bonding with cyano-ligand at the centre. On the other hand, at the bottleneck, there also are orbital overlaps between cesium 5s orbital and cyano-ligand in MO120α and MO120β. From Onishi chemical bonding rule, it is found that cesium ion forms covalent bonding with cyano-ligand at the bottleneck. However, higher total energy was given at the bottleneck. It is because the effect of steric repulsion is more dominant than the stabilization coming from covalent bonding formation.

The selected molecular orbitals (MOs) related to the outer shell of cesium ion (5s and 5p orbitals) for CsMnII8(CN)12 model: (a) the cubic centre (x = 0.0Å), and (b) bottleneck(x = 2.65Å).
Let us consider the cesium-adsorption mechanism under applied voltage, in Cs1-xNaxMnII(CN)3. Figure 8 shows the schematic picture on the ion exchange between cesium and sodium ions in Cs1-xNaxMnII(CN)3, under applied voltage. Sodium ion easily migrates into cesium-contaminated water, due to the small activation energy for sodium ion-conduction. When there exists anions, which causes precipitation with sodium ion, in contaminated water, cesium ion is coercively adsorbed into the cubic centre, due to charge compensation. Once cesium ion is captured into the cube, it is kept fixed, due to the large stabilization.

The schematic picture on the ion exchange between cesium and sodium ions in Cs1-xNaxMnII(CN)3, under applied voltage.
Note that proton-migration from contaminated water into the cube is impossible. It is because the covalent bonding formation between proton and cyano-ligand is required, to stabilize the proton [30]. However, proton cannot form the covalent bonding, due to the existence of the stable covalent bonding between manganese and cyano-ligand.
Hybrid Kohn-Sham DFT calculations were performed to investigate the cesium and sodium ion-conduction mechanism for Cs1-xNaxMnII(CN)3. The activation energies for cesium and sodium ion-conductions were 4.14 eV and 0.19 eV, respectively. Sodium ion forms ionic bonding during sodium ion-conduction. It was concluded that sodium ion-conduction easily occurs, under applied voltage. On the other hand, cesium ion is kept fixed in the cubic centre.
5.2 Ion exchange between cesium andsodium ionsA new coercive cesium-adsorption mechanism was proposed in Cs1-xNaxMnII(CN)3. In Prussian blue, cesium is weakly adsorbed to iron vacancy. It is due to the existence of water defect and because the spontaneous cesium-adsorption is limited to the part of the surface. On the other hand, in our theoretically proposed Cs1-xNaxMnII(CN)3, cesium can be adsorbed, through ion exchange between cesium and sodium ions, under applied voltage.
The more stable and efficient cesium-adsorption can be realized in Cs1-xNaxMnII(CN)3. It is because the use of voltage forces that all sodium ions neighbouring the surface migrates into the contaminated water, and then cesium is coercively adsorbed into the cubic centre, due to charge compensation. Combined with the higher percentage of sodium ion (x) inCs1-xNaxMnII(CN)3 and nano size-crystallizing, the more efficient cesium-adsorption can be realized. It is due to the increase of the surface.
5.3 FutureThe synthesis of the perfect Cs1-xNaxMnII(CN)3 without water defect and manganese vacancy is much expected. A technique how to apply voltage must be also developed. In addition, it is concluded that Cs1-xNaxMnII(CN)3 can be also applicable as sodium ion-conductor in secondary batteries, due to the sufficiently small activation energy.
This work was partially supported by SUZUKI FOUNDATION and Research Foundation for the Electrotechnology of Chubu.