2014 Volume 56 Issue 4 Pages 252-259
2014 Volume 56 Issue 4 Pages 252-259
Objective: We evaluated the percutaneous absorption of N,N-dimethylformamide (DMF) in DMF-exposed workers in the summer and winter by assessing their urinary levels of DMF metabolites. Methods: Breathing-zone concentrations of DMF and workers' urinary levels of N-methylformamide (NMF) and N-acetyl-S-(N-methylcarbamoyl)-cysteine (AMCC) were simultaneously measured in the summer and winter in 193 male workers wearing a respirator and chemical protective gloves. Results: The mean breathing-zone concentrations of DMF in both seasons were below the occupational exposure limit of 10 ppm. Although there was no significant seasonal difference in the breathing-zone concentrations of DMF, workers' urinary levels of NMF and AMCC were significantly higher in the summer than in the winter. Log-transformed urinary levels of the metabolites were significantly correlated with log-transformed breathing-zone concentrations of DMF in the summer, whereas no significant correlation between AMCC and DMF was found in the winter. The urinary levels of AMCC were dispersed more widely than those of NMF, suggesting that urinary AMCC reflected the cumulative exposure to DMF over a workweek. Conclusions: Percutaneous absorption was the principal route of exposure to DMF for the respirator-wearing workers. Increased urinary levels of NMF and AMCC in the summer were attributed to increased percutaneous absorption of DMF resulting from the increased amount of water-soluble DMF absorbed by sweaty skin caused by the increased summertime room temperature and humidity.
(J Occup Health 2014; 56: 252-259)
N,N-Dimethylformamide (DMF) has been widely used as an organic solvent for synthetic textiles and leathers and polar polymers such as polyurethane and polyacrylonitrile1). Japan's production of DMF in 2011 was reported to amount to 50,000 tons2). Occupational exposure to DMF has been reported to cause hepatic damage3–6), pancreatic disorders7), and intolerance to alcohol8, 9). For the protection of workers from excessive exposure to DMF, an occupational exposure limit (OEL) of 10 ppm for DMF has been recommended by the American Conference of Governmental Industrial Hygienists (ACGIH)10) and the Japan Society for Occupational Health (JSOH)11). The Deutsche Forschungsgemeinschaft (DFG) has recommended a MAK value (“maximale Arbeitsplatz-Konzentration”: maximum workplace concentration) of 5 ppm for DMF12). The recommended OEL for DMF was designated with the “skin” notation10–12), since DMF is highly permeable through the skin. The ACGIH13) and DFG14) recommended biological exposure indices (BEIs) of 15 and 35 mg N-methylformamide (NMF)/l in workers' urine collected at the end of a shift, respectively. The ACGIH13) also recommended a BEI of 40 mg N-acetyl-S-(N-methylcarbamoyl)-cysteine (AMCC)/l in urine collected at the beginning of the last shift of a workweek. AMCC is the final product of the conjugation reaction between a metabolic intermediate of DMF and glutathione. Urinary AMCC seems to be more suitable for assessment of cumulative whole-body exposure to DMF over a workweek15, 16) than NMF, since the elimination half-life of AMCC was reported to be 22.1 hours17) or 23 hours18) in comparison with the short elimination half-lives of 4.75 hours and 2.42 hours for NMF through the lung and skin, respectively19).
The present study was undertaken to explore a possible seasonal difference in percutaneous absorption of DMF due to altered climatic conditions for workers handling DMF-containing products in a resin-product manufacturing factory. All of the workers were required to wear respirators and protective gloves for their entire 8-hour work shifts, and we measured their personal exposure concentrations of DMF and its urinary metabolites simultaneously in both the summer and winter. We evaluated the contribution of percutaneous absorption to the total body burden of DMF to which the workers were exposed through the lung and skin by simultaneously measuring the workers' personal exposure concentrations of DMF and their urinary levels of NMF and AMCC. In this report, we also discuss causative factors for the increased urinary levels of DMF metabolites in the summer and some other problems associated with biological monitoring for assessments of the total exposure to DMF.
The present survey was conducted in a resin-products manufacturing factory twice a year during the 10 years from March 2001 to August 2010 in compliance with the Ordinance on Prevention of Organic Solvent Poisoning20). The subjects were a total of 193 male workers aged 36.7 ± 11.9 (mean ± SD) years with work durations of 10.3 ± 6.2 (mean ± SD) years at the participations. All but two of the workers participated twice or more in simultaneous measurements of breathing-zone concentrations of DMF and urinary levels of NMF and AMCC. The subjects were asked not to drink alcohol one day before urine sampling. The work-environment temperature measured during work shifts was 34.1 ± 2.6°C (mean ± SD), ranging from 30.1°C (min.) to 37.0°C (max.), in the summer and 17.1 ± 4.8°C, ranging from 13.0 to 18.6°C, in the winter. The relative humidity was 51.6 ± 11.6% (mean ± SD), ranging from 40.6 to 76.0%, in the summer and 31.5 ± 17.7%, ranging from 16.0 to 68.4%, in the winter.
The workers were engaged in three different processes, including exchange of containers for the resin-dissolved material, mixing and molding of viscous DMF-containing resins and handling (moisturizing, heating and drying) of half-finished products. A local exhaust ventilation system was installed in the factory, and its exhaust hoods were set up upstream in each process.
The present study was approved by the Ethics Review Committee of the Shinshu University School of Medicine. All the subjects participating in the present survey gave us written informed consent, and the results regarding the exposure concentrations of DMF, urinary levels of NMF and AMCC and mask fitness were reported to all the subjects.
Use of personal protective equipmentAll of the workers assigned to these three processes were required to wear an air-purifying elastomeric half-mask equipped with a cartridge containing activated charcoal for collection of organic vapor (Sky Mask GH714, Sanko Chemical Ind. Co., Kanagawa, Japan) as well as chemical protective gloves. The respirator and chemical protective gloves were worn during the workers' entire 8-hour work shifts. The workers were also required to take a training course once a year regarding how to correctly fit and wear the respirator and how to evaluate respirator leaks using a mask fitting tester of the ROKEN Type (MT-03, Sibata Scientific Technology Ltd., Tokyo). A respirator's ratio of leakage through the gap between the facepiece and the worker's face, resulting from interference with the face seal, was measured once a year during the months June through August. The leak ratio was defined as Ci/Co × 100, where Ci and Co represent the photometric concentration of ambient particulate matter measured inside the respirator face-piece and outside the respirator, respectively.
When the leak ratio for DMF vapor was evaluated, the cartridge was replaced with the personal protective equipment (PPE) manufacturer-made particulate filter, which was devised to equalize the negative pressure inside the respirator equipped with the filter to that of a respirator equipped with the cartridge for organic vapor. This filter effectively prevented ambient particulate matter from penetrating through the cartridge into the respirator. Instruction regarding both the mask's fit and the leak test for the workers was performed once a year by personnel responsible for the PPEs with the help of an experienced advisor from a PPE manufacturer. It was confirmed that the mask-wearing workers changed the cartridge regularly at an interval of one week on the basis of the breakthrough curve of cyclohexane attached on the cartridge package. Change out of used respirators and chemical protective gloves was managed by the PPE personnel. All of the workers wore short-sleeved work shirts and trousers in the summer and long-sleeved work shirts with trousers in the winter, but they did not wear any chemical protective clothing.
Personal sampling of DMF in the workplace airDMF vapor was collected in the workers' breathing-zones over their entire 8-hour work shift using a diffusive passive sampler (LiPS) containing distilled water as an absorbent developed by Tanaka et al.21). The personal sampler was worn by individual workers on the collar of their work shirts. After personal sampling, the absorbent was transferred to a glass vial and stored at 4°C until analysis.
Urine samplingThe workers' urine samples were collected for the AMCC and NMF analyses at the beginning and at the end of the last shift of the workweek, respectively, on the same day as collection of breathing-zone air for the DMF analysis. The urine of each worker was collected in a plastic bottle and stored at −20°C until analysis.
Analyses of DMF and urinary NMF and AMCCAn aliquot (1 µl) of the distilled-water absorbent was analyzed for DMF using a combined gas chromatograph-mass spectrometer (GC-MS) equipped with an HP-WAX capillary column (30-minute long, 1-µm film-thick, cross-linked polyethylene, Hewlett Packard Co., Palo Alto, CA, USA). An automatic liquid sample injector (7683 Series Injector, Agilent Technol., Santa Clara, CA) was connected to the GC-MS. The injection port and detector were maintained at 250°C, and the column temperature was programmed to increase from 60 to 160°C at a rate of 10°C/min and from 160 to 220°C at 40°C/min. DMF in the water absorbent was determined by monitoring selected ion fragments at m/z 44 and 73. The quantitative detection limit for DMF was 0.1 ppm (v/v) for a sampling time period of 480 minutes.
Urinary NMF was analyzed according to the methods of Kawai et al.22) and Nomiyama et al23). Briefly, a 0.5-ml sample of urine was added with diethylformamide (4.5 mg/l) as an internal standard, diluted with 2 ml of ethanol and then poured with anhydrous potassium carbonate (1 g) for dehydration. After centrifuging at 3,000 rpm for 10 minutes, an aliquot (1 µl) of the supernatant was analyzed with the GC-MS equipped with an HP-Ultra 2 capillary column (25-minute long, 0.11-µm film-thick). The GC injection port was kept at 250°C, because N-hydroxymethyl-N-methylformamide (HMMF) formed in vivo was heat decomposed by 100% to NMF at 250°C22). The column temperature was programmed to increase from 40 to 140°C at a rate of 10°C/min and from 140 to 220°C at 40°C/min. The detector temperature was maintained at 250°C. NMF levels were determined by monitoring the abundance of selected ion fragments at m/z 30 and 59. Diethylformamide was monitored at 101 m/z. The quantitative detection limit for urinary NMF was 0.1 mg/l.
Urinary AMCC was also analyzed by the method of Mraz24). Briefly, a 1.0-ml sample of urine was diluted with 3 ml of distilled water and then mixed with 1.5 × 10−4 mol quinoline/l as an internal standard in ethanol (2 ml) in a 10-ml centrifuge tube fitted with a glass stopper. Powdered anhydrous potassium carbonate (1.5 g) was added. The mixture was shaken for 2 minutes and centrifuged at 3,000 rpm for 5 min. An aliquot (1 µl) of the solvent layer was analyzed for AMCC with the GC-MS instrument equipped with the same capillary column as that used for NMF. The injection port and detector were maintained at 250°C, and the column temperature was programmed to increase from 40 to 150°C at a rate of 10°C/min and from 150 to 220°C at 40°C/min. The GC peak of AMCC was monitored and quantified as ethyl-N-methylcarbamate at m/z 103. Quinoline was monitored at m/z 129. The quantitative detection limit for urinary AMCC was 0.1 mg/l.
ChemicalsDMF and NMF were purchased from Tokyo Kasei Kogyo (Tokyo, Japan). Ethanol, diethylformamide, quinoline and anhydrous potassium carbonate were obtained from Wako Pure Chemical Industries (Osaka, Japan). AMCC was synthesized by the method described by Mraz and Turecek25).
Data analysisThe observed datasets of both the breathing-zone concentrations of DMF and the workers' urinary levels of NMF and AMCC were examined for logarithmic normal distribution by the Shapiro-Wilk test. Seven values including three for the DMF concentration, two for the NMF level and two for the AMCC level were found to deviate by at least 1.5 times the quartile geometric standard deviation. After excluding the five values from the DMF and NMF assessments, we logarithmically transformed the breathing-zone concentrations of DMF and the urinary levels of NMF and AMCC for further analysis.
The potential seasonal differences in the breathing-zone concentrations of DMF and the urinary levels of NMF and AMCC were tested for statistical significance by the Wilcoxon signed-rank test. We examined the correlations between the log-transformed breathing-zone concentrations of DMF and the log-transformed urinary levels of NMF and AMCC by linear regression using the least-square method, and we tested the correlations for statistical significance by Pearson's product-moment coefficient. A difference in the slope of the regression line for NMF between summer and winter was tested by analysis of variance. We examined the relationship between the urinary levels of AMCC and the breathing-zone concentrations of DMF in each season according to a quartile analysis. Significant differences in the mean urinary levels of AMCC among the four quartile groups were tested by Dunnett's multiple comparison test. The trend of AMCC levels to increase with the breathing-zone concentrations of DMF was examined with the Jonckheere-Terpstra test. All reported p-values were one-tailed (p<0.05). All of the statistical analyses were performed using the SAS 9.2 software (SAS Institute Inc., Cary, NC, USA).
Table 1 shows the seasonal differences in the breathing-zone concentrations of DMF and the workers' urinary levels of NMF and AMCC. No significant difference in breathing-zone concentrations of DMF was found between summer and winter, and the mean breathing-zone concentrations of DMF in both seasons were below the recommended OEL value of 10 ppm. However, the urinary levels of NMF and AMCC were significantly higher in the summer than in the winter. The mean urinary level of NMF in the summer exceeded the recommended BEI value of 15 mg/l, but the mean level of NMF in the winter was below the recommended BEI. The mean urinary levels of AMCC in both seasons were below the ACGIH's BEI value of 40 mg/l. The summer/winter ratio of the AMCC levels was smaller than that of the NMF levels. Notably, the geometric standard deviation (GSD) of AMCC was greater than that of NMF, indicating that the workers' urinary levels of AMCC were dispersed more widely than those of NMF.
| Number of workers examined | GM ± GSD | GM ± GSD | Ratio | |
|---|---|---|---|---|
| Summer | Winter | Summer/Winter | ||
| 100 | 88 | |||
| DMF (ppm) | 7.4 ± 2.2 | 6.2 ± 2.1 | 1.2 | |
| NMF (mg/l) | 33.9 ± 2.2* | 12.9 ± 2.0 | 2.6 | ES |
| AMCC (mg/l) | 23.3 ± 3.2* | 14.3 ± 3.0 | 1.6 | BS |
GM ± GSD represents geometric mean plus or minus the geometric standard deviation. Urine was collected at the end (ES) and at the beginning (BS) of the last shift of the workweek. All the workers wore a respirator and a personal sampler on the collar of workdress during the entire 8-hour work shift.
The log-transformed urinary levels of NMF were found to be significantly correlated with the log-transformed breathing-zone concentrations in both seasons (Fig. 1). The slope of the linear regression in the summer appeared to be steeper than that in the winter, but the seasonal difference in the slope was not significant. We calculated the urinary levels of NMF corresponding to the breathing-zone concentration of 10 ppm DMF on the basis of the two regression lines (Fig. 1), and the geometric means (GM) ± GSD of the NMF levels were 31.7 ± 3.3 mg/l in the summer and 23.1 ± 3.5 mg/l in the winter.

The relationship between the log-transformed NMF levels in the workers' urine at the end of the last shift and the log-transformed DMF concentrations during the 8-h work shift. The solid line with circles indicates the linear regression in the summer, while the dotted line with crosses represents the linear regression in the winter.
Among a total of 100 workers examined in the summer, there were 56 workers (56%) whose urinary NMF levels exceeded the BEI value of 15 mg/l, although their breathing-zone concentrations of DMF were below the OEL value of 10 ppm. On the other hand, among a total of 88 workers examined in the winter, there were 28 workers (32%) whose NMF levels exceeded the BEI value, although their breathing-zone concentrations of DMF were below the OEL value.
The log-transformed urinary levels of AMCC were found to be significantly correlated with the log-transformed breathing-zone concentrations of DMF in the summer not with those in the winter (Fig. 2). There was no significant difference in the slopes of the linear regressions in summer and winter. The quar-tile analysis (Fig. 3) revealed that the urinary AMCC tended to positively increase with the breathing-zone concentrations of DMF in the summer, as evidenced by a significant positive trend according to the Jonckheere-Terpstra test (p<0.01). The urinary level of AMCC was significantly higher in the fourth quartile group than in the first quartile group by Dunnett's multiple comparison test. In the winter, however, neither a significant positive trend of the AMCC levels to increase with the breathing-zone concentrations of DMF nor a statistically significant difference in the urinary AMCC level among the four quartile groups was found.
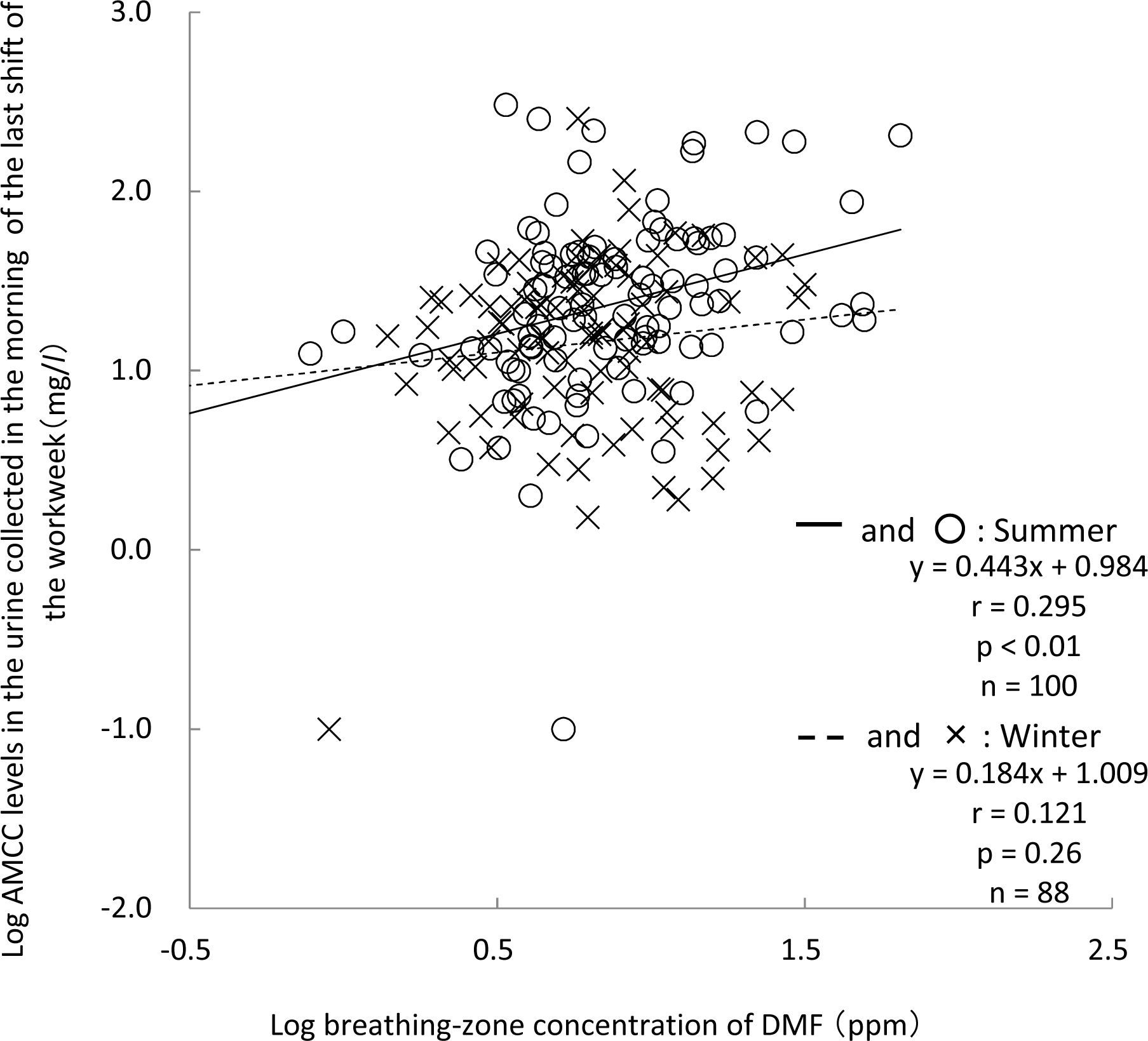
The relationship between the log-transformed AMCC levels in the workers' urine at the beginning of the last shift and the log-transformed DMF concentrations during the 8-hour work shift. The solid line with circles indicates the linear regression in the summer, while the dotted line with crosses represents the linear regression in the winter.

Quartile analysis showing the dependence of the log-transformed AMCC levels in the workers' urine at the beginning of the last shift during the summer and winter on the DMF concentrations during the 8-hour work shift. The 1st, 2nd, 3rd and 4th quartiles span 0.1 to 4.0 ppm, 4.1 to 6.1 ppm, 6.2 to 10.7 ppm and 10.8 to 64.8 ppm, respectively. Horizontal bars with numerical values indicate the means and their values.
As shown in Table 1, the GM ± GSD values of the AMCC levels were 23.3 ± 3.2 mg/l in the summer and 14.3 ± 3.0 mg/l in the winter. Among a total of 100 workers examined in the summer, there were 16 workers (16%) whose urinary AMCC levels exceeded the BEI value of 40 mg/l, although their breathing-zone concentrations of DMF were below the OEL value of 10 ppm. On the other hand, among a total of 88 workers examined in the winter, there were 8 workers (9%) whose AMCC levels exceeded the BEI value, although their breathing-zone concentrations of DMF were below the OEL value.
Among a total of 110 workers who took an annual training course about how to correctly fit the respirator and how to evaluate respirator leaks with the mask fitting tester, the mean ± SD values for the leak ratios were 2.8 ± 2.6%, with the ratios ranging from 0.01 to 10.1%. Twenty-one workers (19%) showed leak ratios ranging from 5.1 to 10.1%, and there were no workers with a leak ratio exceeding 10.1%. We attempted to quantitatively estimate the concentrations of personal exposure to DMF passing through the gap between the facepiece and the worker's face (Cresp), using both the breathing-zone concentration of DMF (Cbz,i) and the leak ratio (LKi [%]) for each worker as follows:
Cresp,i was averaged over the number of workers participating in the mask fitting test, and the mean ± SD values of Cresp,i were 0.3 ± 0.5 ppm in the summer and 0.2 ± 0.3 ppm in the winter. There was no significant seasonal difference in the concentration of respiratory exposure to DMF passing through the gap between the facepiece and the worker's face.
The resin-products manufacturing factory surveyed in the present study was observed to provide almost appropriate management of workplace air concentrations of DMF and to ensure correct respirator use, since the mean breathing-zone concentrations of DMF in both the summer and winter were below the OEL of 10 ppm and since the workers correctly wore their respirators in compliance with proper instruction and management by an experienced advisor from the PPE manufacturer.
We found that the mean leak ratio of the respirator through the facepiece gap was 2.8%, which was lower than the leak ratio of 10%, which corresponds to the US. National Institute for Occupational Safety and Health (NIOSH) Assigned Protection Factor (APF) of 10 for the same type of half-mask26). The APF value is known to be inversely related to the leak ratio. In the present study, we estimated that the mean concentration of respiratory exposure to DMF passing through the facepiece gap was 0.3 ppm in the summer and 0.2 ppm in the winter in comparison with the corresponding breathing-zone concentrations of DMF (7.4 and 6.2 ppm), respectively. Our comparison of these two sets of DMF concentrations suggests that the total body burden of DMF for the respirator-wearing workers resulted predominantly from the percutaneous absorption of DMF vapor on the basis of the finding of Nomiyama et al.19) that the averaged DMF absorptions through the skin and by respiration were 40.4 and 59.6% of the total body burden of DMF, respectively.
We also found that the worker's urinary levels of NMF and AMCC were significantly higher in the summer than in the winter, although there was no significant seasonal difference in the breathing-zone concentrations of DMF. The seasonal difference in the urinary levels of NMF and AMCC is thought to result from increased percutaneous absorption of DMF due to the increased ambient temperature and humidity in the summer. Mraz and Nohova27) demonstrated with human volunteers that the percutaneous absorption of DMF was enhanced by an increase in room temperature and relative humidity, in addition to a decisive role of moisture on the skin. Percutaneous absorption of DMF might also be influenced by skin morphological structures such as the stratum corneum and sweat ducts, barrier-functioning physicochemical properties of the horny layer such as diffusivity28) and microcirculation and molecular properties of DMF such as water-solubility. We thus infer that the increased percutaneous absorption of DMF in the summer observed in the present study is caused by an increased amount of water-soluble DMF absorbed by the sweaty skin, leading to the increased penetration of DMF molecules through the skin into the increased peripheral blood flow under the skin.
We found significant correlations between the log-transformed urinary levels of NMF and the log-transformed breathing-zone concentrations of DMF in both the summer and winter, although a significant correlation between AMCC and DMF was found only in the summer. These results are consistent with those of Sakai et al.29), who observed a lesser correlation between the urinary levels of AMCC and personal exposure concentrations of DMF compared with that of NMF, but our findings are in contrast to the results reported by Kim et al.30), who found no significant relationship between the urinary levels of AMCC and DMF concentrations.
The lesser correlation of the urinary AMCC levels with the single-day breathing-zone concentrations of DMF might be attributed to the long elimination half-life of AMCC (22–23 hours)17, 18), compared with that of NMF (2.4–4.8 hours)19). In the present study, the AMCC levels in the urine collected at the beginning of the last shift (Friday) of the workweek could have been more closely correlated with the mean exposure concentrations of DMF averaged over the four consecutive days (Monday through Thursday) prior to urine sampling in comparison with the single-day concentration of DMF collected during the last 8-hours shift of the workweek (Friday). Thus, we suspect that the lesser correlation of the urinary AMCC levels with the breathing-zone concentration of DMF reflects a large day-to-day variation of the breathing-zone concentrations of DMF. According to the quartile analysis, the workers in the 4th quartile group might usually be exposed to high concentrations of DMF and absorb it percutaneously. This might have caused the significant positive trend and the significant increase in the urinary AMCC levels in the 4th quartile group only in the summer.
The widely disperse urinary levels of AMCC can also be seen from the present finding that the GSDs were larger for AMCC than for NMF in both seasons (Table 1). In this context, a large variation of the urinary levels of AMCC might also result from a large individual difference in the metabolic capacity of the pathway from DMF to AMCC as the induction of P-450 by alcohol consumption. It was previously reported that chronic consumption of alcohol induces cytochrome P-450, leading to enhanced xenobiotic metabolism31, 32). Kim et al.30) reported that the more alcohol consumed on the day before urine sampling, the higher the urinary levels of NMF. Wrbitzky9) pointed out that there seems to be a large range of susceptibility to DMF metabolism in humans and that alcohol consumption influences DMF metabolism.
Nevertheless, AMCC is considered to be an excellent indicator for the monitoring of not only workers' cumulative exposure to DMF but also the health effects of DMF exposure, including liver damage. The formation of AMCC is reported to link with a putative intermediate of methyl isocyanate, a potent hepatotoxicant, which would be carbamoylated with glutathione to S-(N-methylcarbamoyl)glutathione (SMG) and finally to AMCC15, 33–35). He et al.4) reported that the urinary levels of AMCC were positively correlated with serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and γ-glutamyl transpeptidase (γ-GT) among workers exposed to DMF at middle (9.04–19.49 mg/m3) and high (36.61–37.11 mg/m3) levels. However, Sakai et al.29) reported that no apparent correlation between urinary levels of NMF and AMCC and AST or ALT was recognized among workers exposed to DMF at levels below the OEL of 10 ppm. Since the relationship between urinary AMCC levels and serum liver function enzymes in DMF-exposed workers seems to be conflicting, further studies with DMF-exposed workers will be needed to identify the causal relationship between urinary levels of AMCC and liver damage.
Although a total of 193 workers surveyed for a 10-year period were recruited in the present study, 24 workers participated repeatedly. This might pose a limitation of the present study because we could not separate intra- and inter-individual variations of the urinary levels of NMF and AMCC and the exposure concentrations of DMF vapor.
In conclusion, we found that the urinary levels of NMF and AMCC were higher in the summer than in the winter, although there was no significant seasonal difference in the breathing-zone concentrations of DMF. The increased urinary levels of NMF and AMCC in the summer were presumably caused by increased percutaneous absorption of water-soluble DMF absorbed by the sweaty skin resulting from the increased ambient temperature and humidity in the summer. The log-transformed urinary NMF levels were positively correlated with the log-transformed breathing-zone concentrations of DMF in both the summer and winter, while the urinary levels of AMCC were only positively correlated with the breathing-zone concentrations of DMF in the summer. From these points of view, working conditions, especially temperature and humidity in summer, should be improved.
The simultaneous measurements of DMF and the urinary levels of NMF and AMCC revealed that the total personal exposure of the respirator-wearing workers to DMF vapor was predominantly from the percutaneous absorption of DMF. Therefore, the possibility of dermal exposure to DMF vapor should be considered of prime importance for workers laboring under the conditions of high temperature and humidity such as those in the summer, as control of exposure has relied primarily on respiratory protection.
Conflict of interest: The authors declare that they have no conflicts of interest.