2015 Volume 57 Issue 3 Pages 212-221
2015 Volume 57 Issue 3 Pages 212-221
Objectives: The aim of this study was to identify whether arsenic (As) exposure could induce hippocampal neural apoptosis in vivo. Methods: Sixty-four mice were randomly divided into 4 groups of 16 each. Group 1 orally received drinking water alone as a control. Groups 2–4 were given arsenic trioxide (As2O3) orally at the doses of 1 ppm, 2 ppm and 4 ppm, respectively. All the treatments continued for 60 days. Morphological changes in the hippocampus were observed by HE staining. Apoptosis in the hippocampus was examined by TUNEL assay and transmission electron microscopy. The expression levels of Bcl-2 and Bax genes and their proteins in the hippocampus were determined by real-time PCR and Western blotting. The activity of caspase-3 was determined by spectrophotometry. Results: Abnormal histopathological changes and apoptosis were found in the hippocampus of As-exposed mice. The expressions of the Bcl-2 gene and its protein in the hippocampus of As-exposed mice were significantly lower than those in the control group (p<0.05). However, the expressions of the Bax gene and its protein, and the expression ratio of Bax/Bcl-2 in the hippocampus were significantly higher in the groups exposed to As than in the control group (p<0.05). Moreover, the activity of caspase-3 in the hippocampus of mice exposed to As was higher than that in the control (p<0.05). Conclusions: These results indicate that subchronic exposure to As induces apoptosis in the hippocampus of mice by disturbing normal Bax/Bcl-2 regulatory pathways. Meanwhile, it is suggested that the induced apoptosis in the hippocampus may be at least partly responsible for As-induced neurotoxicity.
(J Occup Health 2015; 57: 212–221)
Arsenic (As), a naturally occurring toxic metalloid found in both inorganic and organic forms, is ubiquitous in the environment and contaminates water as a result of geological and industrial pollution1). Moreover, some kinds of arsenide, such as arsenic trioxide (As2O3), are also widely used for the treatment of malignant tumors2). In As-contaminated areas, the As concentration in drinking water or groundwater ranges from 0.25 to 2.1 ppm and even reaches >3.0 ppm in some severely contaminated areas3–5). Exposure to As has been associated with increasing incidences of various chronic diseases, including peripheral vascular disease, cardiovascular disease, diabetes mellitus, and various cancers6). As is also a potent neurotoxicant, and subchronic or chronic As exposure causes severe nervous system dysfunction7–9). Epidemiological studies have demonstrated that exposure to As results in impairments of learning and concentration10) and deterioration in pattern memory and switching attention in humans11). Animals exposed to As showed delay in acquisition and extinction of an operant task12), alterations in locomotor behavior and deficits in spatial learning paradigms13, 14). The above results indicated that As exposure can induce impairment of learning and memory. However, the mechanisms by which As induces neurotoxicity are still unclear.
Recent studies have shown that chronic exposure to As increases apoptosis in the neuronal cells of the brain15). Yen et al. (2011) found that the terminal deoxynucleotidyl transferase deoxy-UTP-nick end labeling (TUNEL)-positive neuronal cells were significantly increased in the cerebral cortex of mice exposed to 0.5 and 5 ppm As for 6 consecutive weeks, indicating that As induces apoptosis in the cerebral cortex of mice16). Pachauri et al. (2013) showed that chronic As exposure induced neuronal apoptosis in the guinea pig brain17). Zhang et al. (2013b) reported that apoptosis was induced by chronic exposure to 50 mg/l As in the brain of rats18). Furthermore, there was a close relationship between the level of As exposure and the proportion of apoptotic cells. Chattopadhyay et al. (2002) showed that human fetal brain explants exposed to As in tissue culture showed apoptosis19). These studies indicated that As exposure induces death of neuronal cells in the brain tissue by apoptotic processes in vivo and in vitro.
The hippocampus is a major component of the brains of humans and other vertebrates, and is involved in memory forming, organizing, and storing20, 21). It is a limbic system structure that is particularly important in forming new memories and connecting emotions and senses, such as smell and sound, to memories20, 21). The hippocampus acts as a memory indexer by sending memories out to the appropriate part of the cerebral hemisphere for long-term storage and retrieving them when necessary21). It has been shown that some exogenous chemical-induced neurotoxicities may be associated with the facilitation of apoptosis in the hippocampus22–25). Lead (Pb) is a common neurotoxicant, and it was found to induce apoptosis in the hippocampus of experimental animals in vivo25). Some studies have shown that other neurotoxicants such as CuO, MnCl2 and ethanol also induced hippocampal neural apoptosis in rats or mice20, 24, 26). These studies indicate that the hippocampus may be a target organ of the neurotoxic agents. Inducing apoptosis of the hippocampal neurons may be one of the mechanisms by which these agents produce neurotoxicity including impairment of learning and memory. However, there have been few reports showing that As exposure induces hippocampal neural apoptosis in vivo.
Apoptosis is tightly regulated by antiapoptotic and proapoptotic effector molecules, including proteins of the Bcl-2 family23). It is well-known that Bax and Bcl-2 are representative members of this family and that Bax promotes apoptosis, while Bcl-2 plays a role in preventing apoptosis27). Therefore, we assumed that subchronic exposure to As may induce apoptosis in the hippocampus through disturbance of normal Bax/Bcl-2 regulatory pathways. In the present study, the morphological changes in the hippocampus were observed by HE staining in mice exposed to 1, 2, and 4 mg/l As2O3. Apoptosis in the hippocampus was examined in mice by the TUNEL assay and transmission electron microscopy. Furthermore, the expression levels of Bcl-2 and Bax genes and their proteins in the hippocampus were determined by real-time PCR and Western blotting. The expression ratio of Bax/Bcl-2 proteins was analyzed by a statistical method. Because caspase activation is an indispensable event in the initiation of mitochondria-mediated apoptosis and caspase-3 is the ultimate enforcer of caspases in apoptosis28), the activity of caspase-3 in the hippocampus of mice was also determined by spectrophotometry. The study aimed to investigate the apoptotic effect of subchronic exposure to As on the hippocampus of mice and explore its molecular mechanism.
As2O3, horseradish peroxidase-conjugated goat anti-rabbit IgG and horseradish peroxidase-conjugated goat anti-mouse IgG were purchased from Sigma Chemical Company (St. Louis, MO, USA). PrimeScript™ RT Reagent Kits and SYBR® Premix Ex Taq™ kits were supplied by TaKaRa (Otsu, Japan). RIPA Tissue Protein Extraction Reagent and BCA protein assay kits were obtained from Biyuntian Company (Shanghai, PR China). Hybond-P PVDF membranes were bought from Millipore in France. Rabbit anti-mouse Bcl-2 and Bax monoclonal antibodies were bought from R & D system in the USA. Mouse anti-mouse β-actin monoclonal antibody was bought from Sigma Chemical Company (St. Louis, MO, USA). Diaminobenzidine (DAB) color reagent kits and UltraSensitive™ S-P kits were obtained from MAIXIN-Bio (Fuzhou, PR China). The TUNEL assay kit and fluorescein were supplied by Roche (Mannheim, Germany). Other reagents were commercially available. When used, As2O3 was weighed and dissolved in dilute NaOH solution, and then the pH of the 40 ppm As2O3 stock solution was adjusted to 7.2.
Animals and treatmentSixty-four male Kunming mice (age 9 weeks) weighting 20 ± 2 g were purchased from the Experimental Animal Center, Dalian Medical University. They were housed four or five per cage under standard conditions of a 12 hour dark-light cycle at 18–22°C and 50% humidity and maintained on a standard diet with water ad libitum. All the animals were randomly assigned to four groups according to their body weight. Group 1 orally received double distilled water alone as a control. Groups 2–4 were given double distilled water containing As2O3 orally at the doses of 1 ppm, 2 ppm and 4 ppm per day, respectively. Experiments were performed in accordance with the Animal Guidelines of Dalian Medical University and in agreement with the Ethics Committee of Dalian Medical University.
Sample collectionAfter 60 days of treatment, all the animals were weighed. Six mice were randomly selected from each group, anesthetized deeply by intraperitoneal injection of sodium pentobarbital and placed in a supine position, and the thorax was then opened through a bilateral incision. A catheter was inserted into the left ventricle, and the right atrial appendage was incised. Physiologic saline was infused until the perfusate from the right atrium was bloodless. The saline perfusion was followed by 4% paraformaldehyde. For observation of histopathology, apoptosis and immunohistochemistry, the hippocampi of three mice of each group were removed and placed in 4% paraformaldehyde. For ultrastructural observation, the hippocampi of the other three mice of each group were removed and placed in 4% glutaraldehyde solution. Moreover, the ten mice left in each group were sacrificed by decapitation for RNA and protein extraction. A skilled technician was designated to remove the hippocampi precisely from the brains of mice under the condition of low temperatures, and the hippocampi were weighed and frozen in liquid nitrogen. Samples were stored at −80°C until use for analysis gene and protein.
Histopathological observationThe formalin-fixed hippocampi were embedded in paraffin, sliced at 5 µm, mounted on glass slides coated with poly L-lysine, and subjected to hematoxylin and eosin staining according to the routine histopathological methods. Histopathological changes were observed under a light microscope.
Ultrastructural observationThe hippocampi were fixed for 2–4 hours with 4% glutaraldehyde solution buffered with 0.1 M cacodylate (pH 7.2) and then postfixed in 1% osmium tetroxide buffered with 0.1 M cacodylate for 2 hours. After fixation, the samples were dehydrated through a graded series of alcohol and embedded in Epon 618. Ultrathin sections were made and stained with uranyl acetate and lead citrate. Then the samples were observed by transmission electron microscopy (TEM) (JEM-2000EX, Olympus, Japan).
TUNEL assayThe TUNEL assay was performed using an In Situ Cell Death Detection Kit and fluorescein (Roche, Mannheim, Germany). All the procedures were based on the manufacturer's protocol with slight modifications. In brief, free-floating hippocampus sections were treated with permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate) for 8 minutes on ice. Afterward, the sections were incubated for 60 minutes with TUNEL reagent mixture at 37°C, and then stained with Hoechst 33342 (2 mg/ml in PBS) for 20 minutes. Finally, the sections were mounted with fluorescence mounting medium and visualized under a confocal microscope (TCS SP5, Leica, Mannheim, Germany). Five fields were randomly selected, and the percentage of positive cells was calculated as the apoptosis index (number of positive cells / total number of cells × 100%).
Quantitative real-time PCRTotal RNA was extracted from mouse hippocampus tissues by using RNAiso Plus according to the manufacturer's instructions (Takara, Japan). The RNA was quantified by using a spectrophotometer. Only RNA samples with an A260/A280 of 1.8–2.2 were used for reverse transcription. One hundred nanograms/microliter of total RNA was reverse transcribed using a Reverse Transcription Kit (Takara, Japan). Quantitative real-time PCR was carried out with a SYBR Green II PCR kit (Takara, Japan) using a TP800 Real-Time PCR Detection System (Takara, Japan). The primers for Bax, Bcl-2 and β-actin are shown in Table 1 (designed by Takara, Japan). The reaction conditions were as follows: an initial denaturation at 95°C for 5 minutes, followed by 40 cycles of 95°C for 30 seconds, 55°C for 30 seconds and 72°C for 30 seconds. The β-actin mRNA was used as the internal control probe.
Western blottingMouse hippocampus tissue was homogenized in ice-cold RIPA Tissue Protein Extraction Reagent (Biyuntian, PR China) supplemented with 1% proteinase inhibitor mix and incubated at 4°C for 1 hour. After incubation, debris was removed by centrifugation at 12,000 × g for 15 minutes at 4°C, and the lysates were stored at −80°C until used. The total protein concentration in the lysates was determined using the BCA protein assay kit (Biyuntian, PR China). The proteins (50 µg per lane) were mixed with an equal volume of SDS-PAGE loading buffer and separated by SDS-PAGE under nonreducing conditions using 10% SDS-PAGE gels and then electrotransferred to a Hybond-P PVDF membrane (Millipore, France). The membrane was blocked with blocking buffer containing defatted milk power for 1 hours and incubated overnight at 4°C with 1 µg/ml rabbit anti-mouse Bcl-2 or Bax monoclonal antibody (1 : 500) (R & D System, Minneapolis, MN). The membrane was washed three times with Tris-buffered saline containing 0.05% Tween-20 (TBST) for 10 minutes and then incubated at room temperature for 1 hour with horseradish peroxidase-conjugated goat anti-rabbit IgG (1 : 2,000) (Sigma, USA) and horseradish peroxidase-conjugated goat anti-mouse IgG (1 : 2,000) (Sigma, USA). The signals were visualized using an enhanced ECL chemiluminescence kit and quantified densimetrically using a UVP BioSpectrum Multispectral Imaging System (UVP, LLC, Upland, CA, USA).
Caspase-3 activity detectionThe activity of caspase-3 was determined using a caspase-3 activity kit (Beyotime Institute of Biotechnology, Haimen, P.R. China) according to the manufacturer's protocol. Briefly, protein was isolated from the spinal cord and sciatic nerve using the lysis buffer supplied with the kit, and 100 mg of protein was incubated in assay buffer containing 10 mM DTT and 0.2 mM caspase substrate, DEVD-pNA, for 2 hours at 37°C. Samples were measured with a microplate reader at an absorbance of 405 nm, with the results representing the caspase-3 activities of the samples.
Statistical analysisValues are expressed as the mean ± SD, and significant differences between mean values were determined by one-way analysis of variance (ANOVA) followed by the Scheffe's test for multiple comparison. All test data were converted and manipulated by using the Statistical Package for the Social Sciences 17.0 (SPSS 17.0) computer package. Differences were considered statistically significant at p<0.05.
Histopathological changes in the hippocampus of the mouse brain in the four groups are shown in Fig. 1. Under light microscope, a reduced number and disordered arrangement of nerve cells, karyopyknosis and karyolysis in the CA1 area of the hippocampus were observed in the mice exposed to As (Fig. 1b, c and d). In particular, there were remarkable abnormal histopathological changes in the CA1 area of the hippocampus in the mice exposed to 4 ppm As2O3 (Fig. 1d). However, the abovementioned phenomena were not observed in the control group (Fig. 1a).
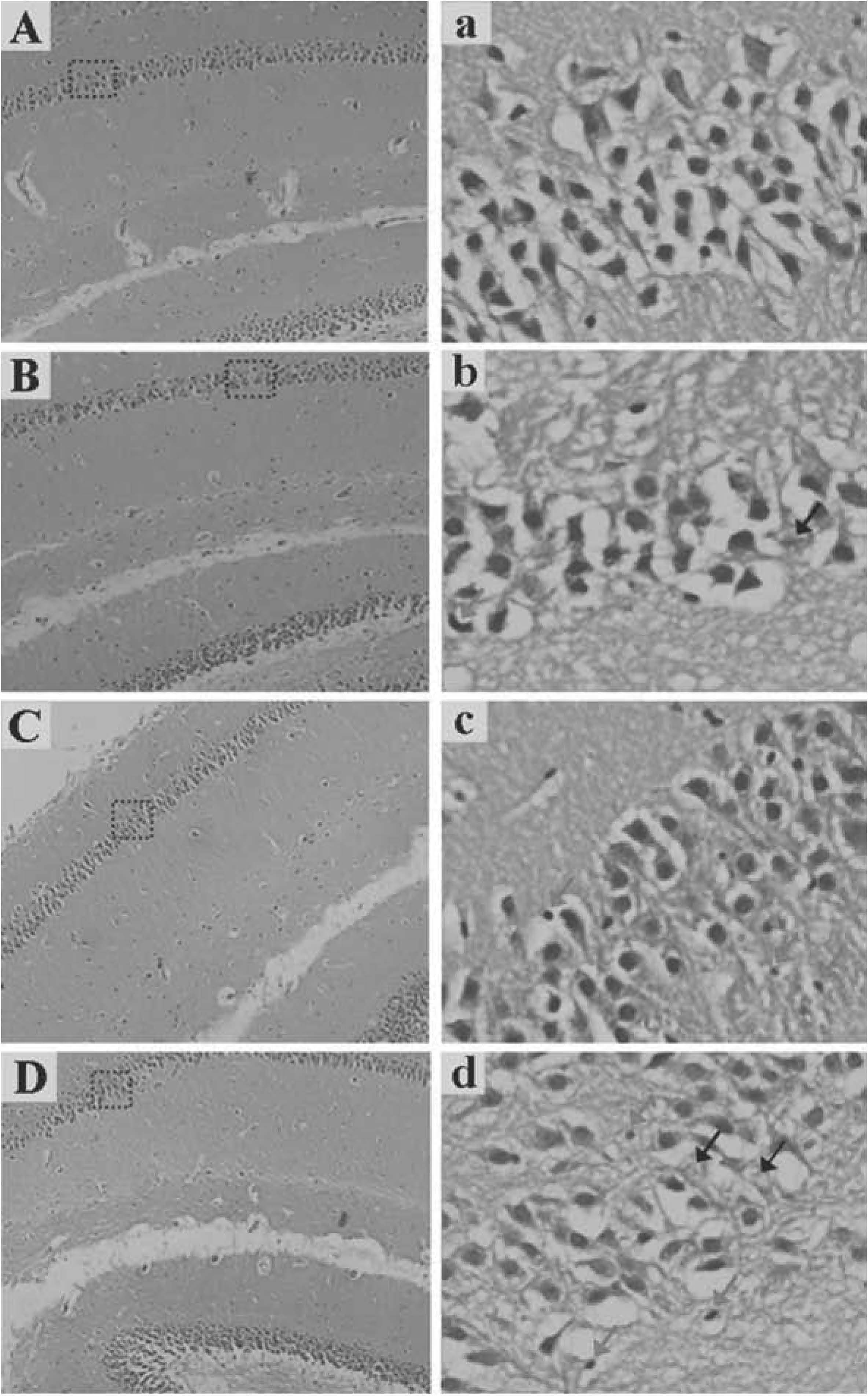
Histopathological changes in the hippocampus of mice in the 4 groups. A, control; B, 1 ppm As2O3; C, 2 ppm As2O3; D, 4 ppm As2O3. Histopathological changes in the hippocampus of mice were examined by H&E staining. A reduced number and messy arrangement of nerve cells, karyopyknosis (indicated by black arrows) and karyolysis (indicated by red arrows) were seen in the CA1 area of the mouse hippocampus in the groups exposed to As.
Apoptotic cells stained by TUNEL in the hippocampus of the mouse brain in the four groups are shown in Fig. 2A. TUNEL-positive neuronal cells were found in the hippocampus of mice exposed to As. Moreover, the apoptotic index showed that there were significantly more TUNEL-positive neuronal cells in the groups exposed to As than that in control group (p<0.05) and that the cells increased in a dose-dependent manner. However, TUNEL-positive neurons were completely absent from the hippocampus of mice in the control group. The morphological features apoptotic neurons in the hippocampus of mice under transmission electron microscope are shown in Fig. 2B. Neurons in the hippocampus appeared to be larger, with intact cell membranes, clear nuclei, and evenly distributed nuclear chromatin in the mice of the control group (Fig. 2B-a). However, the neuronal shrinkage, nuclear membrane incisures, chromatin condensation and margination along the inner nuclear membrane, and karyopyknosis, as features of apoptosis, were found in the hippocampus of mice exposed to As (Fig. 2B-b, c and d).
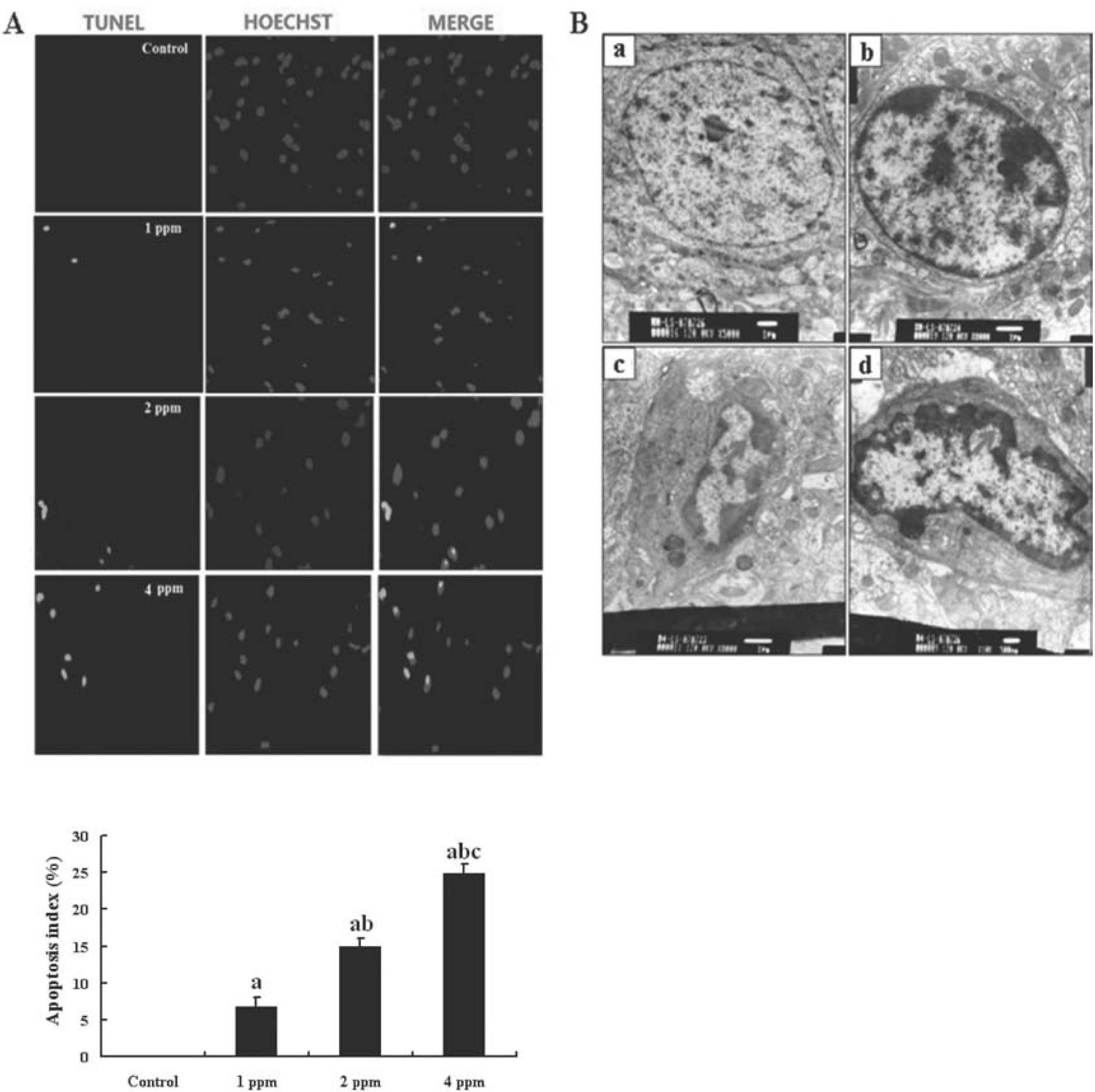
As-induced apoptosis in the hippocampus of the mouse brains
A: Immunofluorescence images of TUNEL labelling in the hippocampus of mice in the 4 groups. Morphological apoptosis was determined by TUNEL assay. The images show cells in the hippocampus of mice subjected to TUNEL and Hoechst 33342 staining (TUNEL, Hoechst 33342 and merged TUNEL-Hoechst). The green color represents TUNEL-positive cells, indicating apoptosis. The blue color represents cell nuclei counterstained with Hoechst 33342. Five fields were randomly selected, and the percentage of positive cells was calculated as the apoptosis index (number of positive cells / total number of cells × 100%). TUNEL-positive cells were found in the hippocampus of mice exposed to As, and the result of apoptotic index showed that the TUNEL-positive neuronal cells increased in a dose-dependent manner in mice exposed to As. Data are shown as the mean ±SD. a: p<0.05 significantly different compared with the control group. b: p<0.05 significantly different compared with the 1 ppm As2O3 treated group. c: p<0.05 significantly different compared with the 2 ppm As2O3 treated group. B: Ultrastructural changes in the hippocampus of mice in the 4 groups. a, control; b, 1 ppm As2O3; c, 2 ppm As2O3; d, 4 ppm As2O3. The control group showed intact cell membranes, clear nuclei and evenly distributed nuclear chromatin in the hippocampus neuron of the mice (a). However, the experimental groups exhibited apoptotic nuclear changes such as neuronal shrinkage, nuclear membrane incisures, chromatin condensation and margination along the inner nuclear membrane (indicated by red arrows), and karyopyknosis in the hippocampus of mice (b, c, d).
The mRNA expression of Bax and Bcl-2 in the mouse hippocampus is shown in Fig. 3. The mRNA expression of Bax in the hippocampus was significantly higher in the groups receiving As than that in control group (p<0.05). Moreover, the mRNA expression of Bax in the hippocampus of mice exposed to As increased in a dose-dependent manner (Fig. 3A). However, the mRNA expression of Bcl-2 in the hippocampus of mice exposed to As was significantly lower than that in the control group (p<0.05) and decreased in a dose-dependent manner (Fig. 3B).
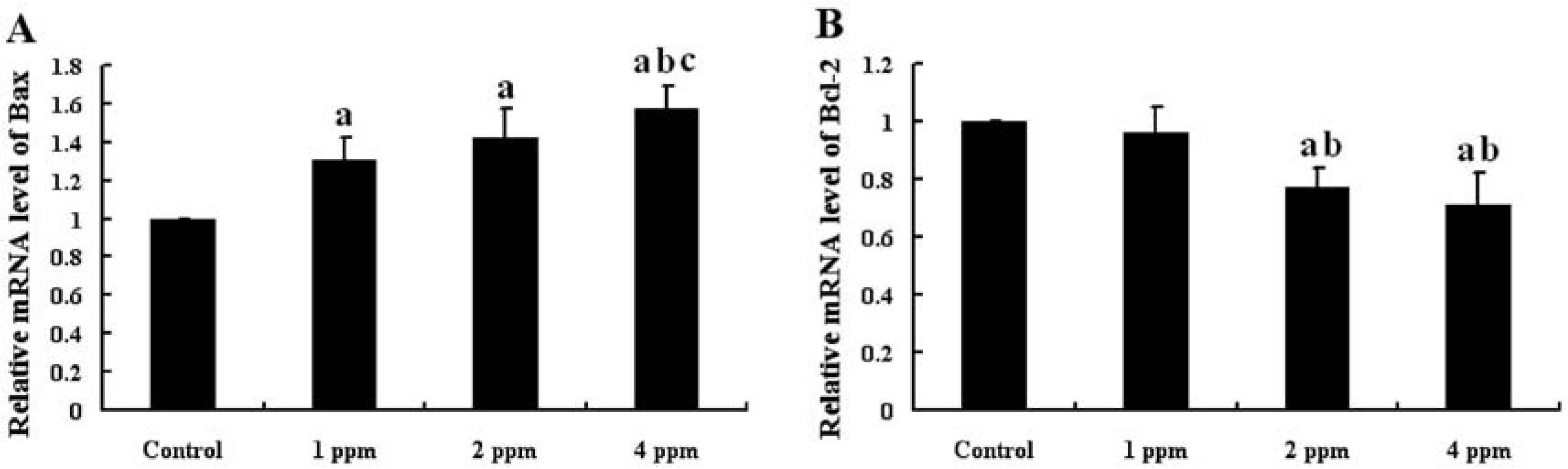
The mRNA expressions of Bax (A) and Bcl-2 (B) in the hippocampus of mice in the 4 groups. The expressions of the Bax and Bcl-2 genes in the hippocampus of mice were analyzed by quantitative real-time RT-PCR. β-actin mRNA was used as the internal control to measure the relative quantitation of the expression of the target gene. Data obtained from six separate analyses are expressed as the mean ± SD (n=6 for each group). a: p<0.05 significantly different compared with the control group. b: p<0.05 significantly different compared with the 1 ppm As2O3 treated group. c: p<0.05 significantly different compared with the 2 ppm As2O3 treated group.
The expression of Bax and Bcl-2 proteins in the hippocampus of mice by Western blot is shown in Fig. 4. The expression of Bax protein in the hippocampus was significantly higher in the groups receiving As than that in the control group (p<0.05) (Fig. 4A). However, the expression of Bcl-2 protein in the hippocampus of mice exposed to As was significantly lower than that in controls (p<0.05) (Fig. 4B). Because alteration of the ratio of Bax/Bcl-2 is a more sensitive and reliable marker than individual Bax and Bcl-2 levels, the expression ratio of Bax/Bcl-2 proteins in the hippocampus of mice was also analyzed. The ratio of Bax/Bcl-2 expression in the hippocampus significantly increased in the experimental groups compared with the control group (p<0.05). In particular, the ratio of Bax/Bcl-2 expression in the hippocampus of mice exposed to 4 mg/l As2O3 was the highest among the 4 groups (Fig. 4C).
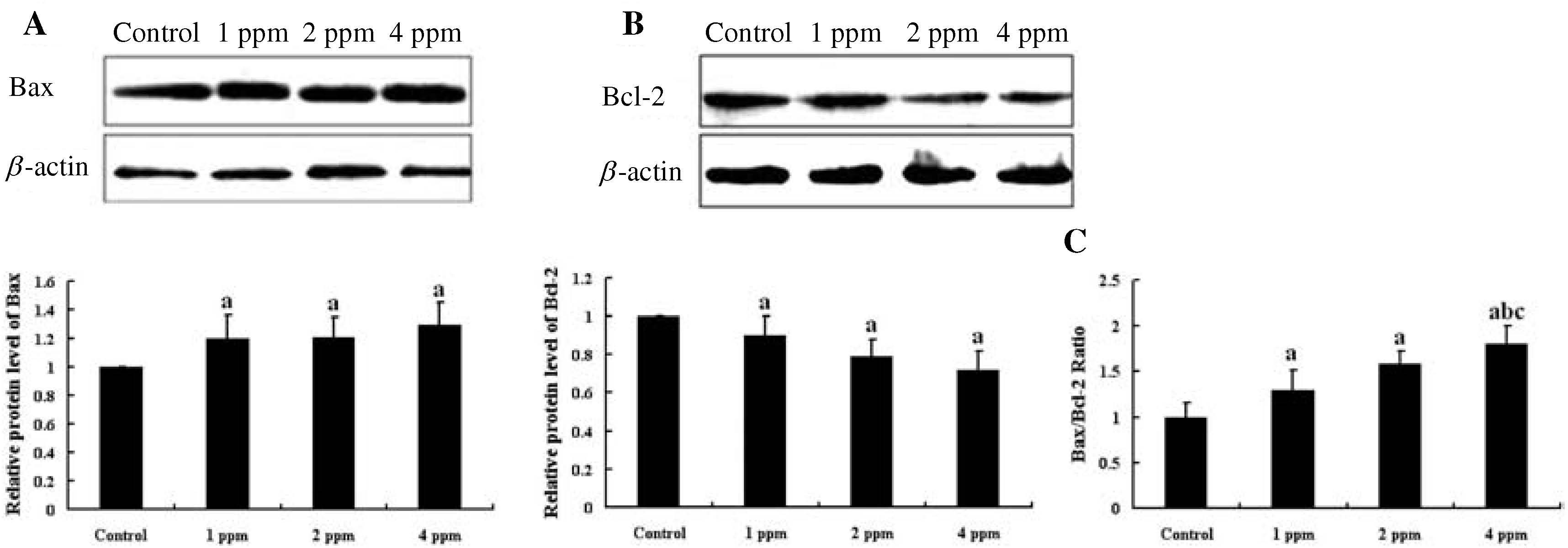
Effect of As on the expression of Bax and Bcl-2 proteins in the hippocampus of mice
A and B: The expressions of Bax (A) and Bcl-2 (B) protein in the hippocampus of mice in the 4 groups. The protein expression level in the hippocampus of mice was analyzed by Western blotting. The relative target protein abundance was determined by the ratio of sample to β-actin. C: The expression ratios of Bax/Bcl-2 proteins in the hippocampus of mice in the 4 groups. The expression ratio of Bax/Bcl-2 proteins was analyzed by a statistical method. Data obtained from six separate analyses are expressed as the mean ± SD (n=6 for each group). a: p<0.05 significantly different compared with the control group.
The activity of caspase-3 in the hippocampus of mice in the 4 groups is shown in Fig. 5. The activity of caspase-3 in the hippocampus of the mice was significantly higher in the groups exposed to As than that in the control group (p<0.05) and increased in a dose-dependent manner.
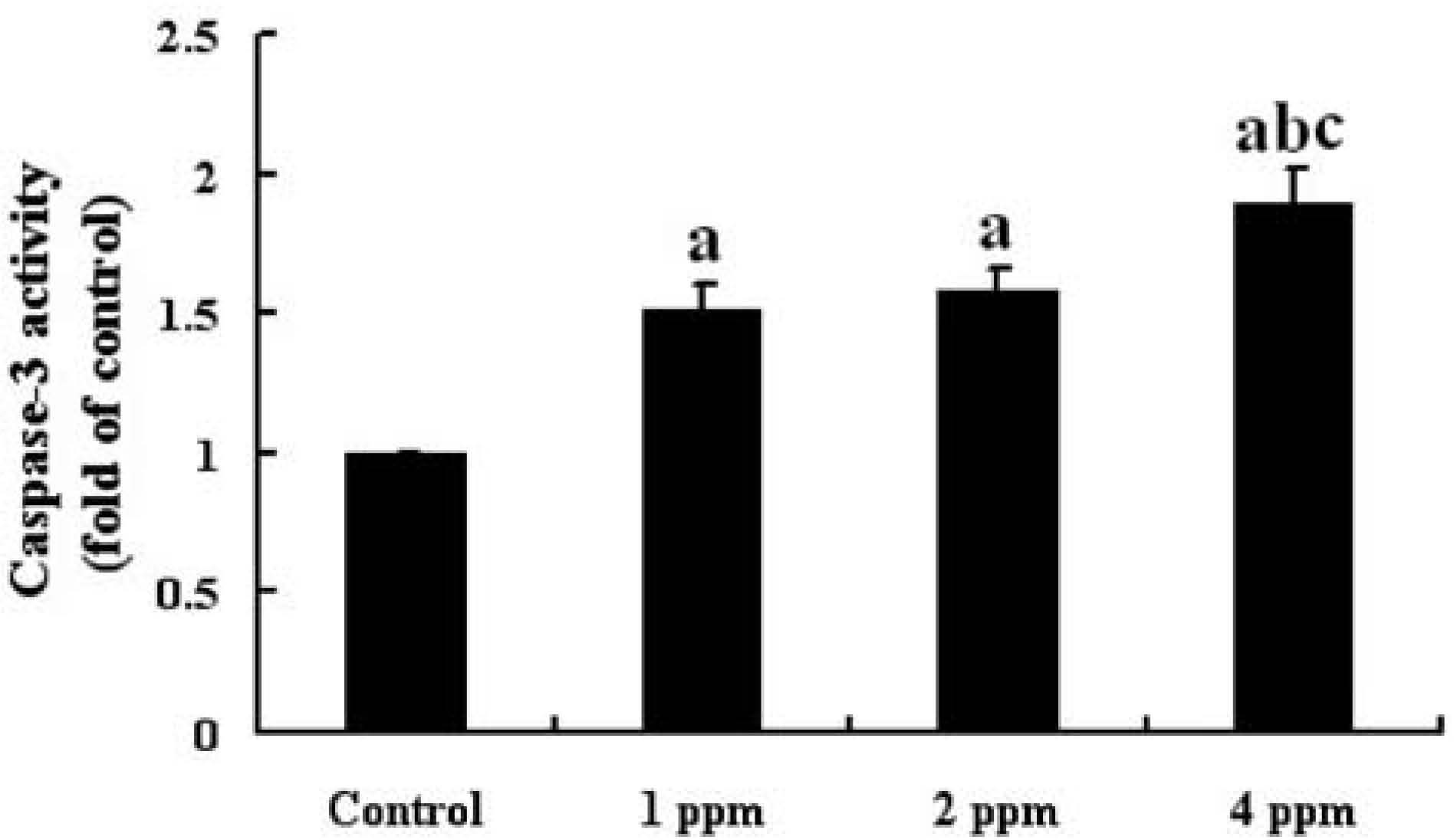
The activity of caspase-3 in the hippocampus of mice in the 4 groups. The activity of caspase-3 in the hippocampus of mice was determined using a caspase-3 activity kit. Data obtained from six separate analyses are expressed as the mean ± SD (n=6 for each group). a: p<0.05 significantly different compared with the control group. b: p<0.05 significantly different compared with the 1 ppm As2O3 treated group. c: p<0.05 significantly different compared with the 2 ppm As2O3 treated group.
Epidemiological investigations have demonstrated that As causes neurotoxicity including impairments of learning and memory10, 11). Experimental studies have shown the alteration of locomotor activity and behavioral disorders in experimental animals exposed to As12–14). In our previous studies, an accumulation of As in the brains of mice and a decrease in memory function were also found in the mice exposed to As at the same doses as used in the present study29, 30). It is well known that the hippocampus is functionally related to vital behaviors and intellectual activities such as memory and learning20). Many studies have shown that some neurotoxicants can adversely affect the hippocampus when administered to rats or mice22–25). In the present study, abnormal histopathological changes including a reduced number and disordered arrangement of nerve cells, karyopyknosis and karyolysis were observed in the hippocampus of mice exposed to As. These results implied that the hippocampus may be a target of neurotoxicants including As and that the resulting damage to it may be involved in As-induced neurobehavioral abnormalities of mice.
Apoptosis, also known as programmed cell death, plays an important role during neuronal development and in the homeostasis of the adult nervous system31). Therefore, disruption of this process or abnormal neuronal apoptosis may have severe effects on the nervous system. A number of studies showed that As exposure could induce apoptosis in a variety of cells32–34). Singh et al. (2010) demonstrated that exposure to arsenate induced apoptosis in the murine embryonic maxillary mesenchymal (MEMM) cells34). It was reported that chronic exposure to As increased apoptosis in the neuronal cells of the brain15). Yen et al. (2011) found that the TUNEL-positive neuronal cells were significantly increased in the cerebral cortex of mice exposed to 0.5 and 5 ppm As for 6 consecutive weeks16). Chattopadhyay et al. (2002) showed that human fetal brain explants exposed to As in tissue culture exhibited apoptosis19). However, there have been few studies reporting that As exposure induced hippocampal neural apoptosis in vivo. In the present study, TUNEL-positive neuronal cells were found in the hippocampus of mice exposed to As and significantly increased in a concentration-dependent manner. Apoptotic effects of As on the hippocampus were further confirmed by transmission electron microscopy. Observation of the ultrastructure showed the features of apoptosis including the neuronal shrinkage, nuclear membrane incisures, chromatin condensation and karyopyknosis in the hippocampus of mice exposed to As. This indicates that As induces cell death in the hippocampus by apoptotic processes. It also suggests that abnormal apoptosis in the hippocampus may be associated with As-induced neurotoxicity.
Apoptosis is a tightly controlled process in which cell death is executed through the activation of specific signaling pathways23). Within cells, there are antiapoptotic and proapoptotic regulatory pathways, and it is the balance between these pathways that determines cell fate. Proteins of the Bcl-2 family are regulatory molecules of apoptosis, and Bax and Bcl-2 are representative members of this family23). The Bax promotes apoptosis, while the Bcl-2 plays a role in preventing apoptosis18). A large body of evidence has demonstrated that As2O3-induced apoptosis in tumor cells has been associated with the downregulation of Bcl-2 and the upregulation of Bax35, 36). Yadav et al. (2010) reported that As exposure induced apoptosis in bone marrow mesenchymal stem cells, downregulation of Bcl-2 mRNA expression and upregulation of Bax mRNA expression37). Singh et al. (2010) demonstrated that exposure to arsenate increased the Bax protein level and decreased the Bcl-2 protein level in murine embryonic maxillary mesenchymal cells34). In the present study, the expression of Bax and Bcl-2 in the hippocampus was also analyzed by real-time PCR and Western blotting. The expression of the Bax gene and its protein in the hippocampus was significantly higher in the groups receiving As than that in the control group. However, the expression of the Bcl-2 gene and its protein in the hippocampus of mice exposed to As was significantly lower than that in the control group. These results indicate that subchronic exposure to As upregulates the expression of Bax and downregulates the expression of Bcl-2 in the treated mice. Because Bcl-2 homodimerizes with itself and forms heterodimers with Bax and Bax is functionally neutralized by heterodimerization with Bcl-2, the ratio of Bcl/Bax protein could ultimately tip the balance toward cell survival or cell death38). Zhang et al. (2013b) reported that the ratio of Bax/Bcl-2 in the liver and brain following exposure to As was significantly increased compared with that of their control group18). In the present study, subchronic exposure to As also increased the ratio of Bax/Bcl-2 expression in the hippocampus of mice, which is consistent with the results reported by Zhang et al. (2013b)18). This indicates that subchronic exposure to As disturbed expression of the Bcl-2 family proteins in the hippocampus of mice. It also suggests that the dysregulated expression of the Bax/Bcl-2 in the hippocampus of mice may be involved in As-induced apoptosis.
It was reported that an increased ratio of Bax/Bcl-2 leads to activation of the caspase cascade and ultimately triggers apoptosis39). Therefore, the present study also examined the effect of As on the activity of caspase-3 as the ultimate enforcer of caspases in the hippocampus of mice. Our results showed that the activity of caspase-3 in the hippocampus of rats increased significantly in the groups exposed to As compared with control group. Yen et al. (2011) reported that after exposure via the drinking water for 6 consecutive weeks, As significantly increased apoptosis of neuronal cells and caspase-3 activation in the cerebral cortex of mice16). Cai et al. (2010) also reported that As2O3 obviously enhanced the caspase-3 activity in a concentration-dependent manner in bone marrow mesenchymal stem cells, supporting our results40). Our results indicates that the caspase-3 in the hippocampus was activated in mice exposed to As and that activation of caspase-3 may be associated with disturbance of the expression ratio of Bax/Bcl-2 by As.
In conclusion, subchronic exposure to As induced abnormal morphological changes and apoptosis in the hippocampus of mice. The expression of the Bcl-2 gene and its protein was downregulated, and the expression of the Bax gene and its protein was upregulated in the hippocampus of mice exposed to As. The expression ratio of Bax/Bcl-2 in the hippocampus was significantly increased by As administration. Moreover, the activation of caspase-3 was found in the hippocampus of mice exposed to As. This indicates that subchronic exposure to As induces apoptosis in the hippocampus of mice by disturbing normal Bax/ Bcl-2 regulatory pathways. It is also suggested that the induced apoptosis in the hippocampus may be at least partly responsible for As-induced neurotoxicity. To explore the detailed molecular mechanisms of As-induced apoptosis, the changes of other downstream key proteins regulating apoptosis should be examined in the hippocampus of mice exposed to As. Meanwhile, it is also necessary to demonstrate further close association between the increased apoptosis in the hippocampus and the As-induced impairment of learning and memory.
Acknowledgments: This study was the supported by the National Natural Science Foundation of China (No. 30571584).
Conflict of interest: The authors declare that they have no conflicts of interest.