2016 Volume 58 Issue 5 Pages 470-476
2016 Volume 58 Issue 5 Pages 470-476
Objective: Hairdressers have an increased risk for airway symptoms especially when using hair-bleaching powder containing persulfate. To minimize exposure, dust-free bleaching powder (DFP) has been made available. We studied the effects of regular powder (RP) or DFP on the airway symptoms of hairdressers with hair-bleaching associated rhinitis. Methods: Twelve hairdressers each performed three hair-bleachings on a wig in an exposure chamber. Half of the subjects used RP and half used DFP. Exposure to persulfate and ammonia was measured. Before and after each bleaching, the participants stated their degree of airway symptoms on a visual analogue scale. Nasal lavage and blood were sampled before exposure, after the last bleaching, and in the morning after exposure to measure inflammatory markers. Results: Exposure to persulfate was higher when using RP compared to DFP, 22 (11-55) vs. 12 (8-13) μg/m3; median (min-max). Exposure to ammonia did not differ between the groups. Both groups reported an increase in asthma-like symptoms and this increase was significant. Neutrophils, lymphocytes, and monocytes increased after exposure in both groups; monocytes decreased the day after. In nasal lavage, IL-8 was increased the morning after for both types of powder, and the increase was significant in the total group. IL-6 increased immediately after exposure and the day after only in the group using RP. Conclusions: Although DFP powder emits lower levels of persulfate, effects are still elicited in symptomatic hairdressers.
Hairdressers have an increased risk for asthma and upper airways symptoms1-3). The exposure that causes airway symptoms is complex and poorly characterized. It may come from different sources such as hair-bleaching products, hair dyes, permanent wave solutions, hair sprays, and various styling products3-6). Thirty-five different airborne compounds, including a large number of elements that may influence the airways, have been identified in hairdressers' work environment7,8).
The hairdressers' respiratory complaints are mainly related to the use of hair-bleaching powders2,9), which contain several components. They are mixed with an oxidizing agent, typically hydrogen peroxide, in concentrations up to 12% prior to the application to the hair.
Persulfate is an important component of hair-bleaching powder, and considerable amounts of potassium-, ammonium- or sodium-persulfate are contained in most powders10). Persulfate may be the reason for much of the hypersensitive airway disorders in hairdressers; however the mechanisms have not yet been elucidated11-17). Hairdressers are exposed to persulfate especially while mixing the hair-bleaching powders, which form dust easily2). In order to minimize exposure, the producers have developed products that generate less dust named dust-free bleaching powders (DFP). However, there have been concerns whether such powders really improve the working environment compared to the traditional ones18).
We recently published a study comparing a bleaching powder labeled as dust-free with a powder without this label, here called regular bleaching powder (RP). Bleaching was carried out under standardized conditions in an exposure chamber, and special attention was paid to the particle exposure19). During the mixing of the powders, there was a particle exposure < 10 μm in diameter only for RP. However, both powders gave an exposure to particles > 10 μm during application to the hair. Although such particles are not classified as respirable, they can directly affect the eyes and the upper airways.
The aim of this study was to determine if two groups of hair-dressers with hair-bleaching associated rhinitis respond differently when using DFP and RP, respectively, in a standardized exposure situation.
Twelve non-smoking and non-atopic hairdressers with nasal symptoms associated with hair-bleaching and without manifest asthma or clear work-related symptoms from the lower airways participated in this study. They were mostly patients from our policlinic who were consecutively asked to participate. To complete the group of participants, symptomatic subjects were consecutively identified by telephone calls to nearby hair salons using a standardized questionnaire containing the same questions as those used for the patients. Before the study, the hairdressers had not been exposed for two days. They were randomly divided into two exposure groups. One used DFP and the other one used RP in a double blind manner. During the study day, two hairdressers performed three hair-bleachings in succession on a wig placed on a temperature-regulated manikin. Each bleaching time was standardized to 40 min.
The exposureThe work was performed in an exposure chamber as previously described20). Filtered air was supplied at a rate of 0.5 air-exchanges/h. Due to the relatively low air exchange rate, the temperature increased during the day (3.7°C) equally for both groups. After the first bleaching, the chamber was ventilated with filtered air until a negligible concentration was obtained, and then hairdresser number two started her first bleaching. When hairdresser number two had finished and the chamber had been emptied, hairdresser number one started her second bleaching and so on. The study took place between nine o'clock a.m. and three o'clock p.m. for hairdresser number one and from ten o'clock a.m. to four o'clock p.m. for hairdresser number two.
To begin, the hairdresser added two scoops (90 g) of bleaching powder into a bowl and mixed it with an appropriate amount of hydrogen peroxide (12%, w/v). She applied the mixture to a clean wig of human hair (Snab Cap, Pivot Point Stockholm, Sweden) using a hair bleaching brush. The thermal manikin was used to imitate any effect that heat convection from a human being may have on the exposure of the hairdresser. The treated hair was successively covered by a commercial folio for hair-bleaching. After 20 min, another scoop (45 g) of powder was mixed. The mass of bleaching powder and hydrogen peroxide used was measured and the amounts did not differ significantly between the two groups.
According to the Safety Data Sheets, the ingredients in RP were dipotassium peroxodisulphate (40-60%), disodium metasilicate (5-< 10%), peroxydisulfuric acid, disodium salt (1-< 10%), kieselguhr, soda ash flux-calcined (1-<5%), cocosfattyacidmonoethanolamide (5-< 10%), sodium dodecyl sulphate (1-< 5%), and magnesium peroxide (1-< 10%). In DFP, they were diammonium peroxodisulphate (10-< 20%), dipotassium peroxodisulphate (30-50%), disodium metacilicate (10-< 15%), kieselguhr, soda ash flux-calcined (5-< 10%), and tetra sodium ethylene diamine tetra acetate (1-< 5%).
During the bleaching procedure, exposure was monitored for ammonia and persulfate. Measurements of persulfate have been described previously19). Briefly, persulfate was sampled in the hairdressers breathing zone on filters (0.8 μm pore size, AAWP03700, Millipore (Solna, Sweden) with a pump flow rate of 7 l/min. The sampled persulfate was hydrolyzed and the ions were separated on a column with quaternary ammonium groups and analysed by ion chromatography. Ammonia was sampled by sulfuric acid-treated silica gel glass tubes, SKC 226-10-06 with flow rates of 0.2 l/min and analysed by an ion chromatograph with conductivity detector according to the standard protocol of the Laboratory of Occupational and Environmental Medicine, Sahlgrenska University Hospital. The exposure to particles is described in details previously19).
Medical examinationsBefore the study, all candidates went through an examination including a medical and work history, spirometry, and a skin-prick test with a standard panel of 13 environmental allergens (ALK, Copenhagen, Denmark) and fresh solutions in sterile water of potassium-persulfate (0.05, 0.1, and 0.5%, w/v). The reactions were read according to Aas and Belin (1973)21). Nobody reacted to the skin-prick tests and lung functions were within the normal limits.
Before and after each bleaching, the participants answered standardized questions about symptoms using a visual analogue scale (VAS)22). No symptoms were scored as 0 and maximal symptoms as 100 mm. Symptoms from the following sites were registered: the eyes (scratching, burning, or itching), running and blocked nose, the pharynx (sensation of dryness, irritation, and/or itching), and from the lower airways (feeling of dyspnoea or chest tightness, in this paper also denoted asthma-like symptoms). Furthermore, spirometry (Spirare 3, Diagnostica, Oslo, Norway) was performed according to the European Respiratory Society23). Forced vital capacity (FVC) and Forced expiratory flow in the first second (FEV1) were registered and compared to reference materials24). Peak nasal inspiratory flow (PNIF) was measured using an inspiratory flow meter (In-check, Clement Clarke International Limited, England) according to the manufacturer's instructions. The mean of three accepted inspirations was recorded. A medical doctor (JN) inspected the eyes, nose, and pharynx, and performed lung auscultation at normal and forced respiration before and after each bleaching.
Nasal lavage and blood samples were collected before the first exposure, after the last one, and on the following morning before the hairdresser's ordinary workday. Nasal lavage was carried out as previously described22) using 18 ml, 0.9% NaCl solution. The liquid was transmitted to the nasal cavity by a syringe and drawn back three times in each nostril. After cell separation, the pellet was suspended to a volume of 500 μl in 0.9% NaCl, and 200 μl each were transformed to glass slides using a cytospin centrifuge (600 rpm for 5 min). The slides were air dried and the supernatant was frozen at -80°C for later analysis in our laboratory.
Laboratory analysesBlood samples were analyzed for hemoglobin and a differential count of white blood cells was performed using Sysmex XE-5000 and Sysmex XE-1800i according to the standard protocol of the laboratory of the University Hospital Lund.
The cytokines IL-6, IL-8, and TNF-α in the nasal lavage and in serum were analysed using a multiplexed Luminex-platform (Bio-Plex 200, Bio-Rad Life Science, Hercules, CA). In the analysis of nasal lavage, bovine serum albumin (BSA) was added to the samples to a final concentration of 0.5% BSA. The results are means of duplicate analyses. The limits of detection in the nasal lavage were 0.38 for IL-6, 0.32 for IL-8, and 1.4 for TNF-α (pg/ml). The corresponding figures in serum were 0.39, 0.43, and 1.7, respectively.
Nasal lavage cells were stained by the May-Grünewald-Giemsa method. A total of 200 cells were counted for each sample. The counting was performed blindly. In 5 (15 %) samples the total number of cells was less than 200.
Ethical considerationsThe study was conducted in accordance with the Declaration of Helsinki and approved by the Regional Ethical Review Board in Lund (2012/413). Written consent was obtained from the participants after written and oral information was provided.
StatisticsChanges in parameters within the groups were evaluated by Wilcoxon Signed Ranks Test. For comparisons between groups (regular vs. dust-free and high vs. low exposure groups for ammonia and persulfate), Mann-Whitney's U-test was employed. Statistical significance refers to p values < 0.05 (two-tailed). The statistical tests were performed in IBM SPSS statistic, version 22 for Windows (SPSS Inc., Chicago, Il, USA).
Exposure to persulfate in the breathing zone was statistically significantly higher when subjects used RP (median, 22 μg/m3; min-max, 11-55 μg/m3) compared to DFP (12 μg/m3; 8-13 μg/m3). No significant difference was found for the ammonia exposure between RP (median, 3.2 mg/m3; min-max, 2.7-9.3 mg/m3) and DFP (4.9 mg/m3; 3.2-7.4 mg/m3).
SymptomsBoth groups developed more asthma-like symptoms following exposure (Fig. 1), and in the total group the increase in symptoms became significant after the second hair-bleaching (p < 0.05). Otherwise, no consistent increase in symptoms from eyes or airways was noted. Contrary to this, there was an almost significant decrease (p = 0.07) in blocked nose in the RP group after the second hair-bleaching.
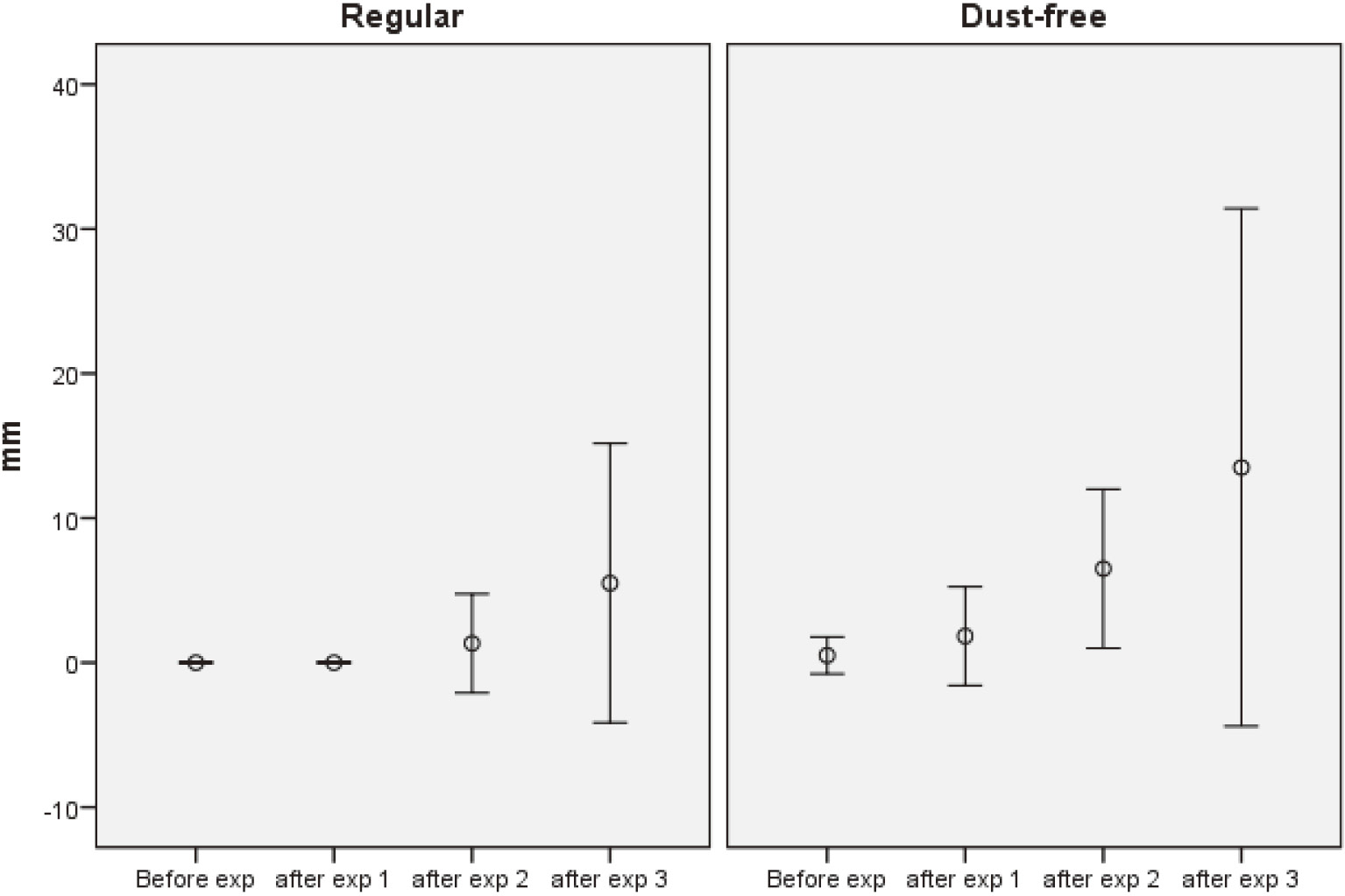
The feeling of dyspnoea and/or chest tightness expressed on a VAS scale (0-100 mm, mean and 95% confidence interval) before and after the first-, the second- and the third hair-bleaching using regular or dust-free hair bleach, respectively.
There were no significant changes within or between the groups after the hair-bleachings in spirometry and PNIF. Lung auscultation was normal in everybody during normal respiration. During forced respiration, slightly expiratory rhonchi were recorded in two subjects in the RP group before and in three subjects after exposure. In the DFP group, no rhonchi were noticed before but were found in two subjects after exposure. In the subjects with increased asthma-like symptoms, there was a non-significant decrease in FEV1 from before exposure (median, 104% of the reference value; min-max 83-127%) to after exposure (100% of the reference value; 94-103%). In those who did not have increased symptoms, the corresponding figures were 100% (83-112%) before exposure vs. 100% (96-103%) after exposure.
Biomarkers in blood and nasal lavageBlood neutrophils, lymphocytes, and monocytes levels increased after exposure in both groups. Monocytes decreased the day after, but only significantly in the total group (Table 1). There were no changes in basophils and eosinophils. The differential cell counts in nasal lavage did not differ between the two groups before exposure. The RP group had a tendency to increase in eosinophils from baseline (median, 0%; min-max, 0-0%) to the day after (1%; 0-3.5%; p = 0.07). No other changes were recorded.
| Total (N=12) | Regular (N=6) | Dust-free (N=6) | |
|---|---|---|---|
| *: p<0.05, **: p<0.01 for changes within the groups compared to values measured before the exposure. | |||
| Neutrophils | |||
| - before | 3.7 (1.5-5.9) | 3.3 (2.0-5.2) | 4.1 (1.5-5.9) |
| - after | 4.9 (2.5-6.8)** | 4.6 (3.3-6.3)* | 5.2 (2.5-6.8)* |
| - morning after | 3.4 (1.9-5.7) | 3.3 (2.1-4.1) | 3.5 (1.9-5.7) |
| Lymphocytes | |||
| - before | 1.8 (1.1-2.8) | 1.6 (1.2-2.3) | 1.9 (1.1-2.8) |
| - after | 2.1 (1.2-3.3)** | 1.9 (1.2-2.5)* | 2.2 (1.2-3.3)* |
| - morning after | 1.8 (1.0-2.4) | 1.7 (1.4-2.1) | 1.8 (1.0-2.4) |
| Monocytes | |||
| - before | 0.53 (0.3-0.8) | 0.48 (0.3-0.7) | 0.57 (0.3-0.8) |
| - after | 0.60 (0.3-0.9)** | 0.55 (0.3-0.8) | 0.65 (0.4-0.9) |
| - morning after | 0.43 (0.3-0.6)* | 0.42 (0.3-0.6) | 0.45 (0.3-0.6) |
| Nasal lavage IL-6 | |||
| - before | 3.9 (0.5-18.3) | 2.1 (0.5-7.7) | 5.7 (0.5-18.3) |
| - after | 3.6 (0.7-12.4) | 2.4 (0.7-7.9)* | 4.8 (0.9-12.4) |
| - morning after | 5.5 (1.6-13.1) | 6.0 (1.6-13,1)* | 5.0 (1.7-8.3) |
| Nasal lavage IL-8 | |||
| - before | 60.3 (15-186) | 51.1 (24-104) | 69.4 (15-186) |
| - after | 65.5 (11-187) | 52.7 (11-122) | 78.3 (12-187) |
| - morning after | 90.8 (25-175)* | 78.6 (36-159) | 103.0 (25-175) |
IL-8 in the nasal lavage increased slightly after exposure and more the next morning in both groups, and was significant in the total group (Table 1). Only the RP group increased significantly for IL-6 in the nasal lavage after the exposure and the next morning. There was no change in the serum and nasal lavage of TNF-α or in serum IL-6 and IL-8.
In this study, we investigated whether two groups of hairdressers with bleaching-associated nasal symptoms reacted differently when using DFP compared to RP during hair bleaching under standardized conditions. The use of RP resulted in higher exposure to persulfate compared to DFP. In spite of that, the hairdressers increased in asthma-like symptoms in both groups and the increase was significant. The groups also reacted similarly regarding total leukocytes, neutrophils, lymphocytes, monocytes and IL-8 in nasal lavage. However, there were increased levels of IL-6 in the nasal lavage only in the RP group.
The ammonia exposure did not differ between the groups. The levels during two hours of exposure were well below the permissible exposure limit in Sweden of 14 mg/m3, 8 h time weighted average25). Thus, it is not likely that the ammonia exposure have influenced the results to any significant degree26). There is no threshold limit (TLV) for persulfate in Sweden, but ACGIH (2008)27) has proposed a TLV of 0.1 mg/m3; this is about twice as high as the highest exposure during 2 h in the present study. The present results are comparable with those from earlier studies2,28,29), although the sampling strategies in these studies were somewhat different and the bleaching powders not specified.
We did not measure the air concentration of hydrogen peroxide, another irritant of the airways, for several reasons. Firstly, the amount used did not differ significantly between the two powders; therefore, it is not likely that the air concentrations should differ to any substantial degree between the two groups. Secondly, an earlier study showed levels far below the present TLV in Sweden25). Thirdly, when hydrogen peroxide was studied in a rabbit model, there were no airway effects in spite of an exposure far above the TLV30).
Strangely, as the hairdressers were selected because of nasal symptoms, no increase in such symptoms was registered and instead asthma-like symptoms appeared, also in the DFP group, which was mainly exposed to non-respirable particles. However, at exposure to such particles, a considerable fraction may also be deposed in the lower airways31). The symptoms were mild and may be explained by something else than airway obstruction. However, mild obstruction was indicated by auscultation as well as by spirometry.
The numbers of white blood cells increased after exposure. It is not straight forward to draw conclusions from this because of the circadian rhythm. Inclusion of a blank exposure in the study would have helped to solve this problem, but was not easy because of difficulty to maintain blindness in the study when using an inert powder. However, during the time period studied, leukocytes and neutrophils were expected to increase about 10%32), whereas lymphocytes and monocytes were expected to increase about 5%. In the present study, the increase was two to three times higher indicating that both exposures induced a response. Furthermore, the monocytes decreased the day after exposure when sampled at the same time as the baseline. Monocytes are easily mobilized from the blood to inflamed tissues to be a reservoir of macrophages, and thus are important components in the early inflammatory process33). This may explain their decrease in the blood the day after the exposure.
The increase in IL-8 in the nasal lavage the morning after exposure also indicates that both powders triggered a measurable response. This cytokine is involved in neutrophil recruitment and may indicate a subclinical reaction in the nasal mucosa in the absence of increased nasal symptoms. IL-6 in the nasal lavage was the only inflammatory marker that differed between the two groups as it increased the morning after the provocation only in the RP group. The reason for that is not clear. No association between IL-6 and exposure level of persulfate was found. One explanation for the difference between the two groups may be the particle size, but much is still not elucidated concerning changes in markers of the effects in nasal lavage34).
In conclusion, RP emits higher levels of persulfate than DFP during the hair bleaching procedure. In spite of that, both powders elicited effects in this group of symptomatic hairdressers. Thus, a change to dust-free powder may not be sufficient to reduce symptoms from the airways in hairdressers. However, to draw general conclusions more powders should be tested. From this study, it is not possible to assess if DFP carries a lower risk than RP for the development of airway disease in healthy hairdressers. Relevant exposure limits are needed for the hairdressers' work environment.
Acknowledgments: We would like to express our gratitude to the volunteers hairdressers who participated in the study. Furthermore, we thank Pia Tallving (RN) and Else Krook Åkerberg (RN) for doing excellent work regarding coordination, medical examinations, and handling of the obtained material; and we also thank bio-medical engineer Gertrud Wohlfart for the skilful analyses of cytokines. Anette Larsson is thanked as a professional hairdresser for advice and help in the organization of the exposure situation.
The study was financed by the Swedish Research Council for Health, Working Life and Welfare (FORTE, 2010-0183) and performed within the framework of the Competence Centre for Medicine and Technology for Working Life and Society (METALUND), Lund University supported by FORTE.