2022 Volume 71 Issue 6 Pages 781-793
2022 Volume 71 Issue 6 Pages 781-793
Castor oil is a vegetable product extracted from Ricinus communis L (castor seed), which is primarily considered an important commercial value for the manufacturing of soaps, lubricants, coatings, etc. It is rich in hydroxylated fatty acids (ricinoleic acid, 89-92%) and is widely used in the cosmetic, pharmaceutical, oleochemical, and agricultural industries. This oil has also been confirmed as a bactericidal, anti-inflammatory, and antiherpetic agents, due to the ricinoleic acid having functional groups, such as -COOH, -OH, and -C=C-. Furthermore, it is converted into various acid derivative compounds with several applications. Therefore, this article reviewed some reaction stages to the preparation of ricinoleic acid from castor oil. Several methods or reaction pathways were employed in the preparation procedure, such as the Twitchell and Colgate-Emery processes, as well as the alkaline catalyzed, transesterification with methyl ricinoleic, and lipase-catalyzed hydrolysis, respectively. Although each of these preparation methods has advantages and disadvantages, the most effective technique was the hydrolysis through the use of the enzyme lipozyme TL IM. Besides being a green method, the conversion rate in the hydrolysis process was 96.2 ± 1.5%
Castor (Ricinus communis L) is an annual herbaceous plant of the family Euphorbiaceae, which is often found in tropical, sub-tropical, and several hot climates around the world, including Indonesia 1) . This species are also widely cultivated in Africa, South America, and Asia 2) , while some of the countries producing the majority of castor oil are Brazil, China, and India 3) .
Castor seeds are further extracted into a vegetable product known as castor oil 1) , 4) , which is primarily considered an important commercial value for the manufacturing of soaps, lubricants, coatings, etc. According to Naik et al. 5) , castor oil production is carried out in 4 methods, namely mechanical (compression), chemical or solvent, aqueous enzymatic, and reactive extractions, which are conducted through ultrasonic-assisted processes. The solvent extraction is often carried out using n-hexane and super-critical carbon dioxide (SC-CO2) 6) , 7) . Meanwhile, reactive extraction offers several advantages, including shorter reaction times, cheaper reagents, less insignificant physical conditions, as well as less expensive and smaller chemical plants.
Castor oil is also composed of triacylglycerols as neutral lipids, free fatty acids, and other constituents such as carotenoids, phenolics, phospholipids, phytochemicals, phytosterols, tocopherols, and tocotrienols 8) . Based on removing impurities and obtaining pure triacylglycerols, the performance of a purification process known as refining crude castor oil is very necessary. This process sequentially includes degumming, neutralization, decolorization, and deodorization, until refined or pure castor oil is obtained. Furthermore, the neutralization process with 30% (w/v) Na2CO3 solution has the ability to neutralize free fatty acids, which is characterized by the value of castor oil acid number <1 9) , 10) .
Castor oil is unique among other vegetable extracts due to containing a hydroxylated fatty compound, namely ricinoleic acid (Fig. 1). This compound also known as cis-12-hydroxy-9-octadecenoic acid or 12-hydroxy oleic acid is an unsaturated fatty concentration, which has a hydroxy group at the C12 atom.

Ricinoleic acid.
The oils reported to be rich in ricinoleic acid with levels of 89-92% 1) , 2) , 8) , 9) , 11) , 12) , 13) , 14) , 15) , 16) , while the other 10% are formed by other monofunctional fatty concentrations 17) . Therefore, castor oil triacylglycerols are 3 molecules of ricinoleic acid, which are bonded as an ester with 1 molecule of glycerol 2) , 18) .
The ricinoleic acid in castor oil contains considerable benefits, especially in the pharmaceutical, oleochemical, cosmetic, medicine, biodegradable polymers, lubricants, coatings, adhesives, and nanoparticle synthesis capping agent industries, respectively 15) , 19) , 20) , 21) , 22) , 23) . Furthermore, the chemical structure of this acid (Fig. 1) shows that it has 3 important functional classes, which includes carboxylic (-COOH), alkene (-C=C-), and hydroxyl (-OH) groups, respectively. These groups are the chemical reactivity positions in ricinoleic acid. Therefore, this compound is able to undergo various rational organic chemical reactions, in order to produce several derivative constituents 24) .
The presence of the -OH group allows ricinoleic acid to undergo dehydration, caustic fusion, pyrolysis, halogenations, alkoxylation, sulfation, and urethane reactions, in order to produce dehydrated, halogenated, alkoxylated, and sulfated castor oils, phosphate ester, sebacic acid and capryl alcohol, urethane polymer, as well as undecylenic acid and heptaldehyde, respectively. Furthermore, the presence of the -C=C- functional group in this compound is a potential for oxidation and polymerization, hydrogenation, epoxidation, halogenations, addition, and sulfonation, in order to produce hydroxy stearate, as well as epoxidized, sulfonated, and polymerized oils, respectively 11) , 15) , 25) . Another potential is the –COOH group of ricinoleic acid, which allows the esterification of 9,10-dihydroxy-9-acyloxystearate with octanol, towards producing octyl 9,10-dihydroxy-9-acyloxystearate as a bio lubricant 12) . This esterification process also produces estolides.
Ricinoleic acid is mainly used as an intermediate in the synthesis of various oleochemical products, such as methyl ricinoleate, ricinoleic alcohol, as well as azelaic and ricinenic acids, which are useful as plasticizers and emulsifiers. Other derivatives of this compound in the cosmetic world are useful as detergent formulations, amphoteric surfactants, hair and skin conditioners, as well as antistatic and softening agents 20) . Based on the pharmaceutical field, it is converted into Ricinoleic-Lactic Acid Copolyester as drug carriers 19) . Furthermore, ricinoleic acid is important in the medical field, due to its ability as bactericidal, anti-inflammatory, and antiherpetic agents 21) . Therefore, this compound is an important hydroxylated fatty acid in the oleochemical, cosmetic, pharmaceutical, and agricultural industries, based on these reviews 24) .
Besides the beneficial capability of ricinoleic acid, it is still found to portray a problem, due to being bound as an ester in castor oil triacylglycerols. These triacylglycerols also contain other minor fatty compounds, such as linoleic (4%), oleic (3%), stearic (1%), and linolenic (<1%) acids, respectively 26) . Furthermore, castor oil contains other components such as carotenoids, phenolics, phospholipids, phytochemicals, phytosterols, tocopherols, and tocotrienols 8) . Therefore, ricinoleic acid should be isolated from castor oil before further use. This review specifically explains several approaches related to the reaction pathway,based on the preparation of ricinoleic acid from castor oil.
The availability of pure ricinoleic acid makes the explanation of the methods to isolate ricinoleic acid from castor oil giving an interesting topic. This section explains various methods that have been successfully developed in the preparation of this compound (Fig. 2). One of the chemical reactions related to the preparation process is known as complete hydrolysis, which involves the breakdown of a substrate with a water molecule.
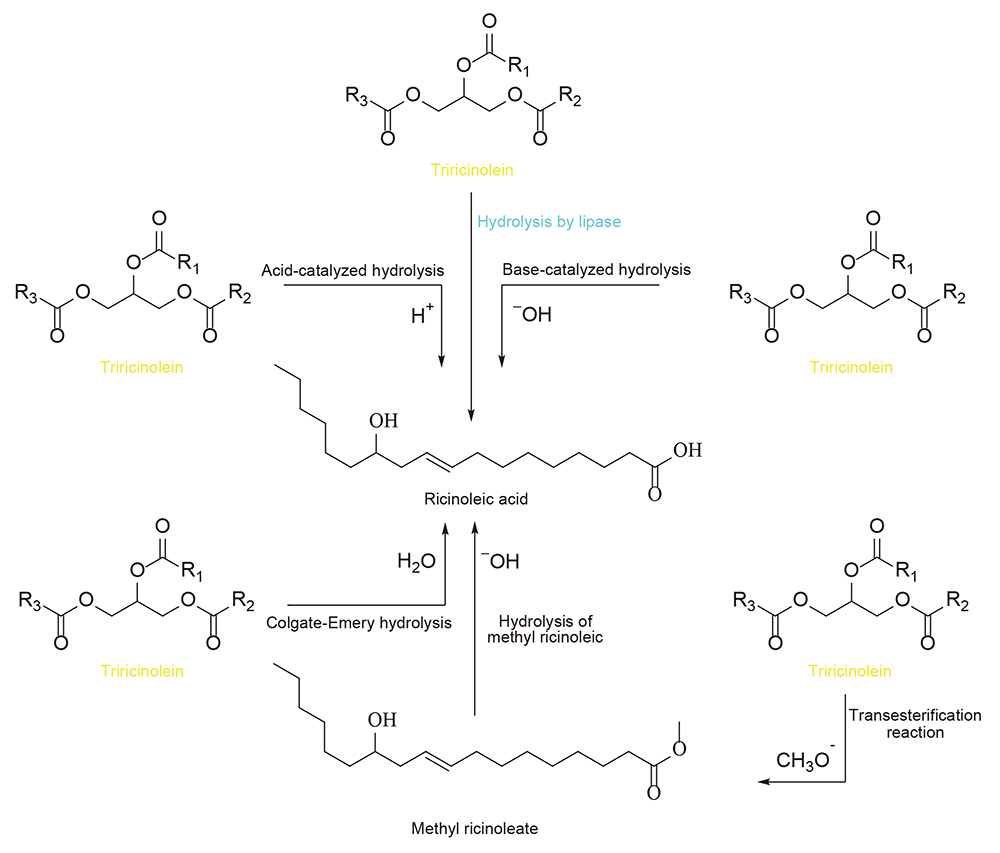
Schematic of some methods for the hydrolysis reaction of castrol oil.
Based on the type of substrate, ricinoleic acid as a carboxylic compound is obtained from an ester constituent through 2 reaction approaches, including,
1. direct hydrolysis of triesters, i.e. castor oil triacylglycerols
2. ester hydrolysis of castor oil transesterification product, namely methyl ricinoleate
Based on direct hydrolysis, the utilized substrate was a triacylglycerols obtained from castor oil. The castor oil triacylglycerols were considered as triesters of ricinoleic acid and glycerol, due to being bound with the acidic compound in large quantities. Therefore, ricinoleic acid was isolated through the hydrolysis reaction of castor oil triacylglycerols 20) , 21) , 27) , with glycerol as a by-product. Furthermore, the base-catalyzed hydrolysis reaction of methyl ricinoleate compounds (esters of ricinoleic acid) produced pure ricinoleic acid. The compound was obtained through the transesterification reaction of castor oil with methanol, through the use of an alkaline catalyst 9) .
Based on using triacylglycerols substrate from castor oil, several methods of ricinoleic acid preparation are described as follows,
2.1 Twitchell processThis process is a conventional method of castor oil hydrolysis, as the feature involves the use of Twitchell reagent, which occurred in batch mode. Twitchell reagent is a lipophilic sulphonic acid (alkyl aryl sulfonated) that is prepared from a variety of fatty (oleic acid, stearic acid, and palmitic acid) and sulfuric compounds, as well as aromatic hydrocarbons (benzene, phenol, naphthalene, m-xylene). This is due to both sulfonic groups and fatty acids being bound as substituents on the aromatic hydrocarbons, as shown in Fig. 3. The sulfonic groups allowed the reagents to function as acidic catalysts with weaker catalytic activity, compared to the sulfuric compound.

Twitchell Reagent 46) .
The combination of lipophilic alkyl and aryl groups with hydrophilic sulfonate classes, allowed Twitchell reagent to function as emulsifiers. Therefore, this reagent was able to unite castor oil and water, as the hydrolytic process became more effective to produce ricinoleic acid.
Based on the hydrolytic process, castor oil, water (50%), Twitchell reagent (0.75-1.25%), and sulfuric acid (1-2%) were heated in a wooden container (an acid-resistant tank), by using exposed steam at atmospheric pressure for 36-48 h 15) . Furthermore, the reagent enabled the conversion rate of catalyzed hydrolysis to reach 93%, at a reaction time of 6 h. Incrementing the amount of the reagent also increased the hydrolytic conversion rate of castor oil to 98%. This increase ensured the effective union of castor oil and water reactants with different phases, as the reaction process and conversion rate became faster and higher, respectively. Each process produced a by-product in the form of glycerol, which is soluble in water.
The hydrolysis of castor oil through the Twitchell process was classified as an acid-catalyzed direct reaction, by using an emulsifier from the reagent. According to Parekh et al. 20) , the hydrolysis of an ester including triacylglycerols from castor oil using an acid catalyst, was a reversible reaction that occurred in the SN1 pathway, which used H2O as a nucleophile.
The reaction mechanism for the hydrolysis of triacylglycerols from castor oil with an acid catalyst (Fig. 4), is described as follows,

Acid-catalyzed hydrolysis of castor oil Acid-catalyzed hydrolysis of castor oil: (a) Hydrolisis of Tricinolein to Diricinolein, (b) Hydrolysis of Diricinolein to Monoricinolein, and (c) Hydrolysis of Monoricinolein to glycerol.
A. Protonation of each oxygen from the carbonyl group, by using H+ ions from the sulfuric acid catalyst or Lipophilic Sulfonic Acid. This protonation enabled the carbon atoms in the carbonyl group to be more positive for attack by weak nucleophiles, such as H2O.
B. The nucleophilic attack of H2O by a lone pair of electrons on each of the electrophilic C atoms of the carbonyl group, formed a tetrahedral intermediate containing oxonium ion.
C. The deprotonation of the oxonium ion in the tetrahedral intermediate by a water molecule produced a neutral molecule that still contained the alkoxy group of glycerol.
D. Protonation of each alkoxy oxygen from the glycerol skeleton by H+ ions.
E. The donation of lone pair electrons from each hydroxyl group formed a bond π on the carbonyl classes. This encouraged the release of glycerol as a leaving group, while forming 3 molecules of intermediate, namely ricinoleic acid, which was protonated on the carbonyl oxygen. Therefore, this was the step involved in the breakdown of castor oil triacylglycerols molecules, in order to produce glycerol and candidate ricinoleic acid.
F. H2O attracted H+ ions from each protonated oxygen in the intermediate, based on forming 3 molecules of ricinoleic acid and Oxonium ions. The deprotonation of this ion also regenerated an acidic catalyst, as 1 molecule of castor oil acid triacylglycerols produced 3 ricinoleic acid and 1 glycerol products, respectively.
The hydrolysis of castor oil by the Twitchell process had a disadvantage, due to forming a by-product, namely estolide. This formation interfered with the hydrolytic reaction, due to reducing the solubility of water and oil, therefore, taking a long time to reach equilibrium 28) .
2.2 Colgate-Emery processThis process commercially has an important role in the production of fatty acids from vegetable oil and fat 10) . In fact, it is a method for breaking vegetable oil triacylglycerols or fats into fatty acids and glycerol, by using water vapour at high temperatures and pressures, in a continuous process. The reaction temperature ranges from 150-350°C, while the pressure reaches 60 bar.
The hydrolysis of castor oil through the pure Colgate-Emery process used a reagent in the form of water vapour, without a catalyst. Based on being conducted at high temperatures and pressures, this hydrolytic pathway is known as high temperature-pressure splitting castor oil. The literature search showed that this process was reported by Lakshminarayana et al. 29) . Furthermore, the reactor in the continuous utilized process was a steel autoclave with a capacity of 2 L. Based on controlling the path of the reaction, the autoclave was equipped with a heating jacket, stirrer, shut-off valve, pressure and temperature gauges, as well as sampling devices. The hydrolytic process was declared to have started, when the system in the autoclave began to reach the predetermined pressure and reaction temperature. Some of the optimized parameters were the ratio variation of oil and water weights, as well as the reaction time, pressure, and temperature, at 1:0.4-1:1, 1-10 h, 20-40 kg/cm2, and 213-250°C, respectively. The highest conversion rate of the process at 92% was achieved when the ratio variation of oil and water weights, time, pressure, and temperature were 1:0.8, 8 h, 40 kg/cm2, and 250°C, respectively. Furthermore, the preparation procedure of the final product (Ricinoleic Acid) was carried out by extraction through diethyl ether as a solvent, while the by-product (glycerol) was separated in the washing process of the organic phase with water. Based on this research, the hydrolytic reaction of castor oil was monitored by determining the parameters of acidic, hydroxyl, and saponification numbers.
The reaction mechanism of the Colgate-Emery process (Fig. 5) is explained as follows,

Hydrolysis of castor oil by Colgate-Emery: (a) conversion of Triricinolein to Diricinoleion, (b) conversion of Diricinolein to Monoricinolein, and (c) conversion of Monoricinolein to glycerol.
A. Based on being at high temperature and pressure, 1 molecule of water vapor cleaved one of the ester groups of castor oil triacylglycerols, namely triricinolein, in order to form ricinoleic acid and a diacylglycerol product. This stage had an increase in the value of the acidic and hydroxyl numbers, based on the monitoring results of the reaction products.
B. Based on the second step, the water vapour continued to cleave another ester group on the diacylglycerol, in order to yield a different molecule of ricinoleic acid and a monoacylglycerol, which continued to increase the value of the acidic and hydroxyl numbers. Furthermore, the presence of diacylglycerols and monoacylglycerols continued to increase the value of the hydroxyl number. However, the potential for an esterification reaction of a carboxylic acid with a monoacylglycerol was reported, in order to form a by-product compound, namely estolide. The possibility of this by-product reaction was further monitored by determining the saponification number.
C. Based on the third step, the water vapour broke the ester group on the monoacylglycerol, in order to produce ricinoleic acid, as a significant increase and decrease in the values of acidic and hydroxyl numbers were detected, respectively. This was due to an increase and a decrease in the formation of ricinoleic acid and values of monoacylglycerols and triacylglycerols, respectively. Therefore, estolides were formed at this stage.
The higher the temperature, pressure, and time, the quicker the occurrence of the hydrolytic reaction of Castrol oil, as the product of ricinoleic acid increases. However, these conditions triggered an esterification reaction between ricinoleic acid and the -OH group in the hydrolytic intermediates (diacylglycerols and monoacylglycerols), in order to form estolides. Another disadvantage of the Colgate-Emery process was the waste of energy, due to the utilized high temperatures and difficult handling of the reaction. This was due to the occurrence in a reactor that required high level of safety.
2.3 Base catalyzed hydrolysisAnother method used in isolating ricinoleic acid from castor oil is through an alkaline catalyzed hydrolysis reaction, with the base catalyst being KOH 30) , 31) and NaOH 15) . This process occurred under reflux, at a temperature range of 70-100°C.
Vaisman et al. 30) carried out the preparation process of ricinoleic acid from castor oil, by using a KOH solution as catalyst in ethanol solvent. When the triacylglycerol were considered to be tririsinolein with the molecular formula and weight of C57O9H101 and 929 g/mol, the mole ratio of castor oil to the utilized KOH was 1:4. Furthermore, the reaction was confirmed to have occurred at the boiling point of ethanol (78°C) for 1 h. Based on the evaporation of the ethanol solvent, the hydrolytic product was acidified with concentrated HCl to pH=1. According to the research of Anwar and Wahyuningsih 31) , that acidified the hydrolytic product of castor oil with H2SO4, acidification was reported to neutralize KOH by converting it into a salt, which was dissolved in the water phase. Furthermore, the ethyl acetate solvent was used to dissolve and separate the isolated ricinoleic acid and glycerol, respectively, i.e., dissolved in the aqueous phase (reverse phase). The remaining water in the organic phase was dried with anhydrous MgSO4, while the decolorization process of the ricinoleic acid product was carried out with Vaisman et al. 30) , also reported a method of purification, namely the salting-out approach (SOA), based on the utilization of monobasic alkali salt of ricinoleic acid. Fractional precipitation also contained isopropyl ether:ethanol (IPE:EtOH) 65:35 v/v, which was applied at a CO:co-solvent system mass:volume ratio of 1:5 that produced a high quality purified ricinoleic acid (purity 97.9-98.6%).
Based on further examination, the preparation of ricinoleic acid from castor oil was a simple reaction, which was carried out using an alkaline catalyst. This was due to occurrence of the reaction at low temperatures (<100°C), through the use of a KOH catalyst that was quite inexpensive. The time was also fast, as the reaction produced ricinoleic acid with a high conversion rate and purity (87.50-88.10%). However, the alkaline catalyst process had several drawbacks as follow,
A. It produced ricinoleic acid with a distinctive color and odor,
B. The neutralization reaction produced a large amount of salt, which was discharged into the environment as waste
The hydrolysis of castor oil with a base catalyst followed the SN2 reaction mechanism, which irreversibly occurred by using a nucleophile from hydroxyl ions. This ion produced from the KOH or NaOH catalyst was a strong nucleophile, which directly attacked the electrofolic C atom of the carbonyl group, on the tririsinolein molecule.
The reaction mechanism for the hydrolysis of castor oil with an alkaline catalyst (Fig. 6) is explained as follows,

Hydrolysis of alkaline catalyzed castor oil from: (a) Triricinolein to Diricinolein, (b) Diricinolein to Monoricinolein, and (c) Monoricinolein to glycerol.
A. The three hydroxyl ions attacked each of the electrophilic C atoms on the triricinolein, which caused the breakdown of the pi bond and formation of a tetrahedral intermediate, with a negative charge on each carbonyl oxygen.
B. The donation of lone electron pairs from each oxygen in the tetrahedral intermediate re-formed the pi bond on the carbonyl group, while encouraging the release of the leaving class, which is an anion form of glycerol. This is referred to as the triricinolein breakdown step, in order to produce 3 molecules of ricinoleic acid and glycerol.
C. Rapid deprotonation of the carboxylic group of each ricinoleic acid also produced 3 ricinoleic acid anions and 1 glycerol molecule.
2.4 Base catalyzed hydrolysis of methyl ricinoleateThe process of isolating ricinoleic acid through hydrolysis of methyl ricinoleate includes 2 stages, namely,
A. Transesterification reaction of Castrol oil with methanol using an alkaline catalyst, in order to produce methyl ricinoleate,
B. Hydrolysis reaction of methyl ricinoleate with a base catalyst, towards the production of ricinoleic acid.
2.4.1 Castrol oil transesterification reaction
Based on the transesterification reaction, triacylglycerols from castor oil were lysed with methanol and alkaline catalysts, such as sodium methoxide 9) , 32) , NaOH 33) , 34) , and KOH 35) , 36) , in order to produce methyl ricinoleate. This reaction often occurred easily at a temperature <100°C, with a time span of 1-3 h. Furthermore, the methyl ricinoleate product is a yellowish liquid with a lower viscosity than castor oil, and was isolated using an organic solvent such as n-hexane, petroleum ether, and ethyl acetate.
Based on the analytical results obtained by GC-MS, the purity and yield of methyl ricinoleate from several previous research was 86.99-100% and 68.70-93.32%, respectively. Neeharika et al. 33) showed that methyl ricinoleate was produced with 100% purity, when isolated through the use of a liquid-liquid extraction technique, with a combination of organic medium and aqueous polar solvents, respectively. Also, the base catalyst was neutralized with a strong acid. Based on this condition, both salt and glycerol were separated within the distilled water and disposed. This was the disadvantage of synthesizing methyl ricinoleate from castor oil using a homogeneous base catalyst, as these reactants were not reused and disposed off as waste into the environment. Meanwhile, the transesterification of castor oil to produce methyl ricinoleate was catalyzed by using heterogeneous base catalysts and lipase enzymes. Based on the use of sodium methoxide as a catalyst, the methyl ricinoleate produced from the reaction was 86.10% 9) . When the catalyst was replaced with NaOH, the purity and yield of the product were 96.26 and 95.38%, respectively. Based on this condition, 20% methanol and 1.5% NaOH were used at a temperature and time of 30°C and 90 mins, respectively.
The conversion reaction into a highly purified methyl ricinoleate was caused by the purity of castor oil as a reactant 9) . Based on this condition, transesterification occurred in the triacylglycerols, as several impurities such as free fatty acids were highly removed, in order not to interfere with the reaction. Furthermore, castor oil was initially purified by neutralization, through the use of a weak base catalyst such as Na2CO3, before being used in a transesterification reaction 10) , 37) . This neutralization process removed free fatty acids in castor oil, by converting them into salts. However, the approach did not degrade triacylglycerols. Besides free fatty acids, several other impurities such as sterols and phospholipids, were separated from castor oil through neutralization. Furthermore, high purity methyl ricinoleate were hydrolyzed with a strong base catalyst, in order to obtain ricinoleic acid. The reaction mechanism for the transesterification process is shown in Fig. 6.
2.4.2 Hydrolysis of methyl ricinoleate
Methyl ricinoleate is an ester compound being hydrolyzed to ricinoleic acid. When the transesterification process produced methyl ricinoleate with 100% purity 33) , it was found to be very advantageous in the hydrolytic reaction. These compounds were also hydrolyzed by using alkaline catalysts or lipase enzymes. Musphianti et al. 32) carried out hydrolysis of methyl ricinoleate by using a NaOH catalyst with a yield of 93.44%. This reaction was carried out for 2 h in ethanol solvent, with the ratio of methyl ricinoleate to NaOH being 1:2. Furthermore, ricinoleic acid was isolated in diethyl ether solvent, after the acidification process to pH=2. The data from IR and H NMR spectra showed that the compound was successfully isolated from methyl ricinoleate. Several studies related to the hydrolysis of methyl ricinoleate using Candida Antarctica Lipase B, were carried out through varying reaction temperature (40-60°C) and enzyme concentration (2-5%) 33) . The conversion rate of the reaction at 98.5% was achieved at a time, temperature, buffer to methyl ricinoleate ratio, and enzyme concentration of 6 h, 60°C, 2:1 (v/w), and 4%, respectively.
The transesterification and base hydrolysis reaction of methyl ricinoleate was conclusively a quite effective pathway, in order to produce ricinoleic acid with high yield and purity. Several advantages of this pathway are as follow,
A. Avoiding the formation of by-products such as estolides, which were produced during direct hydrolysis,
B. Avoiding the use of high temperatures and pressures, as observed in the Colgate-Emery process,
C. There was an alternative to the use of a green catalyst from Lipase, both at the transesterification and hydrolytic stages.
However, this reaction pathway also had disadvantages as follow,
A. The reaction occurred in 2 stages, therefore making it less efficient
B. When used with inorganic base catalysts, such as sodium methoxide, NaOH, and KOH, wastes were deposited into the environment
The reaction mechanism of the base-catalyzed hydrolysis of methyl ricinoleate was also found to support that of the alkaline-catalyzed process. Based on this condition, castor oil triacylglycerols was replaced with methyl ricinoleate. The mechanism of the methyl ricinoleate-catalyzed hydrolysis reaction is shown in Fig. 7.

Castor oil transesterification reaction of (a) Triricinolein, (b) Diriconelin, and (c) Monoricinolein.
Enzymatic hydrolysis is an interesting part of this presently developed research, which included the development of a reaction pathway for the preparation of ricinoleic acid. The use of toxic chemical solutions was one of the reasons the hydrolytic reaction of castor oil led to an enzymatic process. Also, lipases were often used during these reactions. Safe conditions were also an advantage of this process, although it was known to have high selectivity region. Furthermore, Table 1 provided information on several studies, which were related to the use of lipase as a catalyst, in the hydrolytic reaction of castor oil.
The extraction of lipase derived from Ground Oat seed (Avena sativa L.) using organic solvents, was previously reported by Piazza and Farrell 38) . Furthermore, the results showed that the castor oil concentration was proportional to that of the solvent, even at low levels producing good lipolysis. Meanwhile, the effects of additives such as solvents and salt solutions, were studied by Rathod and Pandit 39) . Aspergillus oryzae used as a source of lipase also showed that the solvent effect was not significant on the reaction. The presence of isooctane solvent was also able to increase emulsification, as the enzymatic interaction and hydrolytic reaction with the substrate were closer and more effective than acetone and methanol, respectively. Additionally, the negative effect on the reaction was indicated by the presence of a salt solution. This was confirmed by the decrease in the value (%) of hydrolysis, when the concentration of the salt solution increased.
Gamayurova et al. 21) also succeeded in using candida rugosa in the hydrolytic reaction of castor oil. The optimum conditions obtained were the enzymatic load of 1 mg/mL with an oil-water ratio of 40:6, as the reaction lasted for 16 h at 45°C, while also producing a conversion of 47%. Further studies involving the use of Candida rugosa were carried out by Zhao et al. 40) Glutaraldehyde immobilization was also performed on Candida rugosa, which continued with the activation process and Fe2O3-chitosan. The results showed that the enzymatic concentration and water/oil ratio were 3.27% and 1.60:1, as the reaction pH was in neutral conditions that produced a fairly good conversion of 46.81%.
The hydrolytic reaction carried out through the green and simple method was also found to be continuously developing. Sun and Guo 41) successfully used Lipozyme TL IM as a biocatalyst, during the hydrolysis of castor oil. Furthermore, the optimum conditions achieved at the enzymatic concentration, water/oil ratio, and temperature of 8.9%, 40:1, and 41.3°C, were confirmed to provide a satisfactory conversion of 96.2±1.5%, respectively. The use of Rhizopus oryzae as a biocatalyst was also confirmed to provide satisfactory results 42) . This hydrolytic reaction lasted for 12 h at 37°C, as the oil-water ratio of 1:4 produced a conversion rate at approximately 90%.
Bose et al. 43) also succeeded in using porcine pancreatic lipase as a catalyst, in the hydrolysis of castor oil. Based on this reaction, approximately 4 mg of enzyme per gram of oil in pH 8 provided a conversion of 52% in 2.5 h. The use of an ionic solution, [BMIM] [PF6], was also shown to increase the activity of lipase. Furthermore, the modification of porcine pancreatic lipase after being cross-linked and entrapped with glutaraldehyde and calcium alginate, showed an increase in conversion, respectively 44) . The use of Span 80 as a nonionic surfactant was also confirmed to affect lipase activity by 3.7 fold, compared to a reaction without the catalyst. Therefore, the presence of this surfactant formed a good emulsion, as the interactions of enzyme and substrate were more effective.
Lipozyme TL IM was the cheapest lipase produced by Novozym, having high activity in the modification of fats and oils, as well as acting as a green catalyst 45) . Lipozyme TL IM is the lipase of immobilized Thermomyces laguginosus (TLL) on silica solid through the ionic adsorption process 47) . In oil and lipid modification, Lipozym TL IM is the most effective in the catalytic reaction of Castrol oil hydrolysis to produce ricinoleic acid 48) . The optimum condition of catalytic reaction achieves at pH 8 and temperature 27°C. These data show that Lipozym TL IM is an alternative catalyst and excellent in oil and lipid modification both laboratorium and industry scale. Some of the advantages of using Lipozym TL IM in the process of preparing ricinoleic acid are,
A. It has very high catalytic activity and selectivity in the modification of fats and oils,
B. Cheap,
C. Reusable
D. The reaction occurs at a low temperature of 41.3°C,
The hydrolysis reaction of Castrol oil involves water and oil interaction. The mixture between water and oil produces surface tension. It could be challenging when the reaction is carried out. One solution executed is using a surfactant. The presence of surfactant reduces surface tension and permits interaction between hydrophobic face and hydrophobic residues of Lipozym TL IM. This interaction stimulates the active site of the enzyme moving to the reaction medium, called interfacial activation. It makes the process catalytic more effective 45) . In addition, this article reported that TLL is the single chain of proteins containing alpha helix. It has an actives site of amino acid residues such as serine, histidine, and aspartic acid. These amino acid residues allow the role and some interaction of functional group in the hydrolysis reaction that is a hydroxyl group of serine and a carboxyl group of histidine and aspartic acid. Also, proton donating of imidazole ring in aspartic acid can breakdown ester bond in triacylglycerols molecules such as the conversion of triricinolein to diricinolein, monoricinolein, glycerol, and also ricinoleic acid as main product (Fig. 8).

Mechanism of hydrolysis reaction of methyl ricinoleate.

Mechanism of hydrolysis reaction of Triricinolein using TLL 45) .
Castor (Ricinus communis L) was a renewable resource with wide roles and benefits in various fields of life. Furthermore, castor oil was extracted from this plant species (Ricinus communis L), and contained ricinoleic acid at a fairly high level of 89-92%. This acid was known as a hydroxylated fatty acid with several functional groups, such as -COOH, -OH, and -C=C. The presence of these groups allowed ricinoleic acid to be converted into various derivative compounds with several applications in the cosmetics, pharmaceuticals, and oleochemical industries. Therefore, the purification of ricinoleic acid became an urgent matter to be developed. Furthermore, various published data that were reviewed showed that the preparation of ricinoleic acid from castor oil was carried out through several methods, such as chemical, physical and enzymatic processes. Based on the chemical processes, this preparation was carried out through the Twitchell method, as well as alkaline catalyzed, transesterification, and methyl ricinoleate hydrolysis, respectively. Although several methods were able to produce ricinoleic acid with high purity and yield, the chemical preparation of the compound still produced residual catalysts and by-product (estolides) as wastes to the environment. This acid was also physically isolated through the Colgate-Emery process, which produced high purity and yield. However, this process occurred at high temperatures and pressures. Additionally, the use of lipase enzymes in the preparation of ricinoleic acid was a promising alternative, due to the use of a green process approach. The hydrolysis of castor oil using lipase enzyme type, lipozyme TL IM, also produced the acidic compound with a conversion rate of 96.2±1.5%. Based on being readily available and inexpensive with high catalytic activity, this enzyme (Lipozyme TL IM) is an effective choice for the hydrolysis of castor oil, in order to produce ricinoleic acid with high purity and yield.
Authors acknowledge to Kementerian Pendidikan dan Kebudayaan Republik Indonesia for research grant in 2021.