ABSTRACT
Food intake affects poultry productivity. A complete understanding of these regulatory mechanisms provides new strategies to improve productivity. Food intake is regulated by complex mechanisms involving many factors, including the central nervous system, gastrointestinal tract, hormones, and nutrients. Although several studies have been conducted to elucidate regulatory mechanisms in chickens, the mechanisms remain unclear. To update the current knowledge on feeding regulation in chickens, this review focuses on recent findings that have not been summarized in previous reviews, including spexins, adipokines, neurosecretory proteins GL and GM, and central intracellular signaling factors.
INTRODUCTION
Food intake affects poultry productivity. For example, early post-hatch fasting inhibits skeletal muscle growth, resulting in lower body weight during the finishing period compared to that of chicks fed earlier[1,2,3]. Delaying post-hatching feeding impairs meat and egg quality in chickens[4,5]. Therefore, in addition to the early initiation of feeding, neonatal chicks that start voluntary food intake early are preferable in the chicken industry. However, broiler chickens genetically selected for rapid growth and meat yield do not adequately regulate their voluntary food intake to meet their energy requirements[6]. Broiler chickens eat more than twice as much feed as layer chickens from 2 days of age[7]. Consequently, overconsumption of feed causes excessive accumulation of abdominal fat, which is deemed an animal byproduct or waste[6,8]. Restricted feeding is a method used to control food intake; however, it also causes stress in broiler chickens. Collectively, these data highlight the need to develop a method to control voluntary food intake in chickens. However, the mechanisms underlying initiation of food intake and overfeeding in chickens remain unclear.
Food intake is regulated by several factors, including the central nervous system, gastrointestinal tract, hormones, and nutrients[9,10,11]. Regulatory mechanisms are complicated, but fundamentally similar between vertebrates. However, several factors have different effects on the food intake of chickens and other vertebrates. For example, ghrelin, an orexigenic hormone found in mammals, inhibits food intake in chickens[12], whereas leptin, an anorexigenic hormone in mammals, is not involved in feeding regulation in chickens[13,14]. As it is not always possible to extrapolate the findings of mammalian studies to chickens, studies on feeding regulation in chickens are required. To date, several studies have reviewed feeding regulation in chickens[15,16,17,18,19,20,21,22,23,24] and many of these refer to neuropeptides and gut hormones. In mammals, adipokines and cellular signaling pathways have been known to be involved in feeding regulation for two decades[9,25,26,27,28,29,30,31]. Similar studies have recently been reported in chickens. Moreover, novel food regulation factors, such as spexins and neurosecretory proteins have been reported in chickens.
To update the current knowledge of feeding regulation in chickens, this review focuses on current information on spexins, adipokines, neurosecretory proteins, and cellular signaling pathways involved in the regulation of food intake in chicken. Additionally, it suggests future perspectives for research in chicks.
SPEXIN
Spexin (SPX), also known as neuropeptide Q, was discovered in 2007 using a bioinformatics search tool[32]. Later, its paralog, SPX2, was identified in non-mammals, including chickens, using genome BLAST search information; consequently, the original SPX was identified as SPX1[33]. Phylogenetic and synteny analyses have revealed that the SPX family is closest to the galanin (GAL) family, followed by the kisspeptin family[33]. The mature SPX1 peptide is composed of 14 amino acids and is evolutionarily conserved across vertebrates; it is identical in humans, mice, and chickens[34]. The mature peptide of SPX2 also contains 14 amino acids; however, its sequence differs from that of SPX1 at four positions[33]. Luciferase reporter assays have demonstrated that SPXs activate GAL receptors, GALR 2 and GALR3, but not GALR1 in humans, zebrafish[33], and chickens[35,36]. Interestingly, the potency of SPXs against GALR2 and GALR3 is similar to or greater than those of GAL in humans and zebrafish[33]. In contrast, the potency of SPX1 on GALR2 is lower than that on GAL in chickens, whereas the potency on GALR3 is greater than that on GAL[36]. In addition, of the five GALR subtypes, chicken SPXs activate the GALR2-like receptor (GALR2L) to the greatest extent. These findings indicate that SPXs are endogenous ligands of GALR2 (likely GALR2L in chickens) and GALR3 in vertebrates.
SPX mRNA is widely distributed in the central nervous system and peripheral tissues of chickens[34,35,36]. Fasting significantly increases the plasma SPX concentration[34] and hypothalamic mRNA levels of SPXs in chickens[35,36]. Generally, fasting upregulates orexigenic factors and downregulates anorexigenic factors; for example, the expressions of orexigenic neuropeptides such as neuropeptide Y (NPY) and agouti-related peptide (AgRP) are significantly increased by fasting in chicken hypothalamus[37,38], whereas fasting significantly decreases those of proopiomelanocortin (POMC, the precursor of α-melanocyte stimulating hormone (α-MSH), an anorexigenic peptide) in the hypothalamus and gut hormone such as cholecystokinin (CCK) and peptide YY in chicken gastrointestinal tract[37,38,39,40]. However, intracerebroventricular (ICV) and intravenous injections of SPXs significantly decrease food intake in a dose-dependent manner in chicks[35,36,41], in contrast to GAL, an orexigenic peptide in vertebrates[24]. The decrease following ICV injection of SPX is attenuated by the co-injection of GALR3 antagonists, but not GALR2 antagonists, suggesting that SPX inhibits food intake via GALR3 in chicks[41]. Furthermore, SPXs control food intake via the gene expression of appetite-regulating neuropeptides in the hypothalamus of chicks[35,36]. Intravenous injection of SPXs increases the mRNA levels of cocaine- and amphetamine-regulated transcript (CART, an anorexigenic neuropeptide) in the hypothalamus of chicks, whereas those of AgRP decrease after injection, although no significant change is observed in the levels of NPY and POMC[35,36]. These findings, including those from the luciferase reporter assay[35,36], suggest that SPXs act in an endocrine manner to inhibit food intake by regulating hypothalamic AgRP and CART via GALR2L and GALR3 in chicks.
In mammals, SPX exerts various functions, such as inhibiting food intake, lipid absorption, reducing body weight, and improving insulin resistance[42]. However, there is no information regarding the function of SPXs in chickens other than feeding regulation. Similar to SPXs, GALR2 and GALR3 are widely expressed in peripheral tissues[34], suggesting that SPXs perform various functions in chickens and mammals. The expression of SPX and GALRs is regulated in a tissue-specific manner in chickens[34]. For example, SPX1 mRNA levels in the liver are significantly increased by fasting, whereas they are significantly decreased in adipose tissue and breast muscle[34]. Similarly, fasting significantly increases hepatic GALR2 mRNA levels, but decreases GALR3 mRNA levels in breast muscle[34]. These results raise the possibility that SPXs act in both an endocrine and autocrine/paracrine manners in chickens. Further in vitro studies using cells derived from peripheral tissues are required to elucidate the role of SPX in chickens.
ADIPOKINES
“Adipokine” is a generic term for bioactive polypeptide secreted by adipose tissue. Several studies have elucidated the physiological roles of adipokines in appetite regulation, glucose and lipid metabolism, inflammation, and blood pressure control, particularly in mammals[25,26,27]. Several adipokines, such as apelin, leptin, nesfatin, and vaspin reduce food intake in mammals[25,26,27]. A few studies have reported the effects of adipokines on food intake in chickens.
LeptinBungo et al. (1999) have reported that ICV injection of murine leptin does not affect food intake in chicks[43]. However, studies using mammalian leptin have yielded conflicting opinions[43,44,45,46,47]. In 2016, the chicken leptin mRNA sequence was identified[13] and it shares approximately 25% identity with mammalian amino acids[13]. In contrast to its almost exclusive expression in mammalian adipose tissue, leptin expression is barely detectable in chicken adipose tissue, providing strong evidence that leptin is not an important adipokine in chickens[13]. Although leptin receptors are expressed in the hypothalamus of chickens, they are predominantly expressed in the pituitary gland[13]. The following year, ICV injection of a synthetic chicken partial leptin peptide, whose sequence corresponds to region 22-56 of human leptin, which inhibits feeding in rats when injected into the lateral ventricle, was reported to not affect food intake in chicks[14]. These findings suggest that leptin does not regulate food intake in chicks. However, further studies using full-length chicken leptin are required to confirm this hypothesis.
AdiponectinAdiponectin is the most abundant adipokine in the plasma and is known to regulate insulin sensitivity, atherosclerosis, glucose uptake, and lipid metabolism in mammals[25,48]. Adiponectin concentrations in the plasma and cerebrospinal fluid significantly decrease after 12 h of fasting followed by 3 h of refeeding[49]. However, the effects of adiponectin on food intake in rodents are conflicting, and this discrepancy may be attributed to different feeding conditions. Central injection of adiponectin inhibits and promotes food intake under fasting and feeding conditions, respectively[48]. Similar to mammals[48], adiponectin is highly expressed in chicken adipose tissues[50,51]. However, in contrast to mammals, the plasma concentration of adiponectin is significantly higher in chicks refed for 2 h after 10 h of fasting than in those fasted for 12 h[50]. ICV injection of mouse adiponectin, whose identity to chicken adiponectin is 60%[51], significantly increases food intake in a dose-dependent manner in chicks after fasting for 3 h[52]. Therefore, adiponectin is likely to regulate food intake in chicks in a direction opposite to that observed in mammals.
Other adipokinesIn contrast to the adipokines described above, chemerin and apelin-13 reduce food intake in chicks[53,54] and rodents[55,56]. Daily intraperitoneal injections of recombinant chicken chemerin significantly decrease the food intake of chicks[53]. Central injection of mammalian apelin-13, whose sequence is different from that of chicken only at the first residue of the N-terminus[57], significantly decreases food intake in chicks. Interestingly, the inhibitory effects on food intake appear relatively late in both rodents and chicks[53,54,55,56]; for example, ICV injection of apelin-13 significantly decreases food intake at 8 h post-injection in rat under ad libitum feeding condition[56] and at 4 h post-injection in chicks fasted for 3 h[54]. Therefore, these adipokines may not act directly on the central nervous system. Further studies are required to clarify the mechanisms by which these adipokines reduce food intake in chickens.
Interestingly, comparative omics analyses indicate more direct crosstalk between chicken visceral fat and the reproductive system and lower involvement in the regulation of appetite, inflammation, and insulin resistance[58]. In this study, RNA-seq analysis reveals that the most prominent adipokines in mammals, including leptin, tumor necrosis factor α, interferon-γ, and interleukin-6, are expressed at low levels in adipose tissue of both broiler and layer chickens[58]. However, several adipokines, such as adipolin, adiponectin, adipsin (complement factor D, CFD), retinol-binding protein 4, and visfatin (nicotinamide phosphoribosyl transferase, NAMPT, which has an orexigenic effect in chicks[59]), are expressed at much higher levels than those described above[58]. In addition, qPCR analyses have revealed that fasting significantly reduces the expression of adipolin and adiponectin in both types of chickens, and CFD in layer chickens, whereas NAMPT expression is significantly increased in layer chickens[58]. Therefore, these adipokines may regulate energy homeostasis, including appetite, in chickens.
NEUROSECRETORY PROTEINS GL AND GM
Neurosecretory protein GL (NPGL) has been identified in the arcuate nucleus, formerly known as the infundibular nucleus, of chicks using a cDNA subtractive screening method[60]. A subsequent search of the genome database suggested the presence of a paralogous NPGL, named neurosecretory protein GM (NPGM)[60,61]. Both proteins are conserved in vertebrates[60,61]. A previous study in mice showed that a single ICV injection of murine NPGL stimulates food intake and that NPGL expression in the mediobasal hypothalamus is upregulated during fasting[62]. Morphological analysis reveals that NPGL-immunoreactive fibers contact POMC neurons in the lateral part of the arcuate nucleus[62]. In rats, NPGL overexpression in the mediobasal hypothalamus increases daily food intake of normal chow and cumulative caloric intake in a high-calorie diet[63]. Similar to that in mice[62], fasting promotes NPGL expression in the mediobasal hypothalamus of rats[63] whereas intraperitoneal injection of insulin inhibits NPGL expression in the mediobasal hypothalamus of rats[63]. These findings suggest that hypothalamic NPGL regulates food intake via POMC neurons in response to peripheral insulin concentration.
In chicks, chronic ICV injection of chicken NPGL stimulates food intake[64]. However, no significant changes in NPGL expression are observed in the mediobasal hypothalamus after fasting[65]. In contrast, a single ICV injection of NPGM suppresses food intake in chicks[66] and fasting promotes NPGM expression in the mediobasal hypothalamus[65]. Therefore, it is likely that NPGM plays a physiologically important role in the regulation of feeding in chicks.
Interestingly, overexpression and chronic injection of NPGL induces fat accumulation in chicks[67], mice[68,69], and rats[63,70]. NPGL also alters the expression of genes involved in lipid metabolism of the white adipose tissue; however, the altered genes have been inconsistently identified. Further studies are required to clarify the mechanism by which hypothalamic NPGL promotes abdominal fat accumulation.
CELLULAR SIGNALING PATHWAYS
In the mammalian hypothalamus, cellular signaling pathways, such as insulin, protein kinase B (Akt), mammalian target of rapamycin, Janus kinase (JAK)/signal transducers and activators of transcription (STAT), and AMP-activated protein kinase (AMPK) contribute to the regulation of food intake[9,28,29,30,31]. These pathways are regulated by hormones (adiponectin, insulin, and leptin) and nutrients (glucose and leucine) and control the gene expression of appetite-regulating neuropeptides, such as NPY, AgRP, and POMC. In vitro studies using hypothalamic cell lines suggest that α-MSH phosphorylates cyclic AMP response element binding protein via extracellular signal-regulated kinase (ERK) activation[71,72]. In addition to the hypothalamus, the hindbrain plays an important role in feeding regulation because it contains the nucleus of the solitary tract, where the vagal afferent neurons that are activated by gastric distension and gut hormones terminate, and the area postrema, which receives vagal input and circulating signals[9,73,74,75]. Although few studies on the hindbrain have investigated the cellular signaling pathways involved in feeding regulation, ERK signaling in the dorsal vagal complex, which includes the area postrema, nucleus of the solitary tract, and dorsal motor nucleus of the vagus, plays a key role in CCK and insulin-induced inhibition of food intake[76,77,78,79,80]. A previous study in rats suggests that the activation of the glucagon-like peptide-1 receptor in the hindbrain suppresses food intake through protein kinase A-mediated suppression of AMPK and activation of ERK[81].
Recently, studies on chickens have suggested that central cellular signaling pathways are involved in feeding regulation. For example, fasting increases the AMPK phosphorylation rate in the chicken hypothalamus[82] and an AMPK inhibitor attenuates dexamethasone-induced hyperphagia in chicks[83]. These findings suggest that hypothalamic AMPK activation stimulates food intake in chicks, similar to mammals. ICV injection of recombinant human transforming growth factor-β1 induces hypothalamic Smad2 phosphorylation and suppression of food intake in chicks[84]. However, phosphorylated Smad2 is not detected under ad libitum feeding, fasting, and refeeding conditions in the hypothalamus of chicks[84]. These findings suggest that hypothalamic Smad activation suppresses food intake in chicks, but may not occur in response to feeding conditions[84]. A recent study using a synthetic inhibitor suggests that JAK2/3 stimulates food intake in chicks[85]. However, it remains unclear whether hypothalamic JAK2/3 and its downstream factor, STAT, respond to feeding conditions and hormones in chickens. Further studies are required to clarify the physiological roles of hypothalamic JAK/STAT signaling in the regulation of feeding in chickens.
Interestingly, previous studies have shown that the central cellular signaling factors respond differently to feeding conditions and hormones in broiler and layer chicks. ICV injection of porcine insulin and refeeding promotes hypothalamic Akt phosphorylation in both broiler and layer chicks and promotes Forkhead box O1 (FOXO1) phosphorylation in layer chicks, but not in broiler chicks[86,87]. FOXO1 is phosphorylated by Akt, which induces the nuclear export of FOXO1 and thus abrogates FOXO1-mediated inhibition of POMC expression[28]. Furthermore, the ICV injection of insulin increases POMC mRNA levels in the hypothalamus of layer chicks, but not in broiler chicks[87]. ICV injection of insulin and refeeding lead to the phosphorylation of both Akt and ERK in the medulla oblongata of layer chicks[88], and the phosphorylation of Akt, but not ERK, in broiler chicks (unpublished data). These differences between broiler and layer chicks may be one of the causes of the differences in food intake.
CONCLUSIONS AND FUTURE PERSPECTIVES
Several studies have elucidated regulatory mechanisms underlying food intake in chickens. However, research on adipokines and intracellular signaling factors in the feeding regulation of chickens has been delayed compared to that of mammals, even though differences between mammals and chickens, and between broiler and layer chicks, have been reported. Further studies are required to clarify whether adipokines are physiologically involved in feeding regulation in chickens, which signaling pathways are activated or inhibited by appetite-regulating neuropeptides and hormones, and how they contribute to the gene expression of neuropeptides, such as NPY, AgRP, and POMC.
Neonatal chicks (usually 4–8 days old) are preferred for studies on feeding regulation. Because the early initiation of feeding affects poultry production as described above, studies on neonatal chicks are required. However, the abdominal fat, an adipokine-secreting site, is undeveloped in chicks during this period, because the tissue weight is extremely low and adipocyte size is small[89,90,91]. Similarly, the gastrointestinal tract, the peripheral site that secretes gut hormones, develops in chicks during the first week after hatching[92]. Therefore, it remains unclear whether the adipokines and gut hormones that regulate food intake are produced and secreted in neonatal chicks, even though central injection of these molecules affects food intake. Further studies are required to examine the production of adipokines and gut hormones in peripheral tissues of neonatal chicks.
As commercially-available chicken biomolecules, including adipokines and cytokines, are extremely scarce, several studies have substituted mammalian biomolecules. A previous study reported that central injection of porcine and chicken insulin, but not human and bovine insulin, suppresses food intake in chicks[93], indicating that mammalian peptides/proteins cannot be necessarily substituted in chicken studies. Therefore, chicken peptides/proteins are preferable. However, when using mammalian peptides/proteins, preliminary studies to examine whether these molecules activate or inhibit downstream pathways or gene expression, are required.
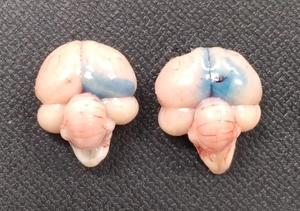
Many studies on central feeding regulation in chickens have adopted the method of ICV injection into chicks, which was developed by Davis et al. (1979)[94]. This method is simple and a successful injection is easy to verify (Fig. 1). In addition, the ICV injection does not affect food intake[95]. In this method, the solution is injected into the lateral ventricle and diffuses through the ventricle. Several studies have shown that responses to feeding conditions and hormones differ between neuronal nuclei[29,30,96] Therefore, simple methods for direct injection into the third and fourth ventricles (located in the center of appetite regulation) and the neuronal nucleus of chicks should be developed to expand this research field.
In conclusion, a complete understanding of the regulatory mechanisms underlying food intake in chickens will provide new strategies to improve production efficiency and quality in the poultry industry.
ACKNOWLEDGMENTS
This study was partially supported by JSPS KAKENHI (grant numbers 19K06353 and 23K05506). The author thanks Ms. Aika Kitayama (Graduate School of Agricultural Science, Kobe University) and Ms. Miyu Kaihatsu (Faculty of Agriculture, Kobe University) for their helpful assistance with the ICV injections into chicks. The author would like to thank Editage (www.editage.jp) for English language editing.
Author Contributions
Takaoki Saneyasu wrote the paper.
Conflict of Interest
The author declared no conflicts of interest.
REFERENCES
- [1] Gonzales E, Kondo N, Saldanha ES, Loddy MM, Careghi C andDecuypere E. Performance and physiological parameters of broiler chickens subjected to fasting on the neonatal period. Poult Sci, 82: 1250–1256. 2003. PMID:12943295, https://doi.org/10.1093/ps/82.8.1250
- [2] Halevy O, Geyra A, Barak M, Uni Z andSklan D. Early posthatch starvation decreases satellite cell proliferation and skeletal muscle growth in chicks. J Nutr, 130: 858–864. 2000. PMID:10736342, https://doi.org/10.1093/jn/130.4.858
- [3] Noy Y andSklan D. Posthatch development in poultry. J Appl Poult Res, 6: 344–354. 1997. https://doi.org/10.1093/japr/6.3.344
- [4] Ijiri D, Hozo N, Shimamoto S, Kawaguchi M, Furukawa A, Tada O, Tomonaga S, Nakashima K andOhtsuka A. Effects of delayed feeding on lipid peroxidation, drip losses, color, and taste of chicken breast meat. Nihon Chikusan Gakkaiho, 89: 191–198. 2018. https://doi.org/10.2508/chikusan.89.191
- [5] Kohrogi R, Nishikoba N, Shimamoto S, Kamimura R, Nakashima K, Tada O, Ohtsuka A andIjiri D. Effects of delayed feeding on the performance of laying hens raised under thermoneutral and high ambient temperature condition. Jpn J Poult Sci, 56: J1–J6. 2019.
- [6] Richards MP andProszkowiec-Weglarz M. Mechanisms regulating feed intake, energy expenditure, and body weight in poultry. Poult Sci, 86: 1478–1490. 2007. PMID:17575199, https://doi.org/10.1093/ps/86.7.1478
- [7] Buzala M andJanicki B. Review: effects of different growth rates in broiler breeder and layer hens on some productive traits. Poult Sci, 95: 2151–2159. 2016. PMID:27194733, https://doi.org/10.3382/ps/pew173
- [8] Ahiwe EU, Omede AA, Abdallh MB andIji PA. Managing Dietary Energy Intake by Broiler Chickens to Reduce Production Costs and Improve Product Quality, in: Banu Yücel and Turgay Taşkin (Eds.), Animal Husbandry and Nutrition, IntechOpen Limited. London. 2018.
- [9] Morton GJ, Cummings DE, Baskin DG, Barsh GS andSchwartz MW. Central nervous system control of food intake and body weight. Nature, 443: 289–295. 2006. PMID:16988703, https://doi.org/10.1038/nature05026
- [10] Morton GJ, Meek TH andSchwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci, 15: 367–378. 2014. PMID:24840801, https://doi.org/10.1038/nrn3745
- [11] Yeo GSH andHeisler LK. Unraveling the brain regulation of appetite: lessons from genetics. Nat Neurosci, 15: 1343–1349. 2012. PMID:23007189, https://doi.org/10.1038/nn.3211
- [12] Geelissen SME, Swennen Q, Geyten SV, Kühn ER, Kaiya H, Kangawa K, Decuypere E, Buyse J andDarras VM. Peripheral ghrelin reduces food intake and respiratory quotient in chicken. Domest Anim Endocrinol, 30: 108–116. 2006. PMID:16054797, https://doi.org/10.1016/j.domaniend.2005.06.005
- [13] Seroussi E, Cinnamon Y, Yosefi S, Genin O, Smith JG, Rafati N, Bornelöv S, Andersson L andFriedman-Einat M. Identification of the long-sought leptin in chicken and duck: expression pattern of the highly GC-rich avian leptin fits an autocrine/paracrine rather than endocrine function. Endocrinology, 157: 737–751. 2016. PMID:26587783, https://doi.org/10.1210/en.2015-1634
- [14] Sims W, Yi J, Cline MA andGilbert ER. Central injection of a synthetic chicken partial leptin peptide does not affect food intake in chicks. Neurosci Lett, 656: 165–168. 2017. PMID:28751205, https://doi.org/10.1016/j.neulet.2017.07.038
- [15] Boswell T andDunn IC. Regulation of the avian central melanocortin system and the role of leptin. Gen Comp Endocrinol, 221: 278–283. 2015. PMID:25583584, https://doi.org/10.1016/j.ygcen.2014.12.009
- [16] Bungo T, Shiraishi J andKawakami SI. Hypothalamic melanocortin system on feeding regulation in birds: A review. J Poult Sci, 48: 1–13. 2011. https://doi.org/10.2141/jpsa.010117
- [17] Furuse M, Yamane H, Tomonaga S, Tsuneyoshi Y andDenbow DM. Neuropeptidergic regulation of food intake in the neonatal chick: A review. J Poult Sci, 44: 349–356. 2007. https://doi.org/10.2141/jpsa.44.349
- [18] Honda K. Glucagon‐related peptides and the regulation of food intake in chickens. Anim Sci J, 87: 1090–1098. 2016. PMID:27150835, https://doi.org/10.1111/asj.12619
- [19] Honda K, Saneyasu T andKamisoyama H. Gut hormones and regulation of food intake in birds. J Poult Sci, 54: 103–110. 2017. PMID:32908415, https://doi.org/10.2141/jpsa.0160100
- [20] Honda K. Peripheral regulation of food intake in chickens: adiposity signals, satiety signals and others. Worlds Poult Sci J, 77: 301–312. 2021. https://doi.org/10.1080/00439339.2021.1898296
- [21] Song Z, Everaert N, Wang Y, Decuypere E andBuyse J. The endocrine control of energy homeostasis in chickens. Gen Comp Endocrinol, 190: 112–117. 2013. PMID:23707377, https://doi.org/10.1016/j.ygcen.2013.05.006
- [22] Tachibana T andTsutsui K. Neuropeptide control of feeding behavior in birds and its difference with mammals. Front Neurosci, 10: 485. 2016. PMID:27853416, https://doi.org/10.3389/fnins.2016.00485
- [23] Tachibana T andCline MA. Biomolecules triggering altered food intake during pathogenic challenge in chicks. J Poult Sci, 60: 2023009. 2023. PMID:36969710, https://doi.org/10.2141/jpsa.2023009
- [24] Tran PV, Chowdhury VS andFuruse M. Central regulation of feeding behavior through neuropeptides and amino acids in neonatal chicks. Amino Acids, 51: 1129–1152. 2019. PMID:31302780, https://doi.org/10.1007/s00726-019-02762-x
- [25] Booth A, Magnuson A, Fouts J andFoster MT. Adipose tissue: an endocrine organ playing a role in metabolic regulation. Horm Mol Biol Clin Investig, 26: 25–42. 2016. PMID:26910750, https://doi.org/10.1515/hmbci-2015-0073
- [26] Pestel J, Blangero F, Watson J, Pirola L andEljaafari A. Adipokines in obesity and metabolic-related-diseases. Biochimie, 212: 48–59. 2023. PMID:37068579, https://doi.org/10.1016/j.biochi.2023.04.008
- [27] Recinella L, Orlando G, Ferrante C, Chiavaroli A, Brunetti L andLeone S. Adipokines: new potential therapeutic target for obesity and metabolic, rheumatic, and cardiovascular diseases. Front Physiol, 11: 578966. 2020. PMID:33192583, https://doi.org/10.3389/fphys.2020.578966
- [28] Belgardt BF, Okamura T andBrüning JC. Hormone and glucose signalling in POMC and AgRP neurons. J Physiol, 587: 5305–5314. 2009. PMID:19770186, https://doi.org/10.1113/jphysiol.2009.179192
- [29] Cota D, Proulx K, Smith KAB, Kozma SC, Thomas G, Woods SC andSeeley RJ. Hypothalamic mTOR signaling regulates food intake. Science, 312: 927–930. 2006. PMID:16690869, https://doi.org/10.1126/science.1124147
- [30] Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, Stuck BJ andKahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature, 428: 569–574. 2004. https://doi.org/10.1038/nature02440
- [31] Ono H. Molecular mechanisms of hypothalamic insulin resistance. Int J Mol Sci, 20: 1317. 2019. PMID:30875909, https://doi.org/10.3390/ijms20061317
- [32] Mirabeau O, Perlas E, Severini C, Audero E, Gascuel O, Possenti R, Birney E, Rosenthal N andGross C. Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res, 17: 320–327. 2007. PMID:17284679, https://doi.org/10.1101/gr.5755407
- [33] Kim DK, Yun S, Son GH, Hwang JI, Park CR, Kim JI, Kim K, Vaudry H andSeong JY. Coevolution of the spexin/galanin/kisspeptin family: spexin activates galanin receptor type II and III. Endocrinology, 155: 1864–1873. 2014. PMID:24517231, https://doi.org/10.1210/en.2013-2106
- [34] Kołodziejski PA, Pruszyńska-Oszmałek E, Hejdysz M, Sassek M, Leciejewska N, Ziarniak K, Bień J, Ślósarz P, Kubiś M andKaczmarek S. Effect of fasting on the spexin system in broiler chickens. Animals, 11: 518. 2021. PMID:33671411, https://doi.org/10.3390/ani11020518
- [35] Meng F, Wu Y, Yu Y, Bu G, Du X, Liang Q, Cao X, Huang A, Zeng X, Huang L, Kong F, Li Y andHan X. Spexin2 is a novel food regulator in Gallus gallus. Int J Mol Sci, 24: 4821. 2023a. PMID:36902252, https://doi.org/10.3390/ijms24054821
- [36] Meng F, Yu Y, Li J, Han X, Du X, Cao X, Liang Q, Huang A, Kong F, Huang L, Zeng X andBu G. Characterization of spexin (SPX) in chickens: molecular cloning, functional analysis, tissue expression and its involvement in appetite regulation. Poult Sci, 102: 102279. 2023b. PMID:36402041, https://doi.org/10.1016/j.psj.2022.102279
- [37] Fang XL, Zhu XT, Chen SF, Zhang ZQ, Zeng QJ, Deng L, Peng JL, Yu JJ, Wang LN, Wang SB, Gao P, Jiang QY andShu G. Differential gene expression pattern in hypothalamus of chickens during fasting-induced metabolic reprogramming: functions of glucose and lipid metabolism in the feed intake of chickens. Poult Sci, 93: 2841–2854. 2014. PMID:25239532, https://doi.org/10.3382/ps.2014-04047
- [38] Kewan A, Shimatani T, Saneyasu T, Kamisoyama H andHonda K. Comparison of the effects of intracerebroventricular administration of glucagon-like peptides 1 and 2 on hypothalamic appetite regulating factors and sleep-like behavior in chicks. Neurosci Lett, 768: 136362. 2022. PMID:34838926, https://doi.org/10.1016/j.neulet.2021.136362
- [39] Aoki K, Kondo M, Okuda M, Saneyasu T, Honda K andKamisoyama H. Identification, expression analysis, and functional characterization of peptide YY in chickens (Gallus gallus domesticus). Gen Comp Endocrinol, 242: 11–17. 2017. PMID:27118705, https://doi.org/10.1016/j.ygcen.2016.04.021
- [40] Reid AMA, Wilson PW, Caughey SD, Dixon LM, D’Eath RB, Sandilands V, Boswell T andDunn IC. Pancreatic PYY but not PPY expression is responsive to short-term nutritional state and the pancreas constitutes the major site of PYY mRNA expression in chickens. Gen Comp Endocrinol, 252: 226–235. 2017. PMID:28694054, https://doi.org/10.1016/j.ygcen.2017.07.002
- [41] Farzin M, Hassanpour S, Zendehdel M, Vazir B andAsghari A. Effects of intracerebroventricular injection of spexin and its interaction with NPY, GalR2 and GalR3 receptors on the central food intake regulation and nutritional behavior in broiler chickens. Neurosci Lett, 777: 136589. 2022. PMID:35346778, https://doi.org/10.1016/j.neulet.2022.136589
- [42] Sun X, Yu Z, Xu Y, Pu S andGao X. The role of spexin in energy metabolism. Peptides, 164: 170991. 2023. PMID:36914115, https://doi.org/10.1016/j.peptides.2023.170991
- [43] Bungo T, Shimojo M, Masuda Y, Tachibanab T, Tanaka S, Sugahara K andFuruse M. Intracerebroventricular administration of mouse leptin does not reduce food intake in the chicken. Brain Res, 817: 196–198. 1999. PMID:9889365, https://doi.org/10.1016/S0006-8993(98)01223-2
- [44] Cassy S, Picard M, Crochet S, Derouet M, Keisler DH andTaouis M. Peripheral leptin effect on food intake in young chickens is influenced by age and strain. Domest Anim Endocrinol, 27: 51–61. 2004. PMID:15158534, https://doi.org/10.1016/j.domaniend.2004.01.004
- [45] Denbow DM, Meade S, Robertson A, McMurtry JP, Richards M andAshwell C. Leptin-induced decrease in food intake in chickens. Physiol Behav, 69: 359–362. 2000. PMID:10869603, https://doi.org/10.1016/S0031-9384(99)00258-9
- [46] Kuo AY, Cline MA, Werner E, Siegel PB andDenbow DM. Leptin effects on food and water intake in lines of chickens selected for high or low body weight. Physiol Behav, 84: 459–464. 2005. PMID:15763584, https://doi.org/10.1016/j.physbeh.2005.01.014
- [47] Piekarski A, Nagarajan G, Ishola P, Flees J, Greene ES, Kuenzel WJ, Ohkubo T, Maier H, Bottje WG, Cline MA andDridi S. AMP-activated protein kinase mediates the effect of leptin on avian autophagy in a tissue-specific manner. Front Physiol, 9: 541. 2018. PMID:29867578, https://doi.org/10.3389/fphys.2018.00541
- [48] Tang N, Zhang X, Chen D andLi Z. Chen Dd, Li Z. The controversial role of adiponectin in appetite regulation of animals. Nutrients, 13: 3387. 2021. PMID:34684387, https://doi.org/10.3390/nu13103387
- [49] Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y andKadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab, 6: 55–68. 2007. PMID:17618856, https://doi.org/10.1016/j.cmet.2007.06.003
- [50] Cai J, Hu Q, Lin H, Zhao J, Jiao H andWang X. Adiponectin/adiponectin receptors mRNA expression profiles in chickens and their response to feed restriction. Poult Sci, 100: 101480. 2021. PMID:34700095, https://doi.org/10.1016/j.psj.2021.101480
- [51] Maddineni S, Metzger S, Ocón O, Hendricks G, III andRamachandran R. Adiponectin gene is expressed in multiple tissues in the chicken: food deprivation influences adiponectin messenger ribonucleic acid expression. Endocrinology, 146: 4250–4256. 2005. PMID:15976057, https://doi.org/10.1210/en.2005-0254
- [52] Madadi S, Hasasnpour S, Zendehdel M, Vazir B andJahandideh A. Role of central adiponectin and its interactions with NPY and GABAergic systems on food intake in neonatal layer chicken. Neurosci Lett, 808: 137283. 2023. PMID:37142113, https://doi.org/10.1016/j.neulet.2023.137283
- [53] Estienne A, Ramé C, Ganier P, Chahnamian M, Barbe A, Grandhaye J, Dubois JP, Batailler M, Migaud M, Lecompte F, Adriaensen H, Froment P andDupont J. Chemerin impairs food intake and body weight in chicken: focus on hypothalamic neuropeptides gene expression and AMPK signaling pathway. Gen Comp Endocrinol, 304: 113721. 2021. PMID:33493505, https://doi.org/10.1016/j.ygcen.2021.113721
- [54] Safikhani A, Zendehdel M, Khodadadi M, Rahmani B, Ghashghayi E andMahdavi K. Hypophagia induced by intracerebroventricular injection of apelin-13 is mediated via CRF1/CRF2 and MC3/MC4 receptors in neonatal broiler chicken. Behav Brain Res, 452: 114536. 2023. PMID:37295613, https://doi.org/10.1016/j.bbr.2023.114536
- [55] Brunetti L, Orlando G, Ferrante C, Recinella L, Leone S, Chiavaroli A, Di Nisio C, Shohreh R, Manippa F, Ricciuti A andVacca M. Peripheral chemerin administration modulates hypothalamic control of feeding. Peptides, 51: 115–121. 2014. PMID:24269538, https://doi.org/10.1016/j.peptides.2013.11.007
- [56] Sunter D, Hewson AK andDickson SL. Intracerebroventricular injection of apelin-13 reduces food intake in the rat. Neurosci Lett, 353: 1–4. 2003. PMID:14642423, https://doi.org/10.1016/S0304-3940(03)00351-3
- [57] Zhang J, Zhou Y, Wu C, Wan Y, Fang C, Li J, Fang W, Yi R, Zhu G, Li J andWang Y. Characterization of the apelin/elabela receptors (APLNR) in chickens, turtles, and zebrafish: identification of a novel apelin-specific receptor in teleosts. Front Endocrinol (Lausanne), 9: 756. 2018. PMID:30631305, https://doi.org/10.3389/fendo.2018.00756
- [58] Bornelöv S, Seroussi E, Yosefi S, Benjamini S, Miyara S, Ruzal M, Grabherr M, Rafati N, Molin AM, Pendavis K, Burgess SC, Andersson L andFriedman-Einat M. Comparative omics and feeding manipulations in chicken indicate a shift of the endocrine role of visceral fat towards reproduction. BMC Genomics, 19: 295. 2018. PMID:29695257, https://doi.org/10.1186/s12864-018-4675-0
- [59] Cline M, Nandar W, Prall B, Bowden C andDenbow D. Central visfatin causes orexigenic effects in chicks. Behav Brain Res, 186: 293–297. 2008. PMID:17920135, https://doi.org/10.1016/j.bbr.2007.08.016
- [60] Ukena K, Iwakoshi-Ukena E, Taniuchi S, Bessho Y, Maejima S, Masuda K, Shikano K, Kondo K, Furumitsu M andTachibana T. Identification of a cDNA encoding a novel small secretory protein, neurosecretory protein GL, in the chicken hypothalamic infundibulum. Biochem Biophys Res Commun, 446: 298–303. 2014. PMID:24582750, https://doi.org/10.1016/j.bbrc.2014.02.090
- [61] Ukena K. Avian and murine neurosecretory protein GL participates in the regulation of feeding and energy metabolism. Gen Comp Endocrinol, 260: 164–170. 2018. PMID:28951261, https://doi.org/10.1016/j.ygcen.2017.09.019
- [62] Matsuura D, Shikano K, Saito T, Iwakoshi-Ukena E, Furumitsu M, Ochi Y, Sato M, Bentley GE, Kriegsfeld LJ andUkena K. Neurosecretory Protein GL, a Hypothalamic Small Secretory Protein, Participates in Energy Homeostasis in Male Mice. Endocrinology, 158: 1120–1129. 2017. PMID:28323972, https://doi.org/10.1210/en.2017-00064
- [63] Iwakoshi-Ukena E, Shikano K, Kondo K, Taniuchi S, Furumitsu M, Ochi Y, Sasaki T, Okamoto S, Bentley GE, Kriegsfeld LJ, Minokoshi Y andUkena K. Neurosecretory protein GL stimulates food intake, de novo lipogenesis, and onset of obesity. eLife, 6: e28527. 2017. PMID:28799896, https://doi.org/10.7554/eLife.28527
- [64] Shikano K, Kato M, Iwakoshi-Ukena E, Furumitsu M, Matsuura D, Masuda K, Tachibana T, Bentley GE, Kriegsfeld LJ andUkena K. Effects of chronic intracerebroventricular infusion of neurosecretory protein GL on body mass and food and water intake in chicks. Gen Comp Endocrinol, 256: 37–42. 2018. PMID:28554734, https://doi.org/10.1016/j.ygcen.2017.05.016
- [65] Kato M, Iwakoshi-Ukena E, Narimatsu Y, Furumitsu M andUkena K. Effect of stressors on the mRNA expressions of neurosecretory protein GL and neurosecretory protein GM in chicks. Front Physiol, 13: 860912. 2022. PMID:35370775, https://doi.org/10.3389/fphys.2022.860912
- [66] Shikano K, Bessho Y, Kato M, Iwakoshi-Ukena E, Taniuchi S, Furumitsu M, Tachibana T, Bentley GE, Kriegsfeld LJ andUkena K. Localization and function of neurosecretory protein GM, a novel small secretory protein, in the chicken hypothalamus. Sci Rep, 8: 704. 2018. PMID:29335496, https://doi.org/10.1038/s41598-017-18822-9
- [67] Shikano K, Iwakoshi-Ukena E, Kato M, Furumitsu M, Bentley GE, Kriegsfeld LJ andUkena K. Neurosecretory protein GL induces fat accumulation in chicks. Front Endocrinol (Lausanne), 10: 392. 2019. PMID:31275247, https://doi.org/10.3389/fendo.2019.00392
- [68] Shikano K, Iwakoshi-Ukena E, Saito T, Narimatsu Y, Kadota A, Furumitsu M, Bentley GE, Kriegsfeld LJ andUkena K. Neurosecretory protein GL induces fat accumulation in mice. J Endocrinol, 244: 1–12. 2020. PMID:31536964, https://doi.org/10.1530/JOE-19-0112
- [69] Narimatsu Y, Iwakoshi-Ukena E, Fukumura K, Shikano K, Furumitsu M, Morishita M, Bentley GE, Kriegsfeld LJ andUkena K. Hypothalamic overexpression of neurosecretory protein GL leads to obesity in male C57BL/6J mice. Neuroendocrinology, 112: 606–620. 2022. PMID:34384081, https://doi.org/10.1159/000518969
- [70] Fukumura K, Shikano K, Narimatsu Y, Iwakoshi-Ukena E, Furumitsu M, Naito M andUkena K. Effects of neurosecretory protein GL on food intake and fat accumulation under different dietary nutrient compositions in rats. Biosci Biotechnol Biochem, 85: 1514–1520. 2021. PMID:33851987, https://doi.org/10.1093/bbb/zbab064
- [71] Damm E, Buech TRH, Gudermann T andBreit A. Melanocortin-induced PKA activation inhibits AMPK activity via ERK-1/2 and LKB-1 in hypothalamic GT1-7 cells. Mol Endocrinol, 26: 643–654. 2012. PMID:22361823, https://doi.org/10.1210/me.2011-1218
- [72] Glas E, Mückter H, Gudermann T andBreit A. Exchange factors directly activated by cAMP mediate melanocortin 4 receptor-induced gene expression. Sci Rep, 6: 32776. 2016. PMID:27612207, https://doi.org/10.1038/srep32776
- [73] Cheng W, Gordian D, Ludwig MQ, Pers TH, Seeley RJ andMyers MG, Jr Hindbrain circuits in the control of eating behaviour and energy balance. Nat Metab, 4: 826–835. 2022. PMID:35879458, https://doi.org/10.1038/s42255-022-00606-9
- [74] de Lartigue G. Role of the vagus nerve in the development and treatment of diet‐induced obesity. J Physiol, 594: 5791–5815. 2016. PMID:26959077, https://doi.org/10.1113/JP271538
- [75] Monteiro MP andBatterham RL. The importance of the gastrointestinal tract in controlling food intake and regulating energy balance. Gastroenterology, 152: 1707–1717.e2. 2017. PMID:28193513, https://doi.org/10.1053/j.gastro.2017.01.053
- [76] Abraham MA, Filippi BM, Kang GM, Kim MS andLam TKT. Insulin action in the hypothalamus and dorsal vagal complex. Exp Physiol, 99: 1104–1109. 2014. PMID:24972836, https://doi.org/10.1113/expphysiol.2014.079962
- [77] Filippi BM, Yang CS, Tang C andLam TKT. Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metab, 16: 500–510. 2012. PMID:23040071, https://doi.org/10.1016/j.cmet.2012.09.005
- [78] Filippi BM, Bassiri A, Abraham MA, Duca FA, Yue JTY andLam TKT. Insulin signals through the dorsal vagal complex to regulate energy balance. Diabetes, 63: 892–899. 2014. PMID:24270985, https://doi.org/10.2337/db13-1044
- [79] Campos CA, Wright JS, Czaja K andRitter RC. CCK-induced reduction of food intake and hindbrain MAPK signaling are mediated by NMDA receptor activation. Endocrinology, 153: 2633–2646. 2012. PMID:22508518, https://doi.org/10.1210/en.2012-1025
- [80] Sutton GM, Patterson LM andBerthoud HR. Extracellular signal-regulated kinase 1/2 signaling pathway in solitary nucleus mediates cholecystokinin-induced suppression of food intake in rats. J Neurosci, 24: 10240–10247. 2004. PMID:15537896, https://doi.org/10.1523/JNEUROSCI.2764-04.2004
- [81] Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ andBence KK. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab, 13: 320–330. 2011. PMID:21356521, https://doi.org/10.1016/j.cmet.2011.02.001
- [82] Song Z, Liu L, Yue Y, Jiao H, Lin H, Sheikhahmadi A, Everaert N, Decuypere E andBuyse J. Fasting alters protein expression of AMP-activated protein kinase in the hypothalamus of broiler chicks (Gallus gallus domesticus). Gen Comp Endocrinol, 178: 546–555. 2012. PMID:22771832, https://doi.org/10.1016/j.ygcen.2012.06.026
- [83] Liu L, Song Z, Jiao H andLin H. Glucocorticoids increase NPY gene expression via hypothalamic AMPK signaling in broiler chicks. Endocrinology, 155: 2190–2198. 2014. PMID:24693963, https://doi.org/10.1210/en.2013-1632
- [84] Saneyasu T, Ueda M, Nagata K, Chai J, Honda K andKamisoyama H. Role of hypothalamic TGF-β/Smad signaling in feeding regulation in chickens. J Poult Sci, 59: 357–363. 2022. PMID:36382057, https://doi.org/10.2141/jpsa.0220040
- [85] Adeli A, Zendehdel M, Babapour V andPanahi N. Interaction between leptin and glutamatergic system on food intake regulation in neonatal chicken: role of NMDA and AMPA receptors. Int J Neurosci, 130: 713–721. 2020. PMID:31813315, https://doi.org/10.1080/00207454.2019.1702983
- [86] Saneyasu T, Fujita S, Kitashiro A, Fukuzo S, Honda K andKamisoyama H. Hypothalamic Akt-mediated signaling regulates food intake in chicks. Neurosci Lett, 670: 48–52. 2018. PMID:29360502, https://doi.org/10.1016/j.neulet.2018.01.032
- [87] Saneyasu T, Fukuzo S, Kitashiro A, Nagata K, Honda K andKamisoyama H. Central administration of insulin and refeeding lead to the phosphorylation of AKT, but not FOXO1, in the hypothalamus of broiler chicks. Physiol Behav, 210: 112644. 2019a. PMID:31398442, https://doi.org/10.1016/j.physbeh.2019.112644
- [88] Saneyasu T, Ueno M, Nagata K, Kewan A, Honda K andKamisoyama H. Central administration of insulin and refeeding lead to Akt and ERK phosphorylation in the chicken medulla. Neurosci Lett, 758: 136008. 2021. PMID:34098027, https://doi.org/10.1016/j.neulet.2021.136008
- [89] Bai S, Wang G, Zhang W, Zhang S, Rice BB, Cline MA andGilbert ER. Broiler chicken adipose tissue dynamics during the first two weeks post-hatch. Comp Biochem Physiol A Mol Integr Physiol, 189: 115–123. 2015. PMID:26263851, https://doi.org/10.1016/j.cbpa.2015.08.002
- [90] Saneyasu T, Nakanishi K, Atsuta H, Ikura A, Kamisoyama H, Hasegawa S andHonda K. Age-dependent changes in the mRNA levels of neuropeptide Y, proopiomelanocortin, and corticotropin-releasing factor in the hypothalamus in growing broiler chicks. J Poult Sci, 50: 364–369. 2013. https://doi.org/10.2141/jpsa.0120188
- [91] Honda K, Saneyasu T, Aoki K, Shimatani T, Yamaguchi T andKamisoyama H. Correlation analysis of hypothalamic MRNA levels of appetite regulatory neuropeptides and several metabolic parameters in 28‐day‐old layer chickens. Anim Sci J, 86: 517–522. 2015. PMID:25441031, https://doi.org/10.1111/asj.12320
- [92] Proszkowiec-Weglarz M. Gastrointestinal anatomy and physiology. In: Sturkie’s Avian Physiology. 7th ed. (Scanes CG and Dridi S eds.), Academic Press, London, Chap 21, pp.485-527, 2022.
- [93] Shiraishi J, Yanagita K, Nishikawa F, Tahara Y, Fujita M, McMurtry JP andBungo T. A comparison of the anorexic effects of chicken, porcine, human and bovine insulin on the central nervous system of chicks. J Poult Sci, 46: 144–148. 2009. https://doi.org/10.2141/jpsa.46.144
- [94] Davis JL, Masuoka DT, Gerbrandt LK andCherkin A. Autoradiographic distribution of L-proline in chicks after intracerebral injection. Physiol Behav, 22: 693–695. 1979. PMID:482410, https://doi.org/10.1016/0031-9384(79)90233-6
- [95] Furuse M, Ando R, Bungo T, Shimojo M andMasuda Y. Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br Poult Sci, 40: 698–700. 1999. PMID:10670685, https://doi.org/10.1080/00071669987115
- [96] Villanueva EC, Münzberg H, Cota D, Leshan RL, Kopp K, Ishida-Takahashi R, Jones JC, Fingar DC, Seeley RJ andMyers MG, Jr Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status. Endocrinology, 150: 4541–4551. 2009. PMID:19628573, https://doi.org/10.1210/en.2009-0642