2023 Volume 64 Issue 1 Pages 19-34
2023 Volume 64 Issue 1 Pages 19-34
Species of Hypochnicium (Polyporales, Basidiomycota) collected from Japan were studied on their taxonomy by morphological and phylogenetic approaches. Phylogenetic analyses based on a nrDNA LSU and ITS dataset including the Japanese specimens and other publicly available ones show that Hypochnicium is polyphyletic. Since the clade containing the type species H. bombycinum was well-supported, we defined this clade as Hypochnicium s. str., and emended Hypochnicium to include restricted taxa with only smooth basidiospores. The new genus Neohypochnicium is proposed to accommodate the remaining taxa excluded from the genus Hypochnicium s. str., which includes both species with smooth basidiospores and ornamented ones. Three new species, Gyrophanopsis japonica, N. asiaticum and N. perlongicystidiosum are described and illustrated based on morphological and phylogenetic analyses using an ITS region dataset. In addition, the following 15 new combinations are proposed: N. albostramineum, N. aotearoae, N. capitulateum, N. cremicolor, N. cystidiatum, N. geogenium, N. guineense, N. huinayense, N. michelii, N. microsporum, N. patagonicum, N. pini, N. punctulatum, N. subrigescens and N. wakefieldiae. An identification key to Japanese species of Bulbillomyces, Gyrophanopsis, Hypochnicium and Neohypochnicium is provided.
The genus Hypochnicium J. Erikss., erected by Eriksson in 1958 with H. bombycinum (Sommerf.) J. Erikss. as the type species, is characterized by producing corticioid basidiomes on woody substrates such as dead trunks and branches, and microscopically by having monomitic hyphal system, clamped hyphae, suburniform basidia, and especially thick-walled and cyanophilous basidiospores that are smooth or ornamented (Eriksson, 1958; Eriksson & Ryvarden, 1976). This genus belongs to Polyporales (Basidiomycota) and most similar to Hyphoderma Wallr. in morphology of basidia, cystidia and hyphae, but differs in basidiospore feature: basidiospores of Hyphoderma are thin-walled and not cyanophilous. Some genera, i.e., Granulobasidium Jülich [type species: Gr. vellereum (Ellis & Cragin) Jülich] (Jülich, 1979), Gyrophanopsis Jülich [type species: Gy. zealandica (Cunn.) Jülich] (Jülich, 1979) and Gloeohypochnicium (Parmasto) Hjortstam [type species: Gl. analogum (Bourdot & Galzin) Hjortstam] (Hjortstam, 1987) have been segregated from Hypochnicium. Granulobasidium differs from Hypochnicium in having long and slender basidia with granular contents, lack of cystidia and presence of usually large numbers of chlamydospores in basidiomes. Subsequently Matheny et al. (2006) showed Gr. vellereum belongs to Cyphellaceae of Agaricales by phylogenetic analysis. Gyrophanopsis is distinguishable from Hypochnicium in having heavily encrusted septocystidia and slightly thick-walled basidiospores (Jülich, 1979; Stalpers & Buchanan, 1991). Gloeohypochnicium differs from Hypochnicium in producing sulpho-vanilline positive gloeocystidia. On phylogenetic analyses, Gloeohypochnicium placed in russuloid clade (Russulales) (Larsson & Larsson, 2003). Therefore, the two genera, Granulobasidium and Gloeohypochnicium are excluded here. According to the Index Fungorum fungal database (http://www.indexfungorum.org/names/names.asp, 31 May 2022), based on the morphological and/or phylogenetic data, 38 species are treated as members of Hypochnicium, and of these, 20 species are based on both data.
From Japan, the following eight taxa of Hypochnicium have been reported based on morphological characteristics only: H. analogum (Bourdot & Galzin) J. Erikss., H. eichleri (Bres. ex Sacc. & P. Syd.) J. Erikss. & Ryvarden, H. globosum Sheng H. Wu, H. longicystidiosum (S.S. Rattan) Hjortstam & Ryvarden, H. polonense (Bres.) Å. Strid, H. punctulatum (Cooke) J. Erikss., H. sphaerosporum (Höhn. & Litsch.) J. Erikss. and H. vellereum (Ellis & Cragin) Parmasto (Maekawa, 1994, 1997). Recently, H. pini Y. Jang & J.J. Kim and H. subrigescens Boidin were added to Japanese Hypochnicium based on morphological and phylogenetic analyses (Maekawa, 2021). Of these species, H. eichleri was subsequently placed in synonymy with H. albostramineum (Bres.) Hallenb. (Nilsson & Hallenberg, 2003). As mentioned above, H. analogum and H. vellereum were transferred to Gloeohypochnicium and Granulobasidium, respectively, based on morphological and phylogenetic analyses. Hypochnicium polonense was also transferred to Gyrophanopsis as Gy. polonensis (Bres.) Stalpers & P. K. Buchanan (Stalpers & Buchanan, 1991), however the generic concept of Gyrophanopsis has been unresolved yet. Thus, the currently known species of Japanese Hypochnicium are eight species.
During survey of corticioid fungi in Japan, we collected many specimens of unidentified Hypochnicium species. Here, we elucidate taxonomic position of these specimens and re-evaluate the genus Hypochnicium based on the phylogenetic analyses of the known species. In addition, voucher specimens of the Japanese known species are also morphologically and phylogenetically reexamined.
All of the Japanese specimens examined in this study are deposited at the Tottori University Mycological Herbarium (TUMH) and the isolates are deposited in the fungal culture collection (TUFC) of the Fungus/Mushroom Resource and Research Center, Tottori University, Tottori, Japan. Six foreign specimens of H. subrigescens deposited in the herbarium of University of Gothenburg (GB) were also examined for comparison with the Japanese specimens.
2.2. Morphological observationMorphological descriptions were based on observations of dried specimens. Macro-morphological features of basidiomes were observed using a stereoscopic microscope (SMZ 1500, Nikon Imaging, Tokyo, Japan). For microscopic observations, sections of basidiomes were mounted in Melzer's reagent, lactophenol cotton blue solution, and 3% (w/v) potassium hydroxide (KOH). Measurements, line drawings, and photographs of microscopic elements were made using differential interference contrast (DIC) microscope (Eclipse 80i and Eclipse Ni, Nikon Imaging) equipped with a digital camera (DS-Fi1, Nikon Imaging) and a microscope zoom drawing arm (Y-IDT, Nikon Imaging). For each specimen, the length and width of 30 basidiospores, 20 basidia and 20 cystidia were recorded. The descriptions use the following values: Lm, mean basidiospore length; Wm, mean basidiospore width; Q, L/W; and Qm, mean Q. The surface structure of basidiospores was observed with a scanning electron microscope (SU1510, Hitachi, Tokyo), using dried specimens. Procedures for fixing, dehydrating, and sputtering of the specimens followed Endo et al. (2019).
2.3. Culture studiesPolysporous isolates were obtained from flesh basidiomes of “Neohypochnicium asiacum” (TUMH 60588, TUMH 61220 and TUMH 61227), “N. perlongicystidiosum” (TUMH 40397 and TUMH 63328), and “Gyrophanopsis japonica” (TUMH 61400). Macro- and micro-morphological characteristics of the cultures were observed on malt extract agar [MA: 1.5% (w/v) malt extract, Becton, Dickinson and Company, Franklin Lakes, NJ, USA; 2% (w/v) Bacto agar, Becton, Dickinson and Company] at 25 °C. To determine the optimum growth temperature, the isolates were grown on MA plates at eight different temperatures (5-40 °C).
2.4. DNA extraction, PCR amplification, and sequencingThe procedures for DNA sequencing followed Maekawa et al. (2020). Genomic DNA was extracted from culture mycelia or basidiomata using a Maxwell 16 Tissue DNA Purification Kit (Promega, Madison, WI, USA). The internal transcribed
spacer (ITS) and partial region of nuclear-encoded large subunit rRNA gene (LSU) sequences were PCR-amplified and directly sequenced with primers ITS5/ITS4 for ITS (White, Bruns, Lee, & Taylor, 1990) and LR0R/LR5 for LSU (Hopple & Vilgalys, 1994). Because ITS sequence from TUFC 14478 showed heterozygotic waves, its PCR product was cloned using a pGEM-T Easy Vector System (Promega) with ECOS competent Escherichia coli (Migula) Castellani and Chalmers JM109 cells (Nippon Gene Co., Ltd., Tokyo, Japan). The DNA from transformed clones were amplified and sequenced as described above. The obtained sequences were registered in NCBI GenBank database (ITS: LC663668-LC663687, LSU: LC663688-LC663693) after deleting ambiguous characters.
2.5. Phylogenetic analysesThe phylogenetic analyses using ITS and LSU sequences were performed in two steps: 1) ITS-LSU combined dataset including Hypochnicium, Gyrophanopsis, and the remaining Polyporales lineages; 2) ITS dataset including Hypochnicium, Gyrophanopsis, and their close genera. First analyses aimed to reveal phylogenetic relationships of Hypochnicium s. lat. within a higher taxonomic group, and ITS-LSU dataset was constructed following to the phylogenies focusing on Polyporales by Binder et al. (2013) and Justo et al. (2017) (Table 1). An outgroup is the member of Russulales: Heterobasidion annosum (Fr.) Bref. and Stereum hirsutum (Willd.) Pers. (Justo et al., 2017). The ITS and LSU sequences were independently aligned using MAFFT online (Madeira et al., 2019) and then manually combined and edited on BioEdit Ver. 7.2.5 software (Hall, 1999). The combined sequences were annotated (as ITS1, 5.8S, ITS2, and 28S) by ITSx Ver. 1.1.3 (Bengtsson-Palme, et al., 2013) on PlutoF workbench (Abarenkov et al., 2010). From 74 taxa, we built the alignment of ITS-LSU dataset comprising of 1,932 sites (ITS: 919 sites, LSU: 1,013 sites) with 1,106 distinct alignment patterns (ITS: 662 sites, LSU: 431 sites). Phylogenetic inferences were performed on Maximum likelihood (ML) and Bayesian method. The ML phylogeny was constructed using raxmlGUI Ver. 2.0.5 software (Stamatakis, 2014; Edler, Klein, Antonelli, & Silvestro, 2021) with rapid bootstrap algorithm based on 1,000 replication of nonparametric bootstrap (BS) analyses. For ML analysis on the ITS-LSU dataset, a GTR+G+I substitution model was selected based on the result from ModelTest-NG Ver. 0.2.0 (Flouri et al., 2015; Darriba et al., 2020). For Bayesian inferences (BI) of phylogeny, we estimated the best partitioning schemes and substitution models using PartitionFinder 2 Ver. 1.1 (Lanfear, Frandsen, Wright, Senfeld, & Calcott, 2017) under “all” search parameter and AICc criterion. The best models were GTR+G+I for ITS1, ITS2, and 28S, and K80+G+I for 5.8S, and the four data blocks were independently treated as different subset. The BI phylogenetic analysis was implemented using MrBayes Ver. 3.2.7 software (Ronquist et al., 2012) that computed two runs with four Markov chain Monte Carlo (MCMC) iterations with sampling topology for every 100 generations. After 3,000,000 generations of iterations, conservation of trees was confirmed by Tracer Ver. 1.7.2 software (Rambaut, Drummond, Xie, Baele, & Suchard, 2018) and stopped analysis. We burn-in the first 25% topologies and subsequently constructed a consensus tree from 50% majority topologies. The ML and BI trees were visualized using FigTree Ver. 1.4.4 software, and significant branches were assumed by ≥ 50 of ML bootstrap value (MLBS) and ≥ 0.90 of Bayesian inference posterior probability (BIP).
| Clade in Polyporales | Family | Species | Collection nos. | Accession nos. | |
| ITS | nrLSU | ||||
| /antrodia | Fomitopsidaceae | Antrodia serpens | Vampola-X-1989 | KC543143 | KC543143 |
| Daedalea quercina | FP-56429 | KY948809 | KY948883 | ||
| Fomitopsis pinicola | AFTOL-770 | AY684164 | AY684164 | ||
| /core polypoid | Polyporaceae | Ganoderma tsugae | UBC-F23891 | KJ146707 | KJ146707 |
| Lopharia cinerascens | FP-105043-Sp | JN165019 | JN164813 | ||
| Trametes suaveolens | FP-102529-Sp | JN164966 | JN164807 | ||
| T. versicolor | FP-135156-Sp | JN164919 | JN164809 | ||
| /phlebioid | Irpicaceae | Byssomerulius corium | FP-102382 | KP135007 | KP135230 |
| Hapalopilus ochraceolateritius | Miettinen 16992.1 | KY948741 | KY948891 | ||
| Irpex lacteus | FD-9 | KP135026 | KP135224 | ||
| Meruliopsis albostramineus | HHB-10729 | KP135051 | KP135229 | ||
| Meruliaceae | Aurantiporus croceus | Miettinen-16483 | KY948745 | KY948901 | |
| Climacodon septentrionalis | AFTOL-767 | AY854082 | AY684165 | ||
| Crustodontia chrysocreas | CBS 125.889 | MH864087 | MH875546 | ||
| Hydnophlebia chrysorhiza | FD-282 | KP135338 | KP135217 | ||
| Phlebia radiata | AFTOL-484 | AY854087 | AF287885 | ||
| Phanerochaetaceae | Bjerkandera adusta | HHB-12826-Sp | KP135198 | KP135198 | |
| Phanerochaete rhodella | FD-18 | KP135187 | KP135258 | ||
| /resudial | Cerrenaceae | Cerrena unicolor | FD-299 | KP135304 | KP135209 |
| Radulodon casearius | HHB-9567-Sp | KY948752 | KY948871 | ||
| Spongipellis delectans | HHB-10489-Sp | KP135301 | KP135287 | ||
| Hyphodermataceae | Hyphoderma litschaueri | FP-101740-Sp | KP135295 | KP135219 | |
| H. setigerm | FD-312 | KP135297 | KP135222 | ||
| Meripilaceae | Meripilus giganteus | FP-135344-Sp | KP135307 | KP135228 | |
| Physisporinus vitreus | KHL11959 (GB) | JQ031129 | JQ031129 | ||
| Rigidoporus undatus | Miettinen-13591 | KY948731 | KY948870 | ||
| Panaceae | Cymatoderma sp. | OMC1427 | KY948826 | KY948872 | |
| Panus fragilis | HHB-11042-Sp | KP135328 | KP135233 | ||
| Podoscyphaceae | Abortiporus biennis | CBS 676.70 | MH859896 | MH871686 | |
| FD-319 | KP135300 | KP135195 | |||
| Cymatoderma dendriticum | CBS 207.62 | JN649339 | JN649339 | ||
| Podoscypha bolleana | CBS 333.66 | JN649354 | JN649354 | ||
| P. petalodes subsp. rosulata | CBS 659.84 | MH861805 | MH873498 | ||
| Steccherinaceae | Antrodiella stipitata | FD-136 | KP135314 | KP135197 | |
| Butyrea luteoalba | FP-105786-Sp | KP135320 | KP135226 | ||
| Cabalodontia delicata | 639/19 | MT849297 | MT849297 | ||
| Caudicicola gracilis | X-3081 | KY415962 | KY415962 | ||
| Etheirodon aff. fimbriatum | FP-102075 | KY948821 | KY948864 | ||
| Fibricium subceraceum | H. Loy s.n. (O) | JN710531 | JN710531 | ||
| Flaviporus americana | HHB-4100-Sp | KP135316 | KP135196 | ||
| Junghuhnia pseudozilingiana | Matti Kulju 1004 (H) | JN710561 | JN710561 | ||
| Steccherinum laeticolor | FP-102480-Sp | KY948823 | KY948868 | ||
| Unknown | Bulbillomyces farinosus | FP-100488-T | KY948802 | - | |
| NH 9933 (GB) | - | DQ681201 | |||
| Climacocystis borealis | FD-31 | KP135308 | KP135210 | ||
| Diplomitoporus crustulinus | FD-137 | KP135299 | KP135211 | ||
| Gyrophanopsis polonense | NH-11337 (GB) | DQ677511 | DQ677511 | ||
| G. japonica | TUMH 61400 | LC663668 a | LC663688 a | ||
| Hypochnicium bombycinum | Otto Miettinen 9441 (H) | KY415959 | KY415959 | ||
| H. cremicolor | NH 1149 (GB) | DQ677506 | DQ677506 | ||
| H. erikssonii | NH 9635 (GB) | DQ677508 | DQ677508 | ||
| H. geogenium | NH 10910 (GB) | DQ677509 | DQ677509 | ||
| H. lyndoniae | CBS 125.874 | MH864079 | MH875536 | ||
| H. michelii | MA:Fungi 79155 | FN552535 | NG_060635 | ||
| H. multiforme | TUMH 40197 | - | LC663693 a | ||
| TUMH 64581 | LC663674 a | - | |||
| H. pini | TUMH 61221 | LC663680 a | LC663691 a | ||
| H. punctulatum | FP-101698-Sp | KY948827 | KY948860 | ||
| Hypochnicium sp. | FP-110227-Sp | KY948804 | KY948862 | ||
| WY-DT1 | KP980549 | KY425696 | |||
| H. sphaerosporum | RLG-15138-Sp | KY948803 | KY948861 | ||
| H. subrigescens | KHL11968 (GB) | JQ031128 | JN710546 | ||
| KHL11968 (O) | JN710546 | JQ031128 | |||
| TUMH 64612 | LC663685 a | LC663692 a | |||
| H. wakefieldiae | MA:Fungi 7675 | FN552531 | JN939577 | ||
| Neohypochnicium asiaticum | TUMH 61220 | LC663669 a | LC663689 a | ||
| N. perlongicystidiosum | TUMH 40397 | LC663677 a | LC663690 a | ||
| Pouzaroporia subrufa | BRNM-710164 | FJ496661 | FJ496723 | ||
| Miettinen 14343 (H) | KY948736 | KY948736 | |||
| Candelabrochaete | Unknown | Candelabrochaete africana | FP-102987-Sp | KP135294 | KP135199 |
| Gelatoporiaceae | Gelatoporiaceae | Gelatoporia subvermispora | FD-354 | KP135312 | KP135212 |
| Obba rivulosa | FP-135416-Sp | KP135309 | KP135208 | ||
| Grifolaceae | Grifolaceae | Grifola frondosa | AFTOL-701 | AY854084 | AY629318 |
| G. sordulenta | AFTOL-562 | AY854085 | AY645050 | ||
| Incrustoporiaceae | Incrustoporiaceae | Skeletocutis chrysella | X-319 | FN907916 | FN907916 |
| S. lilacina | HHB-10522-Sp | KY948834 | KY948894 | ||
| Tyromyces chioneus | FD-4 | KP135311 | KP135291 | ||
| Ischnodermataceae | Ischnodermataceae | Ischnoderma resinosum | FD-328 | KP135303 | KP135225 |
| Russulales (outgroup) | Bondarzewiaceae | Heterobasidion annosum | DAOM-73191 | - | AF287866 |
| VL-296 | JF440572 | - | |||
| Stereaceae | Stereum hirsutum | FPL-8805 | AY854063 | AF393078 | |
a Newly obtained sequences. Bold shows Hypochnicium s. lat.
The second analysis using ITS dataset was performed for phylogenetic species delimitation of Hypochnicium s. lat. From the result of the ITS-LSU phylogeny, we included four other genera close to Hypochnicium s. lat.: namely, Abortiporus Murrill, Bulbillomyces Jülich, Cymatoderma Jungh., Podoscypha Pat., and Pouzaroporia Vampola. As an outgroup, we selected five species in /residual clade [Climacocystis borealis (Fr.) Kotl. & Pouzar, Meripilus giganteus (Pers.) P. Karst., and Radulodon casearius (Morgan) Ryvarden] and /phlebioid clade [Bjerkandera adusta (Willd.) P. Karst. and Phanerochaete rhodella (Peck) Foudas & Hibbett]. Total of 78 sequences were included in ITS dataset (Table 2). The dataset was aligned by MAFFT online and manually edited, and then we got the alignment comprising of 774 sites with 516 distinct alignment patterns. The ML and BI analyses were performed under GTR+G+I model, of which ModelTest-NG suggested as the best substitution model for the ITS dataset. The MCMC iterations in MrBayes were stopped after 2,000,000 generations. Other settings for ML and BI analyses were followed ITS-LSU dataset. The alignments and phylograms were submitted in TreeBASE (http://treebase.org/:S29321).
| Species | Specimen nos. | Locality | Accession nos. |
| Abortiporus biennis | FD-319 | USA | KP135300 |
| CBS 676.70 | USA | MH859896 | |
| A. cf. biennis | 3206-2A09 | Hungary | MW620047 |
| Bjerkandera adusta | HHB-12826-Sp | USA | KP135198 |
| Bulbillomyces farinosus | FP-100488-T | USA | KY948802 |
| Climacocystis borealis | FD-31 | USA | KP135308 |
| Gyrophanopsis japonica a | TUMH 61400 (TYPE) | Japan | LC663668 b |
| G. polonensis | FCUG 2262 | Turkey | DQ309065 |
| FCUG 2675 | Russia | DQ309067 | |
| G. zealandica | FCUG 3009 | New Zealand | DQ309068 |
| Hypochnicium albostramineum | 11-4-1 | Japan | AB907586 |
| FCUG 1865 | Spain | AF429421 | |
| FCUG 269 | Sweden | AF429422 | |
| FCUG 1772 | Sweden | AF429423 | |
| H. aotearoae | FCUG 2972 | New Zealand | DQ309071 |
| FCUG 3120 | New Zealand | GQ906536 | |
| H. bombycinum | MA:Fungi 15305 | Spain | FN552537 |
| TUMH 61180 | Japan | LC663687 b | |
| H. cremicolor | FCUG 2151 | Spain | AF429424 |
| FCUG 160 | Denmark | AF429425 | |
| F0012210 | China | KC282470 | |
| H. cystidiatum | FCUG 3086 | Central African Republic | DQ658163 |
| FCUG 3087 | Gabon | DQ658164 | |
| H. geogenium | FCUG 2052 | France | AF429426 |
| MA:Fungi 48308 | Sweden | FN552534 | |
| H. guineensis | MA:Fungi 79156 (TYPE) | Equatorial Guinea | FN552536 |
| H. huinayensis | MA:Fungi 19598Tell (TYPE) | Chile | HG000303 |
| MA:Fungi 13980MD | Chile | HG326616 | |
| H. karstenii | KUC20131022-30 | Korea | KJ668512 |
| KUC11076 | Korea | KJ713989 | |
| H. lundellii | 443 | Sweden | AY781277 |
| H. lyndoniae | FCUG 3029 | New Zealand | DQ309069 |
| FCUG 2979 | New Zealand | DQ309070 | |
| H. michelii | MA:Fungi 79155 (TYPE) | Spain | FN552535 |
| H. microsporum | CG-GUY12-075 | France | KY019169 |
| CG-GUY13-100 | France | KY019171 | |
| H. multiforme | TUMH 64581 | Japan | LC663674 b |
| H. patagonicum | GB 0129149 TYPE | Chile | HG000304 |
| H. pini | NIBRFG0000107453 | Korea | JX217823 |
| F0023763 | China | KC282471 | |
| F0023765 | China | KC282472 | |
| TUMH 61221 | Japan | LC663680 b | |
| TUMH 64587 | Japan | LC663681 b | |
| H. punctulatum | FCUG 1921 | Denmark | AF429410 |
| FCUG 1203 | Norway | AF429412 | |
| TUMH 61188 | Japan | LC663682 b | |
| Hypochnicium sp. | FP-110227-Sp | USA | KY948804 |
| KUC8713 | Korea | HM008934 | |
| WY-DT1 | China | KP980549 | |
| H. sphaerosporum | RLG-15138-Sp | USA | KY948803 |
| H. subrigescens | FCUG-1966 | Denmark | AF429427 |
| 5285 | Norway | JN710546 | |
| KHL11968 (GB) | Norway | JQ031128 | |
| TUMH 64612 | Japan | LC663685 b | |
| TUMH 61539 | Japan | LC663686 b | |
| H. wakefieldiae | FCUG 2194 | Estonia | AF429415 |
| FCUG 1709 | Finland | AF429419 | |
| MA:Fungi 7675 | Spain | FN552531 | |
| Meripilus giganteus | FP-135344-Sp | UK | KP135307 |
| Neohypochnicium asiaticum a | TUMH 64609 | Japan | LC663671 b |
| TUMH 60588 | Japan | LC663670 b | |
| TUMH 61220 (TYPE) | Japan | LC663669 b | |
| TUMH 61227 | Japan | LC663672 b | |
| TUMH 64610 | Japan | LC663673 b | |
| N. perlongicystidiosum a | TUFC 14478 | Japan | LC663677 (c-1) b |
| Japan | LC663678 (c-2) b | ||
| Japan | LC663679 (c-3) b | ||
| TUMH 63328 | Japan | LC663676 b | |
| TUMH 63618 | Japan | LC663675 b | |
| Neohypochnicium sp. | TUMH 63706 | Japan | LC663684 b |
| Phanerochaete rhodella | FD-18 | USA | KP135187 |
| Podoscypha bolleana | CBS 333.66 | Central African Republic | JN649354 |
| P. elegans | CBS 426.51 | Argentina | MH856927 |
| P. petalodes subsp. rosulata | CBS 659.84 (TYPE) | Pakistan | MH861805 |
| Pouzaroporia subrufa | BRNM-710164 | Czech Republic | FJ496661 |
| Miettinen-14343 (H) | Indonesia | KY948736 | |
| Radulodon casearius | HHB-9567-Sp | USA | KY948752 |
a New species. b Newly obtained sequences.
In ITS phylogeny, most of Hypochnicium s. lat. species showed clear resolution of its species boundary, where each species is well-supported by both monophyletic clustering an morphological difference; however, three species [H. albostramineum, “Neohypochnicium asiaticum”, and H. wakefieldiae (Bres.) J. Erikss.] are not defined by this criterion. Hence we did further species delimitation based on relative threshold of genic divergences between inter/intraspecific variation. The procedures were following to Wang et al. (2018). The threshold between inter/intraspecific variations were estimated from the clearly defined species using MEGA7 (Kumar, Stecher, & Tamura, 2016) as the average evolutionary divergence within or net between groups under maximum composite likelihood method (Tamura, Nei, & Kumar, 2004) with 1,000 replications of bootstrap analyses. The highest intraspecific variation and the lowest interspecific one in Hypochnicium s. lat. species were estimated here and compared these values among ambiguous taxa. In addition, RAxML phylogeny with a GTR+G+I model was built from a narrower range of ITS dataset including only the three species.
The ITS-LSU phylogenies were completed when the best likelihood values -InL = 28647.12 for the optimized tree in RAxML and -InL = 28422.11 and 28431.56 for cold chains in MrBayes. The ML and BI phylograms did not show the topology conflict having the supported branches (i.e., MLBS < 50, BIP < 0.90) (Fig. 1). The phylogenetic tree showed closely relationships between /resudial clade, /phlebioid clade, and Candelabrochaete africana Boidin (MLBS/BIP = 82/0.99) within the order Polyporales (MLBS/BIP = 100/1), corresponding to the previous studies (Binder et al., 2013; Justo et al., 2017). Although all Hypochnicium s. lat. species grouped in /residual clade, many species were nested within the genera Abortiporus, Bulbillomyces, Cymatoderma, Podoscypha, and Pouzaroporia. Hypochnicium bombycinum, the type species of Hypochnicium, formed monophyletic clade (MLBS/BIP = 89/0.98) with H. multiforme (Berk. & Broome) Hjortstam, H. lyndoniae (D.A. Reid) Hjortstam, and Hypochnicium sp. WY-DT1. The genus Gyrophanopsis (Gy. polonensis and Gy. japonica) formed monophyletic clade with strong support (MLBS/BIP = 100/0.99) as sister clade of Bulbillomyces farinosus (Bres.) Jülich (MLBS/BIP = 94/1). The remaining Hypochnicium species formed monophyletic clade with moderate support (MLBS/BIP = 72/0.99); namely, H. michelii Tellería, M. Dueñas, Melo & M.P. Martín, H. geogenium (Bres.) J. Erikss., H. pini, H. punctulatum, H. sphaerosporum, H. subrigescens, H. wakefieldiae, and two new species. This monophyletic clade of Hypochnicium s. str. positioned apart from Gyrophanopsis clade.

In the ITS dataset, the best likelihood values were -InL = 9179.27 in RAxML and -InL = 9406.76 and 9408.01 in MrBayes, respectively. Both analyses demonstrated the similar topology (Fig. 2). The ITS phylogenies supported each monophyletic lineage of Hypochnicium s. str. (MLBS/BIP = 88/0.99), Gyrophanopsis (MLBS = 99/1), and the remaining Hypochnicium species (MLBS/BIP = 68/0.90). At least 14 species of Hypochnicium were included in “Neohypochnicium” clade: namely, H. albostramineum, H. aotearoae B.C. Paulus, H. Nilsson & Hallenb., H. cremicolor (Bres.) H. Nilsson & Hallenb., H. cystidiatum Boidin & Gilles, H. geogenium, H. guineense Tellería, M. Dueñas, Melo & M.P. Martín, H. huinayense Tellería, M. Dueñas & M.P. Martín, H. michelii, H. microsporum G. Gruhn, Schimann & M. Roy, H. patagonicum Gorjón & Hallenb., H. pini, H. punctulatum, H. subrigescens, and H. wakefieldiae. All Japanese lineages excepting one species, “Neohypochnicium asiaticum”, formed well-supported monophyletic clade (MLBS ≥ 95, BIP ≥ 0.95) with foreign strains of conspecific taxa. On the complete sequences of ITS region (ITS1-5.8S-ITS2), the interspecific variations among “N. asiaticum”, “N. albostramineum” and “N. wakefieldiae” were relatively smaller (2.1-6.3%; 12-35 bps of differences within 535-537 bps) than those among other species of Hypochnicium s. lat. (≥ 3.5%) and “N. asiaticum” showed paraphyletic clade; however interspecific variations among the three taxa exceed those of intraspecific variation within each single species (≤ 1.4%). Also, the RAxML phylogeny including ITS sequence of only three groups indicated each monophyly of “N. wakefieldiae” (MLBS = 100), “N. albostramineum” (MLBS = 99) and “N. asiaticum” (MLBS = 71) (Supplemental Fig. S1); thus, we treated these three groups as different species.

Neohypochnicium N. Maek. & R. Sugaw., gen. nov.
MycoBank No.: MB 842573.
Diagnosis: Basidiomata resupinate, effused, adnate; hymenial surface smooth to odontoid. Hyphal system monomitic; hyphae hyaline, with clamp connections at all septa. Cystidia often present, aseptate, thin- to thick-walled, enclosed or projecting beyond the hymenial surface. Basidia subclavate to suburniform, normally 4-sterigmate, with a basal clamp. Basidiospores ellipsoid to subglobose, smooth, finely verrucose or finely echinulate, thick-walled (not less than 0.5 µm thick), cyanophilous, inamyloid.
Type species: Neohypochnicium perlongicystidiosum N. Maek., Kogi & Norikura.
Gene sequence ex-holotype: LC663690 (LSU), LC663677 (ITS).
Etymology: “Neohypochnicium” [neo (= new) + Hypochnicium] refers to having morphologically similar features to Hypochnicium.
Hypochnicium J. Erikss., Symb. Bot. Upsal. 16: 100 (1958) emend. N. Maek.
Basiodiomata resupinate, effused, adnate. Hymenial surface smooth, grandinioid to odontoid. Hyphal system monomitic; hyphae hyaline, with clamp connections at all septa. Cystidia often present, aseptate, thin- to thick-walled, enclosed or projecting beyond the hymenial surface. Basidia clavate to suburniform, normally 4-sterigmate, with a basal clamp. Basidiospores ellipsoid to subglobose, smooth, thick-walled (not less than 0.5 µm thick), cyanophilous, inamyloid.
Remarks: Hypochnicium emended is primarily characterized by producing smooth, thick-walled (not less than 0.5 µm thick) and cyanophilous basidiospores, clavate to suburniform basidia, and hyphae with clamp connections at all septa. This genus shares these morphological features with Bulbillomyces and Gyrophanopsis. But Bulbillomyces distinguishes from Hypochnicium in that produces sclerotia (Aegerita state) associated with teleomorph. Gyrophanopsis differs from Hypochnicium in that has heavily encrusted septocystidia and only slightly thick-walled basidiospores (less than 0.5 µm thick).
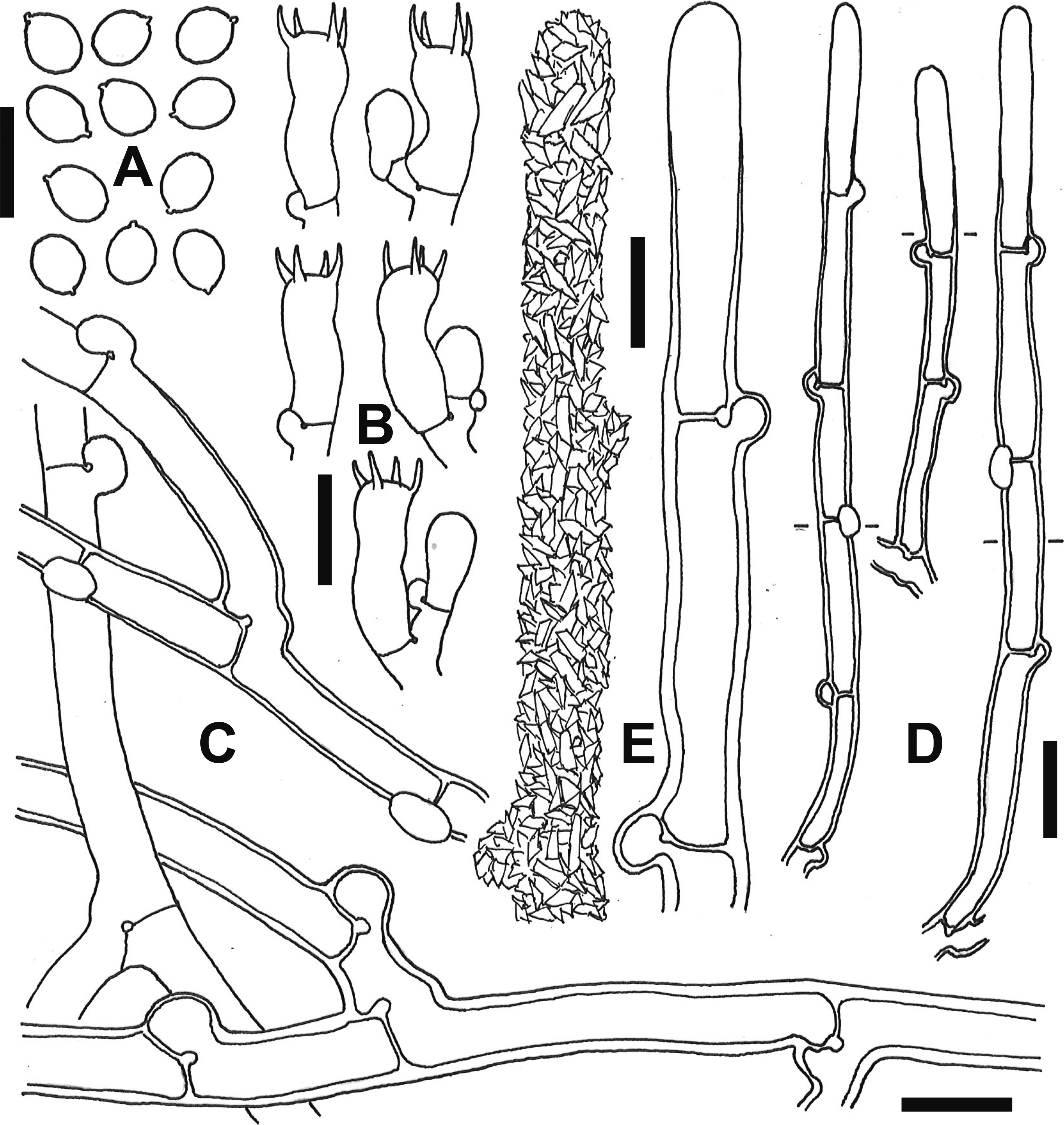
3.2.2. New speciesNeohypochnicium asiaticum N. Maek. & Kogi, sp. nov. (Figs. 3A, 4, 7A, 8A)
MycoBank No.: MB 842561.
Diagnosis: Neohypochnicium asiaticum is characterized by having smooth hymenophores, cylindrical to subfusiform cystidia (52-170 × 9-13 µm), and broadly ellipsoid, finely verrucose, thick-walled (0.5-1 µm thick) basidiospores measuring 7-8(-8.5) × 5.5-6.5 µm.
Holotype: TUMH 61220, on decayed wood of coniferous tree, Kokoge, Tottori City, Tottori Pref., Japan, 8 Nov 1983, collected by N. Maekawa. Ex-holotype culture: TUFC 31698. GenBank: LSU = LC663689; ITS = LC663669.
Etymology: “asiaticum” refers to Asia, where the vouchered specimens were collected.
Description: Basidiomes resupinate, adnate, effused, 100-300 µm thick; hymenial surface white, “Buff” to “Pale Luteous” when drying, smooth, sometimes minutely cracked under the lens (×10); margin concolorous with the hymenial surface, thinning out, indeterminate. In vertical section, subhymenium subhyaline, composed of densely interwoven hyphae; subiculum subhyaline, composed of loosely interwoven hyphae at first and then gradually becoming dense. Hyphal system monomitic; hyphae 3-7.5 µm wide, smooth, thin- to thick-walled (up to 1.5 µm thick), with clamp connection at all septa. Cystidia arising from subicular or subhymenial hyphae, cylindrical to subfusiform with obtuse apex, 52-170 × 9-13 µm, smooth, thin- to slightly thick-walled (up to 0.5 µm), with a clamp connection at the basal septum, projecting up to 80 µm beyond the hymenial surface. Basidia clavate to suburniform, 23-42 × 7-8 µm, thin-walled, usually with oily drops, with a basal clamp, producing 4 sterigmata; sterigmata 5-7 µm in length. Basidiospores broadly ellipsoid, 7-8(-8.5) × 5.5-6.5 µm [Lm = 7.6 ± 0.4 µm, Wm = 5.9 ± 0.3 µm, Q = 1.2-1.4, Qm = 1.3 ± 0.1; n=180/9] finely verrucose, thick-walled (0.5-1 µm thick), cyanophilous, non-amyloid.
Characteristics in culture: The optimum growth temperature for the polysporous isolates TUFC 31698, TUFC 31733 and TUFC 100660 was 25 °C. Isolates could grow between 5 and 30 °C, but no visible growth was observed at 35 °C. Growth on MA was 20-26 mm in radius at 25 °C after 5 d in the dark. Mycelial mats at that time white, cottony; reverse side of mycelial mats not discolored; agar not bleached; odor not noticeable. Aerial hyphae 2-6 µm wide, subhyaline, thin- to slightly thick-walled (up to 0.5 µm thick), with clamp connections at all septa, moderately branched, occasionally encrusted. Marginal hyphae 2-4 µm wide, subhyaline, thin-walled, clamp connections at all septa, sparsely branched. Submerged hyphae dense, 4-7.5 µm wide, subhyaline, thin- to slightly thick-walled (up to 0.5 µm thick), with clamp connections at all septa, relatively much branched. Conidia, chlamydospores and cystidium-like cells not observed after 6 wk of incubation at 25 °C.
Species codes (Stalpers, 1978): 7, (12), 14, 21, 30, 39, 52, 53, 54, 82.
Other specimens and cultures examined: JAPAN. TUMH 60588 on dead and decorticated trunk of a coniferous tree, Hasehiji, Ina City, Nagano Pref., 19 Sep 2013, collected by N. Maekawa (culture: TUFC 100660); TUMH 64607 [as H. punctulatum (TMI 17549)] on decayed wood of Cryptomeria japonica D. Don, Miasa-mura, Kitaazuki-gun, Nagano Pref., 12 Jul 1987, collected by N. Maekawa; TUMH 64605 [as H. punctulatum (TMI 9479)] on decayed wood of Pinus densiflora Sieb. & Zucc., Miasa-mura, Kitaazuki-gun, Nagano Pref., 2 Oct 1987, collected by N. Maekawa; TUMH 64609 on decayed wood of a coniferous tree, Takane-machi, Takayama City, Gifu Pref., 28 Sep, 2015, collected by N. Maekawa; TUMH 61227 [as H. eichleri (TMI 17550)] on decayed wood of C. japonica, Kokoge, Tottori City, Tottori Pref., 17 May 1984, collected by N. Maekawa (culture TUFC 31733); TUMH 64608 [as H. eichleri (TMI 10704)] on decayed wood of P. densiflora, Daisen-cho, Saihaku-gun, Tottori Pref., 28 Oct 1984, collected by N. Maekawa; TUMH 64606 [as H. punctulatum (TMI 17548)] on dead trunk of P. densiflora, Okamasu, Kokufu-cho, Tottori City, 14 Jul 1986, collected by N. Maekawa; TUMH 64610 on dead branch of P. densiflora, Daisen-cho, Saihaku-gun, Tottori Pref., 11 Oct 2015, collected by H. Kogi.
Remarks: Neohypochnicium asiaticum is characterized by smooth hymenial surface, broadly ellipsoid and finely verrucose basidiospores measuring 7-8(-8.5) × 5.5-6.5 µm, and cylindrical to subfusiform cystidia measuring 52-170 × 9-13 µm. Neohypochnicium asiaticum is placed in the H. punctulatum complex designated by Nilsson and Hallenberg (2003), with N. albostramineum, N. huinayense, N. punctulatum and N. wakefieldiae. Phylogenetically, N. asiaticum is very close to N. albostramineum, but basidiospores (8-9.5 × 6.5-7.5 µm, Tellería, Dueñas, Melo, Hallenberg, & Martín, 2010) of H. albostramineum are larger than those of N. asiaticum. Neohypochnicium asiaticum also resembles N. wakefieldiae having broadly ellipsoid to subglobose basidiospores measuring 6.3-8.3 × 5.5-6.3 µm (Martínez & Nakasone, 2014) and 5-6.5(-7) × 4.5-6 µm (Parmasto, 1967, as H. caucasicum Parm.), and slightly thick-walled subicular hyphae. However, the two species have different substrate preferences, viz., N. asiaticum has been collected only from coniferous trees such as C. japonica and P. densiflora, whereas N. wakefieldiae have been recorded from both broad-leaved and coniferous trees (Nilsson & Hallenberg, 2003). Furthermore, ITS sequence data revealed that N. wakefieldiae is phylogenetically different from N. asiaticum, although sequences from the five specimens of the latter species do not form a single clade. At least N. asiaticum must currently be regarded as morphologically cryptic with N. wakefieldiae.
Neohypochnicium perlongicystidiosum N. Maek., Kogi & Norikura, sp. nov. (Figs. 3B, 5, 7B, 8B, 8D)
MycoBank No.: MB 842574.
Diagnosis: Neohypochnicium perlongicystidiosum is distinguished from others in the genus by having odontoid hymenophore, cylindrical and thick-walled cystidia (75-493 × 7.5-14.5 µm), and subglobose to globose, finely verrucose, thick-walled (up to 2.5 µm thick) basidiospores measuring (9-)10-12(-13.5) × (8.5-)9-11(-13) µm.
Holotype: TUMH 63328, on dead branch of a broad-leaved tree, Yakushima-cho (Yakushima Island), Kumage-gun, Kagoshima Pref., Japan, 6 Sep 2018, collected by N. Maekawa. Ex-holotype culture: TUFC 101573. GenBank: LSU = LC663690; ITS = LC663677.
Etymology: “perlongicystidiosum” refers to having significantly long cystidia.
Description: Basidiomes resupinate, adnate, effused; hymenial surface whitish cream, “Buff” to pale ochre, smooth, grandinioid to odontoid (5-18 warts per mm2), byssoid to hispidulous under the lens (×10); margin concolous to the hymenial surface, thinning out, indeterminate. In vertical section, subhymenium subhyaline, composed of densely interwoven hyphae; subiculum subhyaline, composed of loosely interwoven hyphae at first and then gradually becoming dense. Hyphal system monomitic; hyphae 4.5-8 µm wide, smooth, thin- to thick-walled (up to 2 µm thick), with clamp connection at all septa (Fig. 4D). Cystidia arising from subicular hyphae, cylindrical to tubular, 75-493 × 7.5-14.5 µm, smooth, thick-walled (2-4 µm thick) excepting the apical part, with a clamp at the basal septum, projecting up to 250 µm beyond the hymenial surface (Fig. 4C). Basidia clavate to suburniform, 46-70 × 9-12 µm, thick-walled (up to 1 µm thick) in the lower half, with a basal clamp, producing 4 sterigmata; sterigmata 7-9 µm in length (Fig. 4B). Basidiospores subglobose to globose, (9-)10-12(-13.5) × (8.5-)9-11(-13) µm (Lm = 11.2 ± 1.1 µm, Wm = 10.3 ± 1.0 µm, Q = 1.0-1.2, Qm = 1.1 ± 0.1; n=120/6), finely verrucose, thick-walled (up to 2.5 µm thick), cyanophilous, non-amyloid (Fig. 4A).
Characteristics in culture: The optimum growth temperature for the polysporous isolates TUFC 14478 and TUFC 101573 was 25 °C. Isolates could grow between 5 and 30 °C, but no visible growth was observed at 35 °C. Growth on MA was 25-28 mm in radius at 25 °C after 5 d in the dark. Mycelial mats at that time white, cottony to woolly (Fig. 8B); reverse side of mycelial mats not discolored after 2 w; agar bleached after 2 w; odor not noticeable. Aerial hyphae 2.5-8 µm wide, subhyaline, thin- to slightly thick-walled (up to 0.5 µm thick), clamp connections presnt at all septa, sometimes encrusted, moderately branched. Marginal hyphae 2.5-6.5 µm wide, subhyaline, thin-walled, clamp connections at all septa, sparsely branched. Submerged hyphae dense, 2.5-6.5 µm wide, subhyaline, sometimes with pale yellowish oily contents, thin- to slightly thick-walled (up to 0.5 µm thick), with clamp connections at all septa. Chlamydospores limoniform, fusiform to ellipsoid, sometimes irregular shaped, 27-52 × 14-25 µm, thick-walled (1.5-3 µm thick), terminal or intercalary, abundant on aerial hyphae (Fig. 8D). Conidia and cystidium-like cells not observed after 6 wk of incubation at 25 °C.
Species codes (Stalpers, 1978): 6, (12), 14, (21), (22), 30, 37, 39, 52, 53, 54, (55), (57), 82, 85.
Other specimens and cultures examined: JAPAN. TUMH 40397 on dead branch of a broad-leaved tree, Hachijo-machi (Hachijo Island), Tokyo, 8 Sep 2011, collected by N. Maekawa and R. Nakano (culture: TUFC 14478); TUMH 64602 on dead trunk of Quercus acutissima Carruth. (bedlog of shiitake cultivation), Hamatama-machi, Higashimatuura-gun, Saga Pref., 16 Dec 2003, collected by K. Kamohara; TUMH 64603 on dead trunk of Q. acutissima (bedlog of shiitake cultivation), Saigo-son, Higashiusuki-gun, Miyazaki Pref., 6 Dec 2003, collected by S. Komatsu; TUMH 64604 on dead trunk of Q. serrata Murray (bedlog of shiitake cultivation), Miyazaki Pref., 3 Feb 2016, collected by N. Maekawa; TUMH 63618 on dead trunk of Q. acutissima (bedlog of shiitake cultivation), Miyazaki Pref., 8 Jul 2016, collected by E. Nagasawa (culture TUFC 101628).
Remarks: Neohypochnicium perlongicystidiosum is primarily characterized by having grandinioid to odontoid hymenial surface and cylindrical, thick-walled cystidia measuring 75-493 × 7.5-14.5 µm. Neohypochnicium perlongicystidiosum is morphologically similar to H. longicystidiosum (S.S. Rattan) Hjortstam & Ryvarden (as Hyphodontia longicystidiosa S.S. Rattan; Rattan, 1977) and N. patagonicum in producing long cystidia. However, basidiospores of H. longicystidiosum are smooth and 4-5.5 × 4-4.5 µm (Rattan, 1977), whereas N. perlongicystidiosum produces finely verrucose and larger basidiospores measuring (9-)10-12(-13.5) × (8.5-)9-11(-13) µm. Neohypochnicium patagonicum produces also morphologically similar cystidia measuring 100-250(-350) × 8-12 µm, but its basidiospores [8-10 × (7-)7.5-8.5 µm, Gorjón & Hallenberg (2013)] are smaller than those of N. perlongicystidiosum. Furthermore, ITS sequence data showed that N. perlongicystidiosum is phylogenetically distinct from N. patagonicum.
Gyrophanopsis japonica N. Maek. & Kogi, sp. nov. (Figs. 3C, 6, 8C)
MycoBank No.: MB 842575.
Diagnosis: Gyrophanopsis japonica is characterized by byssoid to hispidulous hymenophore, cylindrical septocystidia (105-200 × 6-9 µm) heavily covered with subhyaline crystals, and broadly ellipsoid to subglobose, smooth and slightly thick-walled (up to 0.5 µm thick) basidiospores measuring 5-5.5 × 4-4.5 µm.
Holotype: TUMH 61400, on decayed wood of a broad-leaved tree, Taiwa-cho, Kurokawa-gun, Miyagi Pref., Japan, 21 Sep 2014, collected by N. Maekawa. Ex-holotype culture: TUFC 100860. GenBank: LSU = LC663688; ITS = LC663668.
Etymology: “japonica” refers to Japan, where the vouchered specimen was collected.
Description: Basidiome resupinate, adnate, effused, 100-200 µm thick; hymenial surface “Buff” when drying, smooth to grandinioid, byssoid to hispidulous under the lens (×10); margin concolorous with the hymenial surface, thinning out, indeterminate. In vertical section, subhymenium subhyaline, composed of densely interwoven hyphae; subiculum subhyaline, composed of loosely interwoven hyphae. Hyphal system monomitic; hyphae 4-9.5 µm wide, smooth, thin- to thick-walled (up to 2.5 µm thick), clamp connection at all septa, sometimes heavily encrusted. Cystidia (septocystidia) arising from subicular hyphae, cylindrical with obtuse apex, 2-4 clamped septate, 105-200 × 6-9 µm, thick-walled (up to 2 µm thick), but wall becoming thinner towards the apex, with a clamp connection at the basal septum, projecting up to 130 µm beyond the hymenial surface, heavily encrusted with subhyaline crystalline material of 1-2 µm thick. Basidia subclavate to subcylindric, slightly constricted, 14-20 × 5-5.5 µm, with a basal clamp, producing 4 sterigmata; sterigmata 2-3 µm in length. Basidiospores broadly ellipsoid to subglobose, 5-5.5 × 4-4.5 µm [Lm = 5.4 ± 0.2 µm, Wm = 4.2 ± 0.3 µm, Q = 1.2-1.4, Qm = 1.3 ± 0.1; n=20/1], smooth, slightly thick-walled (up to 0.5 µm thick), cyanophilous, non-amyloid.
Characteristics in culture: The optimum growth temperature for the polysporous isolate TUFC 100860 was 25-30 °C. Isolate could grow between 5 and 35 °C, but no visible growth was observed at 40 °C. Growth on MA was 22-28 mm in radius at 25 °C after 21 d in the dark. Mycelial mats at that time transparent, but whitish around the inoculum, downy; reverse side of mycelial mats not discolored; agar not bleached. Aerial hyphae 2-4 µm wide, subhyaline, thin-walled, usually lacking clamp connections at the septa, moderately branched, occasionally encrusted. Marginal hyphae 2-4 µm wide, subhyaline, thin-walled, lacking clamp connections at all septa, sparsely branched. Submerged hyphae 2-4.5 µm wide, subhyaline, thin-walled, clamp connections at the septa, but occasionally clampless septate, moderately branched. Chlamydospores, conidia and cystidium-like cells not observed after 3 w of incubation at 25 °C.
Species codes (Stalpers, 1978): 9, 13, 14, 17, (30), (39), 52, 53, (82).
Specimen and culture examined: see holotype.
Remarks: Gyrophanopsis japonica is characterized by its wider subicular hyphae (4-9.5 µm wide), heavily encrusted septocystidia, small basidia (14-20 × 5-5.5 µm) and globose, slightly thick-walled basidiospores measuring 5-5.5 × 4-4.5 µm. This species differs phylogenetically from the previously described two species, Gy. polonensis and G. zealandica. In addition, Gy. japonica produces globose basidiospores (5-5.5 × 4-4.5 µm) and basidia measuring 14-20 × 5-5.5 µm, whereas Gy. polonensis produces ellipsoid basidiospores (6.5-8.5 × 4-5 µm) and longer basidia measuring 20-35 × 4-6 µm (Maekawa, 1994). On the other hand, G. zealandica strongly resembles Gy. japonica, but differs in some minor characters. According to Stalpers and Buchanan (1991), septocystidia of G. zealandica are partly covered with a sheath of yellowish-brown crystalline material of about 1 µm thick, whereas those of Gy. japonica are covered with subhyaline crystalline material. The other morphological characteristics, viz. basidiospore shape and size and hyphal features of Gy. japonica are overlapped to those of Gy. zealandica.





Neohypochnicium albostramineum (Bres.) N. Maek., comb. nov.
Basionym: Hypochnus albostramineus Bres., Annls mycol. 1(2): 109, 1903.
MycoBank No.: MB 842576.
Remarks: For descriptions, see Bernicchia and Gorjón (2010), and Nilsson and Hallenberg (2003). This species has broadly ellipsoid, finely verrucose basidiospores measureing 8-10(-12) × 6.5-7(-8) µm (Bernicchia & Gorjón, 2010).
Neohypochnicium aotearoae (B.C. Paulus, H. Nilsson & Hallenb.) N. Maek., comb. nov.
Basionym: Hypochnicium aotearoae B.C. Paulus, H. Nilsson & Hallenb., N.Z. Jl Bot. 45(1): 144, 2007.
MycoBank No.: MB 842577.
Remarks: This species has ovoid to subglobose, verrucose basidiospores measuring 5.6-8.7 × 4.8-7.3 µm (Paulus, Nilsson & Hallenberg, 2007).
Neohypochnicium capitulatum (Boidin & Gilles) N. Maek., comb. nov.
Basionym: Hypochnicium capitulatum Boidin & Gilles, Bull. Soc. Mycol. Fr., 116: 164, 2000.
MycoBank No.: MB 842578.
Remarks: Although sequence data of N. capitulatum has not been obtained, the species belongs to Neohypochnicium because it forms subglobose, warted basidiospores measuring 8-9 × 7-7.5 µm (Boidin & Gilles, 2000).
Neohypochnicium cremicolor (Bres.) N. Maek., comb. nov.
Basionym: Hypochnus cremicolor Bres., Annls mycol. 1(2): 109, 1903.
MycoBank No.: MB 842579.
Remarks: For descriptions, see Bernicchia and Gorjón (2010), and Nilsson and Hallenberg (2003). This species has broadly ellipsoid, finely verrucose basidiospores measuring 6-6.5 × 5-5.5 µm (Bernicchia & Gorjón, 2010).
Neohypochnicium cystidiatum (Boidin & Gilles) N. Maek., comb. nov.
Basionym: Hypochnicium cystidiatum Boidin & Gilles, Cahiers de La Maboké, 9(2): 90, 1971.
MycoBank No.: MB 842580.
Remarks: This species has ovoid, ornamented basidiospores measuring (4.8-)5.2-6.7 × 4.2-5.2 µm (Boidin & Lanquetin, 1971).
Neohypochnicium geogenium (Bres.) N. Maek., comb. nov.
Basionym: Corticium geogenium Bres., Annls Mycol. 1(2): 98, 1903.
MycoBank No.: MB 842581.
Remarks: For descriptions, see Bernicchia and Gorjón (2010), Eriksson and Ryvarden (1976). This species has ellipsoid, smooth basidiospores measuring 6-7.5(-9) × 4-4.5(-5.5) µm (Eriksson & Ryvarden, 1976).
Neohypochnicium guineense (Tellería, M. Dueñas, Melo & M.P. Martín) N. Maek., comb. nov.
Basionym: Hypochnicium guineense Tellería, M. Dueñas, Melo & M.P. Martín, Mycologia 102(6): 1431, 2010.
MycoBank No.: MB 842582.
Remarks: This species has globose, verrucose basidiospores measuring (7-)8-9 × (7-)7.5-8.5(-10) µm (Tellería et al., 2010).
Neohypochnicium huinayense (Tellería, M. Dueñas & M.P. Martín) N. Maek., comb. nov.
Basionym: Hypochnicium huinayense Tellería, M. Bueñas & M.P. Martín, Persoonia 31: 281, 2013.
MycoBank No.: MB 842583.
Remarks: This species has globose, ornamented basidiospores measuring 6.5-8(-9) × 6.5-8 µm (Crous et al., 2013).
Neohypochnicium michelii (Tellería, M. Dueñas, Melo & M.P. Martín) N. Maek., comb. nov.
Basionym: Hypochnicium michelii Tellería, M. Dueñas, Melo & M.P. Martín, Mycologia 102: 1431, 2010.
MycoBank No.: 842585.
Remarks: This species has ellipsoid, smooth basidiospores measuring (7.5-)9-11 × (6-)7-7.5 µm (Tellería et al., 2010).
Neohypochnicium microsporum (G. Gruhn, Schimann & M. Roy) N. Maek., comb. nov.
Basionym: Hypochnicium microsporum G. Gruhn, Schimann & M. Roy, Bull. Soc. Mycol. Fr., 130: 303, 2017.
MycoBank No.: MB 842584.
Remarks: This species has subglobose, smooth basidiospores measuring (3.7-)3.9-5(-5.8 × (3.2-)3.8-5(-5.3) µm (Gruhn, Schimann & Roy, 2014).
Neohypochnicium patagonicum (Gorjón & Hallenb.) N. Maek., comb. nov.
Basionym: Hypochnicium patagonicum Gorjón & Hallenb., Mycol. Progr. 12(2): 188, 2013.
MycoBank No.: MB 842586.
Remarks: This species has broadly ellipsoid to subglobose, verrucose basidiospores measuring 8-10 × (7-)7.5-8.5 µm (Gorjón & Hallenberg, 2013).
Neohypochnicium pini (Y. Jang & J-J. Kim) N. Maek., comb. nov.
Basionym: Hypochnicium pini Y. Jang & J-J. Kim, Mycotaxon 124: 211, 2013.
MycoBank No.: MB 842587.
Description: Basidiomes resupinate, adnate, effused, 50-200 µm thick; hymenial surface white, “Buff” to “Pale Luteous” when drying, smooth to minutely warted. Hyphae 4-6 µm wide, smooth, thin- to thick-walled (up to 2 µm thick), with clamp connection at all septa. Cystidia cylindrical to subfusiform with obtuse apex, 53-132 × 6-11.5 µm, thin- to slightly thick-walled (up to 0.5 µm), with a clamp connection at the basal septum, projecting up to 50 µm beyond the hymenial surface. Basidia clavate to suburniform, 18-37 × 5-8 µm, producing 4 sterigmata. Basidiospores broadly ellipsoid, 5.5-7(-8) × 4.5-6(-6.5) µm, finely verrucose, thick-walled (up to 1 µm thick), cyanophilous, non-amyloid.
Specimens and cultures examined: JAPAN. TUMH 64589 [as H. punctulatum (TMI 6991)] on dead trunk of a broad-leaved tree, Daisen-cho, Saihaku-gun, Tottori Pref., 18 Oct 1981, collected by N. Maekawa; TUMH 64588 [as H. punctulatum (TMI 6771)] on dead trunk of Pinus densiflora, Ichikawa-cho, Kanzaki-gun, Hyogo Pref., 19 Jul 1981, collected by N. Maekawa; TUMH 64590 [as H. punctulatum (TMI 7618)] on dead trunk of Quercus serrata, Higashioro, Tottori City, Tottori Pref., 9 Oct 1983, collected by N. Maekawa; TUMH 64592 [as H. punctulatum (TMI 17547)] on decayed wood of a broad-leaved tree, Daisen-cho, Saihaku-gun, Tottori Pref., 1 Oct 1984, collected by N. Maekawa; TUMH 61221 [as H. punctulatum (TMI 17546)] on dead trunk of Castanopsis cuspidata (Thunb.) Schottky, Ue-machi, Tottori City, Tottori Pref., 1 Jul 1985, collected by N. Maekawa and E. Nagasawa (culture: TUFC 30475); TUMH 64591 on dead branch of Larix kaempferi (Lamb.) Carrière, Miasa-mura, Kitaazumi-gun, Nagano Pref., 1 Oct 1987, collected by N. Maekawa; TUMH 64587 on dead branch of a broad-leaved tree, Yorii-machi, Osato-gun, Saitama Pref., 12 Sep 2014, collected by H. Kogi.
Remarks: Of the Japanese specimens, TUMH 64588, TUMH 64589, TUMH 64590, TUMH 64592 and TUMH 61221 were reported as H. punctulatum by Maekawa (1994), but in this study these specimens were identified as N. pini based on phylogenetic analyses and morphological re-observation.
Neohypochnicium punctulatum (Cooke) N. Maek., comb. nov.
Basionym: Corticium punctulatum Cooke, Grevillea 6(no. 40): 132, 1878.
MycoBank No.: MB 842588.
Description: Basidiomes resupinate, adnate, effused, 70-110 µm thick; hymenial surface white, “Buff” to “Pale Luteous” when drying, smooth. Hyphae 4-6 µm wide, smooth, thin-walled, with clamp connection at all septa. Cystidia cylindrical to subfusiform with obtuse apex, 70-107 × 5-10 µm, thin-walled, with a clamp connection at the basal septum, projecting up to 30 µm beyond the hymenial surface. Basidia clavate to suburniform, 28-35 × 6.5-7.5 µm, with a basal clamp, producing 4 sterigmata. Basidiospores broadly ellipsoid, 7.5-8.5 × 6.5-7.5 µm, finely verrucose, thick-walled (up to 1 µm thick), cyanophilous, non-amyloid.
Specimen and culture examined: JAPAN. TUMH 61188 on dead trunk of dead trunk of a coniferous tree, Iijima-machi, Kamiina-gun, Nagano Pref., 10 Oct 1996, collected by N. Maekawa (culture: TUFC 33598).
Remarks: Basidiospore dimensions of the Japanese specimen (TUMH 61188) fall into those of H. punctulatum s. str. described by Nilsson and Hallenberg (2003). The Japanese specimens reported as H. punctulatum and H. eichleri by Maekawa (1994) were identified N. asiaticum (see specimens of N. asiaticum) or N. pini (see specimens of N. pini) based on the phylogenetic analyses and morphological re-observation.
Neohypochnicium subrigescens (Boidin) N. Maek., comb. nov.
Basinonym: Hypochnicium subrigescens Boidin, Cahirs de La Maboké, 9(2): 90, 1971.
MycoBank No.: MB 842589.
Description: Basidiomes resupinate, adnate, effused, 100-200 µm thick; hymenial surface white to “Buff” when drying, smooth. Hyphae 3-8 µm wide, smooth, thin- to slightly thick-walled (up to 0.5 µm thick), with clamp connections at all septa, densely arranged in the subiculum. Cystidia cylindrical to subfusiform with obtuse apex, 42-180 × 6.5-10 µm, thin-walled, with a clamp connection at the basal septum, projecting up to 100 µm beyond the hymenial surface. Basidia clavate to suburniform, 18-36 × 5-8 µm, with a basal clamp, producing 4 sterigmata. Basidiospores subglobose to broadly ellipsoid, (5.5-)6-7(-7.5) × (5-)5.5-6.5(-7) µm, smooth, thick-walled (up to 1 µm thick), weakly cyanophilous, non-amyloid.
Specimens examined: JAPAN: TUMH 61539 on decayed wood of Cerasus sargentii (Rehder) H. Ohba, near Hanadate, Osaki City, Miyagi Pref., 22 Sep 2014, collected by N. Maekawa (culture: TUFC 100892); TUMH 64583 [as H. sphaerosporum (TMI 10702)] and TUMH 64584 [as H. sphaerosporum (TMI 17551)] on a shiitake bedlog of Quercus serrata, Daito-cho, Higashiiwai-gun, Iwate Pref., 5 Nov 1986, collected by N. Maekawa; TUMH 64585 [as H. sphaerosporum (TMI 17552)] on a shiitake bedlog of Q. crispula Blume, Daito-cho, Higashiiwai-gun, Iwate Pref., 5 Nov 1986, collected by N. Maekawa; TUMH 64586 [as H. sphaerosporum (TMI 17553)] on decayed wood of a broad-leaved tree, Misakubo-cho, Iwate-gun, Iwate Pref., 21 Sep 1986, collected by N. Maekawa, collected by N. Maekawa; TUMH 64582 [as H. sphaerosporum (TMI 9477)] on dead trunk of Pinus sp., Miasa-mura, Kitaazumi-gun, Nagano Pref., 1 Oct 1987, collected by N. Maekawa; TUMH 64612 on dead and decorticated trunk of a coniferous tree, Takane-machi, Takayama City, Gifu Pref., 27 Sep, 2015, collected by N. Maekawa. DENMARK: GB-0150192 on log of Betula sp., Jutland, Ebeltoft, Ahl, 1 Sep 1987, collected by N. Hallenberg. NORWAY: GB-0104172 on dead Alnus incana (L.) Moench, Akershus, Skedsmo, Holmen Nature Reserve, 1994, collected by H. Kauserud. SWEDEN: GB-0087503, Västergötland, Medelplana par., Rabäck, Munkängarna, 8 Oct 2008, collected by K.H. Larsson; GB-0090069 on deciduous wood, Västergötland, Nolhaga, 19 Sep 1994, collected by K. Hjortstam; GB-0090501 on wood of angiosperm, Västergötland. V:a Tunhem par., Halleberg, Hallesnipen-Ovandalen, 5 Oct 2006, collected by K.H. Larsson; GB-0090583 on Betula sp., Jämtland, Kall par., Skäckerfjällen Nature Res., 6 Sep 2006, collected by K.H. Larsson.
Remarks: This species is characterized by densely arranged relatively thin-walled hyphae in the subiculum and smooth, subglobose to broadly ellipsoid basidiospores measuring (5.5-)6-7(-7.5) × (5-)5.5-6.5(-7) µm (Fig. 7C). Hypochnicium erikssonii Hallenb. & Hjortstam resembles H. subrigescens morphologically, but differs in having larger basidiospores measuring 7-8 × 5.5-7 µm (Hallenberg & Hjortstam, 1990). In Japan, Maekawa (1994) reported a Hypochnicium species with smooth, subglobose to globose basidiospores measuring 6-7.5 × 5-6.5 µm as H. sphaerosporum. But H. sphaerosporum has been treated a synonym of H. punctulatum because its type specimen produces weakly ornamented basidiospores (Hallenberg & Hjortstam, 1990; Nilsson & Hallenberg, 2003). In the present study, reexamination of the Japanese specimens of H. sphaerosporum (TUMH 64582, TUMH 64583, TUMH 64584, TUMH 64585 and TUMH 64586) showed their smooth basidiospores. In addition, the morphological features of these Japanese specimens were identical to those of the six specimens of H. subrigescens collected from Denmark, Norway and Sweden.
Neohypochnicium wakefieldiae (Bres.) N. Maek., comb. nov.
Basionym: Corticium wakefieldiae Bres., Annls mycol., 18(1/3): 48, 1920.
MycoBank No.: MB 842590.
Remarks: For descriptions, see Bernicchia and Gorjón (2010), Nilsson and Hallenberg (2003), and Tellería et al. (2010). This species has broadly ellipsoid, finely verrucose basidiospores measureing 6.5-8 × 5.5-6 µm (Bernicchia & Gorjón, 2010).
3.2.4. Previously unreported species from JapanHypochnicium multiforme (Berk. & Broome) Hjortstam, Windahlia 23: 2, 1998.
Description: Basidiomes resupinate, adnate, effused, 50-150 µm thick; hymenial surface grayish white to pale yellowish white when drying, smooth; margin concolous with the hymenial surface, thinning out, indeterminate. Hyphae 2-5(-6) µm wide, smooth, thin- to slightly thick-walled (up to 1 µm thick), with clamp connections at all septa, densely arranged in the subiculum. Cystidia lacking. Basidia subclavate to suburniform, 22-45 × 7.5-12 µm, with a basal clamp, producing 4 sterigmata. Basidiospores subglobose to globose, (7-)9-11(-12) × (6.5-)8-10(-11) µm, smooth, thick-walled (up to 2 µm thick), weakly cyanophilous, non-amyloid.
Specimens and cultures examined: JAPAN. TUMH 64580 [as H. globosum (TMI 10705)] on decayed wood of Pinus densiflora, Ue-machi, Tottori City, Tottori Pref., 10 Jul 1986, collected by N. Maekawa; TUMH 64579 [as H. globosum (TMI 15329)] on decayed wood of P. densiflora, Nishigo-mura, Nishishirakawa-gun, Fukushima Pref., 2 Nov 1986, collected by N. Maekawa; TUMH 64578 [as H. globosum (TMI 15330)] on dead branch of a broad-leaved tree, Aihara, Sagamihara City, Kanagawa Pref., 6 Oct 1991, collected by S. Kigawa; TUMH 61219 on dead trunk of Trochodendron aralioides Siebold & Zucc., Kumage-gun (Yakushima Island), Kagoshima Pref., 25 Oct 1995, collected by N. Maekawa; TUMH 40197 on dead branch of a broad-leaved tree, Midorigaoka, Iwamizawa City, Hokkaido, 25 Sep 2010, collected by N. Maekawa (culture: TUFC 12763); TUMH 64581 on dead branch of Quercus crispula Blume, Takane-machi, Takayama City, Gifu Pref., 28 Sep 2015, collected by N. Maekawa.
Remarks: The Japanese specimens are primarily characterized by having ovoid to subglobose, smooth, thick-walled (up to 2 µm thick) and weakly cyanophilous basidiospores measuring (7-)9-11(-11.5) × (6.5-)8-10(-11) µm, and lacking cystidia. These characteristics conform the descriptions of H. karstenii (Bres.) Hallenb. (Hallenberg, 1983), a synonym of H. multiforme.
Paulus et al. (2007) suggested based on ITS region of rDNA that Hypochnicium forms a monophyletic group and that Gyrophanopsis is a synonymy of Hypochnicium. Subsequently, Tellería et al. (2010) detected two major clades (one clade containing taxa with smooth basidiospores; another ones with ornamented basidiospores) within Hypochnicium by phylogenetic analysis using 43 sequences from 15 species of this genus with two Hyphoderma species as out groups. These molecular phylogenetic analyses showed the monophyly of Hypochnicium, but they performed phylogenetic analyses using only Hypochnicium species without phylogenetically related genera. Justo et al. (2017) showed that Hypochnicium is polyphyletic lineage located in the “/residual clade” of Polyporales based on molecular phylogenetic analyses using LSU, ITS and rpb1 genes of Hypochnicium and its related genera. Namely, the genus was divided into a clade, “/hypochnicium clade”, containing the type species H. bombycinum and H. karstenii producing smooth basidiospores and another clade containing H. punctulatum and H. wakefieldiae having ornamented basidiospores located in Podoscyphaceae. Our molecular phylogenetic analyses also revealed that Hypochnicium s. lat. (hereinafter referred to as hypochnicioid species) is clearly polyphyletic. Hypochnicium s. str. emended in the present study is consisted of restricted species with smooth basidiospores, whereas Neohypochnicium includes both species with smooth basidiospores and ones with ornamented basidiospores. Based on our phylogenetic analyses, we proposed two new species and 15 new combinations (11 species with ornamented basidiospores and four with smooth ones) for Neohypochnicium. However, concerning hypochnicioid species with smooth basidiospores, we have not found morphologically discriminative criteria that distinguish between Hypochnicium and Neohypochnicium. Without molecular phylogenetic analysis, at present it is not possible to identify the genus to which a hypochnicioid species with smooth basidiospores belongs. Therefore, remaining hypochnicioid species with smooth basidiospores lacking molecular data are retained in Hypochnicium. Phylogenetic position of these morphologically circumscribed species will have to be assessed on the basis of future molecular studies.
The genus Gyrophanopsis was established by Jülich (1979) as the monotypic genus with G. zealandica. This genus is primarily characterized by having septocystidia and smooth, slightly thick-walled basidiospores (Jülich, 1979). Paulus, Nilsson and Hallenberg (2007) treated Gyrophanopsis as a synonym of Hypochnicium based on phylogenetic analysis using ITS region. In our studies, Gyrophanopsis and Bulbillomyces appeared as sister clades, not inside Hypochnicium. Gyrophanopsis japonica, Gy. polonensis and Gy. zelandica have septocystidia and smooth basidiospores (cell wall: less than 0.5 µm thick), and the three species form a monophyletic clade with strong support. The genus Bulbillomyces established by Jülich (1974) is monotypic, containing only B. farinosus (Bres.) Jülich. Bulbillomyces, Gyrophanopsis and Hypochnicium share smooth, thick-walled and cyanophilous basidiospores, subclavate to suburniform (slightly constricted) basidia, and clamped-septate hyphae. However, Bulbillomyces and Gyrophanopsis can be distinguished from Hypochnicium by forming sclerotia (Aegerita state) and septocystidia, respectively. In addition, B. farinosus produces sclerotia also in culture (Nakasone, 1990; Stalpers, 1978), whereas sclerotium production has not been reported in cultures of hypochnicioid species with smooth basidiospores (Nakasone, 1990; Stalpers, 1978). Thus, the phylogenetic and morphological differences strongly suggest that each of the three genera is an independent genus.
In the present study, we obtained the following new findings on species circumscription of H. multiforme, N. pini and N. subrigescens. Hypochnicium multiforme is morphologically very similar to H. globosum Sheng H. Wu, but the two species differ in cyanophily of basidiospores. Hypochnicium multiforme produces cyanophilous basidiospores whereas those of H. globosum are not cyanophilous (Wu, 1990). The three Japanese specimens, TUMH 64578, TUMH 64579 and TUMH 64580, were reported as H. globosum without information of basidiospore cyanophily (Maekawa, 1994). In the present observation, however, we revealed that these specimens have weakly cyanophious basidiospores. The phylogenetic analysis using ITS sequence data also supported that the Japanese specimen (TUMH 64581) was included in H. multiforme. These results suggest that the Japanese specimens belong to H. multiforme. Since there is no sequence data (ITS) of H. globosum, it is necessary to obtain the DNA information of this species and to examine the phylogenetic relationship with H. multiforme in the future. Neohypochnicium pini belongs to the N. punctulatum group and is morphologically most similar to N. cremicolor, but differs from the latter in basidiome thickness, cystidium size and host (substrate). According to Jang et al. (2013), N. pini (as H. pini) produces thinner basidiomes (up to 100 µm thick) and cystidia measuring 40-80 × 5.5-8.5 µm, and occurs on Pinus spp. On the other hand, H. cremicolor has thicker basidiomes (100-300 µm thick) and larger cystidia [70-150 × 7-10 µm, as H. punctulatum, Eriksson & Ryvarden (1976)], and has been reported from both broad-leaved trees and coniferous trees including Pinus spp. (Eriksson & Ryvarden, 1976; Bernicchia & Gorjón, 2010). However, all of the Japanese specimens of H. pini, excepting TUMH 64591, examined in this study have longer cystidia (over 100 µm long) and thicker (100-250 µm thick) basidiomes than the original description (Jang et al., 2013). In addition, the Japanese specimens of H. pini have been collected from both coniferous and broad-leaved trees. Therefore, it is difficult to distinguish H. pini from H. cremicolor by these morphological features and substrate specificity. In our phylogenetic tree using ITS region, the phylogeny showed a polyphyletic N. subrigescens, with some Japanese and Norwegian sequences (N. subrigescens-I) located within Neohypochicium core clade, and a second group (N. subrigescens-II: Denmark and USA) on a distant position outside the core clade. The voucher specimen (GB-0150192) of Danish sequence (AF429427) has morphologically similar to the Japanese specimen (TUMH 64612) excepting their basidiospore dimensions; the Japanese specimen has slightly larger basidiospores (6-7 × 5.5-6 µm) than those [5.5-6(-6.5) × 4.5-5(-5.5) µm] of the Danish specimen. In addition, the morphological characteristics, including the basidiospore dimensions, of both specimens are not inconsistent with the original description of this species by Boidin and Lanquetin (1971). Therefore, sequencing of the type specimen or newly collected specimens from the type locality (Central African Republic) are needed for elucidating phylogenetic relationships of true H. subrigescens with morphologically similar unknown taxa. Neohypochicium sp. (TUMH 63706) also cannot be distinguished from N. subrigescens only by its morphological characteristics, but molecular phylogenetic analysis suggested that it is clearly different from N. subrigescens. Thus, in Hypochnicium and Neohypochnicium, molecular phylogenetic analysis is indispensable for discriminating morphologically similar species.
In our cultural study of the three new species, we recognized abundantly formation of chlamydospores in cultural mycelia of the isolates of N. perlongicystidiosum. According to cultural studies of hypochnicioid fungi by Boidin and Lanquetin (1971), Nakasone (1990) and Stalpers (1978), examined cultures of H. bombycinum, H. cystidiatum, H. eichleri, H. lundellii (Bourdot) J. Erikss., H. punctualatum and H. sphaerosporum did not produce chlamydospores. Therefore, chlamydospore formation in culture might be useful as a criterion for discriminating N. perlongicystidiosum.
A key to species of Bulbillomyces, Gyrophanopsis, Hypochnicium and Neohypochnicium known from Japan is shown below.
Key to species of Bulbillomyces, Gyrophanopsis, Hypochnicium and Neohypochnicium for Japan
1. Sclerotia (Aegerita state) present associated with basidiomes; basidiospores smooth, ellipsoid, 6.5-7.5 × 4.5-5.5 µm; cystidia conical to subcylindrical tapering toward the apex, heavily encrusted (Maekawa, 2002)......Bulbillomyces farinosus
1. Sclerotia (Aegerita state) absent associated with basidiomes......2
2. Septocystidia present......3
2. Septocystidia absent......4
3. Basidiospores smooth, ellipsoid, 6.5-8.5 × 4-5 µm......Gyrophanopsis polonensis
3. Basidiospores smooth, subglobose, 5-5.5 × 4-4.5 µm......Gy. japonica
4. Basidiospores smooth......5
4. Basidiospores ornamented......8
5. Cystidia absent......6
5. Cystidia present......7
6. Basidiospores ovoid to ellipsoid, (8-)8.5-11(-13) × 7-11(-12) µm ......Hypochnicium bombycinum
6. Basidiospores subglobose, (7-)9-11(-11.5) × (6.5-)8-10(-11) µm ......H. multiforme
7. Basidiospores globose, 4.5-5 µm diam; cystidia cylindrical, 120-320 × 5-7 µm (Maekawa, 1994)......H. longicystidiosum
7. Basidiospores globose to suglobose, (5.5-)6-7(-7.5) × (5-)5.5-6.5(-7) µm; cystidia cylindrical to subfusiform with obtuse apex, 42-180 × 6.5-10 µm......Neohypochnicium subrigescens
8. Basidiospores globose, (9-)10-12(-13.5) × (8.5-)9-11(-13) µm; cystidia cylindrical, 75-493 × 7.5-14.5 µm; chlamydospores produced in culture......N. perlongicystidiosum
8. Basidiospores globose to ellipsoid, less than 9 µm long; cystidia cylindrical to subfusiform, less than 170 µm long......9
9. Distribution worldwide; basidiospores subglobose to ellipsoid, 7.5-8.5 × 6.5-7.5 µm......N. punctulatum
9. Distribution Asia; basidiospores smaller......10
10. Basidiospores 5.5-7(-8) × 4.5-6(-6.5) µm; substrate broad-leaved trees and coniferous trees......N. pini
10. Basidiospores 7-8(-8.5) × 5.5-6.5 µm; substrate coniferous trees......N. asiaticum
The authors declare no conflicts of interest. All the experiments undertaken in this study comply with the current laws of Japan.
We are grateful to Dr. Ellen Larsson, University of Gothenburg, for providing valuable specimens on loan. We also thank Ms. Sachiko Ueta for experimental support. Polysporous isolates examined in this study were provided by FMRC, Tottori University, through the National BioResource Project of the MEXT, Japan. This study was partially supported by Grants-in-Aid from the Institute for Fermentation, Osaka (IFO).