2020 年 2020 巻 4 号 p. 11-16
2020 年 2020 巻 4 号 p. 11-16
The white grub beetle, Dasylepida ishigakiensis Niijima et Kinoshita (Coleoptera: Scarabaeidae), is an indigenous pest of sugarcane in the Miyako Islands, Okinawa, Japan. The mouthparts of the adult beetles are known to degenerate and they are assumed to have limited nutritional resources. On the basis of this, we studied changes in the quantity of stored lipids in the adult lifetime. For observing changes of stored lipid quantity, we adopt a commercial kit to conduct methylation of induced fatty acids in the whole insect body and purify them for analysis. We individually extracted and methylated whole lipids in the bodies of male beetles, both in laboratory-reared and feral individuals, and analyzed the methylated fatty acids by gas chromatography for quantification. In laboratory-reared males, we found no difference between newly eclosed beetles and those just after reproductive diapause, only the males who intensively flew for mating had lower levels of fatty acids. Feral males who have flown vigorously for mating possessed significant lower levels of fatty acid. From these results, we are able to know that there is certain relationship between male’s flight capability and stored lipids quantity in non-migratory insects.
In long-distance migratory insects, triacylglycerol (TG) has been recognized as the major energy source fueling their flight activity (Beenakkers et al., 1985; Blacklock and Ryan 1994). TG is a neutral lipid that stores body fat and is transported to flight muscles in the hemolymph in the form of diacylglycerol bound to lipophorin (Chino, 1985; Soulages and Wells, 1994). TG is composed of glycerol and three fatty acids, and fatty acids used as flight are known to stored mainly in TG. Quantities of fatty acids stored in TG have been used in estimating the flight capability of insects. Compared to long-distance migratory insects, such as the common cutworm Spodoptera litura (Murata and Tojo, 2004), there is little information available on the relationship between flight capability and the quantities of fatty acid stored in the whole body (mainly in TG) of non-migratory insects (Stanley-Samuelson et al., 1986). The fatty acid level in TG has been recognized as a barometer of stored energy; however, researchers have to use the timeconsuming preliminary procedure of preparative thin-layer chromatography to separate and isolate TG. To simplify this process, we used a commercial kit that can isolate and methylate the fatty acids in the whole body of insects. In this study, we used this kit to compare the total levels of free fatty acids and fatty acids included in TG in both laboratory-reared and feral specimens of the white grub beetle, Dasylepida ishigakiensis Niijima et Kinoshita (Coleoptera: Scarabaeidae).
D. ishigakiensis is a destructive sugarcane pest in the Miyako Islands, Japan (Sadoyama et al. 2001). This beetle has a two-year life cycle. Its mating season is from late January to early March, and the larvae hatch in March and April and feed on sugarcane roots. Third-instar larvae that appear around September feed vigorously on the roots and underground stems of sugarcane, often killing the plants shortly before harvest (Oyafuso et al., 2002). The larvae continue to feed until the following March, and they then move to a deeper soil layer to aestivate from late March to mid-April (Kijima and Tarora, 2010; Oyafuso et al., 2011), pupating in this location in October and reaching adulthood in November. Adults remain in the soil for a further two months, emerging from soil for just a short time in the warm winter evenings to mate (Tanaka et al., 2008; Fukaya et al., 2009). They use the cues of decreasing light intensity and temperature at 18:00 (over 18 °C) to synchronize the timing of their mating flights, which take 20–30 minutes (Oyafuso et al., 2002; Arakaki et al., 2004; Fukaya et al., 2009). As adult D. ishigakiensis do not feed, the nutrients that are obtained during the larval stage and allocated for mating behavior (mate-searching flight and copulation) and egg production are limited (Oyafuso et al. 2001; Harano et al. 2010). Therefore, the adult white grub beetles have a nutritionally “closed” ecology, which may offer a good experimental model to investigate the relationship between their behaviors and consumed amount of stored lipids in their body, particularly in males who reemerge from the soil to mate multiple times and perform the matesearching flight several times until exhaustion of their flight energy.
In this study, we prepared both laboratory-reared and feral males of different physiological conditions and compared their body weights and stored fatty acids. We derivatized all fatty acids stored in both laboratory-reared and feral males to fatty acid methyl esters (FAME) and then purified them. We analyzed the derivative FAMEs by gas chromatography for quantification. By observing the quantitative changes of fatty acids in the bodies of the beetles throughout their adulthood,we tried to elucidate the relationship between flight capability and the quantity of stored lipids in the adult stage.
Insects
Third-instar larvae of D. ishigakiensis were collected from the soil in sugarcane fields on Miyako Island, Okinawa Prefecture, Japan in early February 2012. The larvae were kept individually in plastic cups (inner diameter, ca. 5.7 cm; height, 3.5 cm), each containing humus and fertile soil as a substrate and a piece of sugarcane stem (diameter, ca. 1.5 cm; length, 2 cm) as food, and they were transported to our laboratory in Tsukuba and maintained at 24 °C on a long photoperiod (14L:10D). We changed the sugarcane stems every three weeks until the larvae stopped feeding. After six to eight weeks, the larvae pupated, and they became adult approximately four weeks’ later. Adults terminate reproductive diapause and become sexually mature when they are kept at 25 °C for one week and subsequently at 15-20 °C for 8 weeks (Tanaka et al. 2008). Laboratory-reared individuals before their reproductive diapause, those immediately after reproductive diapause, and those with three or four times of flight experiences in a flight tunnel were frozen and kept at -30 °C until lipid extraction. As described in Fujiwara-Tsujii et al. (2014), the male adults started flying to search for mates when they were introduced to a flight tunnel in the presence of a lure impregnated with a synthetic female pheromone, (R)-2-butanol and the light intensity was decreased gradually from 175 to 0.91 lx in 20min. By using this procedure, we prepared males with an experience of extensive flights as much as possible.
Furthermore, we collected feral male beetles by using traps baited with the synthetic pheromone in sugarcane fields in early February 2011. The beetles were transported to our laboratory, and 11 of them were killed immediately by freezing and kept at -30 °C until lipids extraction. Others were kept for some days in plastic cups (90 ml) with a piece of moist tissue paper at 18 °C under a 12L–12D photoperiod and were forced to fly several times in a flight tunnel by using the above-described procedure until they completely lost their flight ability. Those who lost their flight ability were also killed by freezing and kept at -30 °C until lipid extraction.
Methylation and purification of fatty acids in total insect lipids
We measured the fresh weights of frozen males. We then cut off the head part of each frozen beetle, placed the residual body parts immediately in a dry chamber of a freeze-dryer (FD-5N, EYELA, Tokyo), and dried them for 24 hours. We removed exoskeleton of dried male beetles and weighed the residual parts including dried muscles, digestive tract, and hemolymph.
We used commercial kits for methylation and purification (fatty acid methylation kit and methylated fatty acid purification kit, Nacalai tesque, Kyoto). This enabled us to methylate all the fatty acids in the insects’ bodies (both free fatty acid and fatty acid included in TG). We placed a dried sample of each beetle without exoskelton into a 4 ml glass screw vial and then added 0.5 ml of reagent A (a 52: 48 mixture of toluene and methanol) and 0.5 ml of reagent B (7% methanol solution of undisclosed chemicals) to the vial, which was then kept at 37 °C for 1 hour. We added methylation reagent C (30 wt. % boron trifluoride methanol solution) to the vial, mixed it for 30 seconds using a voltex mixer, and then kept it at 37 °C for 20 minutes. We subsequently added 1.0 ml of n-hexane to the vial, mixed it for 30 seconds using a voltex mixer, and then transferred the organic layer to a new vial. We added deionized water to the vial, mixed it again, and then transferred the hexane layer to another new vial. To purify the methylated fatty acid (fatty acid methylated esters; FAME) we used silica gel cartridges of the kit. We applied the n-hexane layer including FAME to the cartridge which were pre-washed with 3 ml of n-hexane. We applied another 3 ml of washing solvent (n-hexane) and subsequent 3 ml of eluent (2% ethyl acetate in n-hexane) to provide the purified FAME solution. We diluted these samples to an appropriate concentration and then conducted GC analysis.
GC analysis
For quantification of FAME, we conducted GC analysis on an Agilent HP6890 GC equipped with a DB-23 fuzed silica column (60 m × 0.25 mm ID × 0.15 μm film thickness, J&W Scientific, Folsom, CA, USA) and a flame ionization detector. The carrier gas was helium at a constant pressure of 230kPa. Splitless injections were made into the GC column at 250°C. The GC oven temperature was held at 50 °C (for 1 min), increased from 50 °C to 175 °C at 25 °C min−1, increased from 175 °C to 230 °C at 4 °C min−1, and held at 230 °C for 10 min. We analyzed authentic samples of FAME (supelco 37-compound FAME Mix, Supelco, Bellefonte, USA) using the same method, and used retention times and peak areas to characterize and quantify FAME. All methylated fatty acids were also analyzed by GC/MS and checked their mass spectrum for identification.
Statistics
To compare quantities of FAME, we determined the total peak area of GC analysis using Scheffe’s test following a oneway analysis of variance at a 5% significance level.
Changes in fresh body weight of male body
In the laboratory-reared individuals, the fresh weight of newly emerged male beetles was significantly heavier than that of the beetles after reproductive diapause. There was also a significant difference between the fresh weights of the beetles just after reproductive diapause without flight experience and those with three to four times of flight experiences (Fig 1-a).
We also found significant differences in fresh weights between feral adult males collected in early mating season and those enforced on mate-finding flights after collection (Fig 1-b).

Comparison of FAME quantities
We detected the same six fatty acids methyl eaters— palmitic acid (C16:0), palmitoleic acid (C16:1), oleic acid (C18:1), linoleic acid (C18:2), linolenic acid (C18:3), and arachidonic acid (C20:4) —in all individuals. In all samples, oleic and linoleic acids were the predominant fatty acids, followed by palmitic acid.
The total quantity of FAME per 1 mg dry weight of each individual is shown in Fig 2. In the laboratory-reared individuals, we did not find any significant difference between newly eclosed males and those just after reproductive diapause, but the quantity of FAME was significantly lower in males after the experience of the flight activities. In feral beetles, similarly, individuals enforced on the flight-treatment had a lower level of FAME than did the beetles collected in the early mating season without the treatment.
The FAME levels of males just after reproductive diapause were the almost equivalent in both laboratory-reared and feral beetles in the early mating season. Similarly, the FAME levels in laboratory-reared and feral males with the flight-treatments were the comparable and were significantly lower than those in feral males in the early mating season. Even males after the experience of vigorous flight activities retained approximately half the amounts of whole-body lipids of newly eclosed males.
Our study has demonstrated that quantitative analysis of total lipids can be conducted for the non-migratory insect, D. ishigakiensis. Formerly, extraction of total lipids and separation of TG and analysis in the form of FAME have required complicated experimental steps (Itoyama et al. 1999; Folch et al. 1957), such as extracting total lipids via chloroformmethanol (2:1, v/v) and applying the concentrated extract for preparative thin-layer chromatography to separate and isolate TG with subsequent conversion of the separated fatty acids to their methyl esters. The use of commercial kits simplified these steps and made them easier.
Among laboratory-reared beetles, the fresh weights of newly emerged individuals were significantly heavier than those of beetles just after reproductive diapause (Fig. 1). However, at both these stages, the FAME quantities per body dry weight were equivalent. The differences in the fresh weight may be attributed to the body water content. Although the mouthpart of adult D. ishigakiensis is known to degenerate (Arakaki et al., 2004), it remains unclear whether this beetle can take in water during adulthood. We have never observed either feeding or drinking behavior in these beetles in the field (personal observation). Our findings are consistent with the idea that adult beetles cannot take in water.
The amounts of FAME in the males that had undertaken several mating flights were significantly less than those in males with either no or little flight experience (Fig. 2). This trend was observed both in laboratory-reared and feral males.
These findings show, as in migratory insects, that quantities of stored lipids in non-migratory insects are linked to their flight capabilities. The quantities of fatty acids confirmed in our experiments include both free fatty acids and fatty acids stored in TG (whole-body lipids). In our analysis, we found significant differences between beetles with different flight experiences. Half of their whole-body lipids are likely to be consumed during their mating flights. The mating season of D. ishigakiensis is generally from late January to early March, but the periods that are suitable for mating are greatly influenced by the temperature prevailing in any given year. It is therefore difficult to predict the exact timing of their mating during the season. From our results, the quantity of lipids in male body could be used as the barometer for knowing the seasonal variation of this beetle’s emergence. We could know which timing of mating season (early, middle or late of their mating season) by analyzing stored lipids extracted from feral beetles.
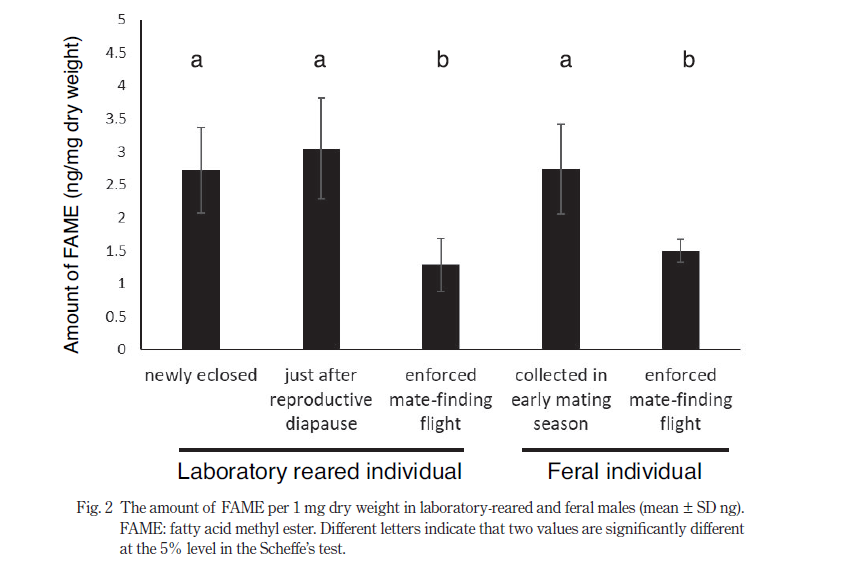
Furthermore, we did not find any difference in whole-body lipid quantities between newly eclosed males and those just after reproductive diapause. D. ishigakiensis has evolved two diapauses in its life cycle, and the significance of these physiological events has been discussed by Tanaka et al (2008). From adult eclosion to the first mating flight timing (2 months or more), these beetles might reduce their metabolism to save energy, resulting that they could use more energy for matesearching flights and copulation. During diapause, insects are known to be characterized by extremely low metabolic rates (Danks, 1987), which is also to be expected in reproductive diapause of D. ishigakiensis. In this study, we have demonstrated reasonable energy consumption by this beetle. Tanaka et al. (2008) observed flight muscle development in D. ishigakiensis, i.e., the muscle fiber width and its color in both newly eclosed beetles and those several days after eclosion. During the first 30 days after eclosion, muscle fiber width increased rapidly, and its color turned to pinkish. Our findings revealed that the development of flight muscles had almost no influence on the quantity of whole-body lipid.
Males who experienced extensive flights and lost flight activity retained a certain amount of fatty acids, i.e. approximately half of the amount of fatty acids in newly eclosed individuals. The use of the retained fatty acids remains unclear. However, this study has revealed how their whole-body lipids are allotted during adulthood, and it might shed lights on the metabolism strategy of “nutritionally closed insects” like D. ishigakiensis.
This work was partly supported by the Research and Development Projects for Application and Promotion of New Policy for Agriculture, Forestry, and Fisheries (no. 23021).
We thank late Hiroaki Oroku, ex-director of the Miyakojima Branch of OPARC, for his kind support of this project. The authors also would like to thank Ayako Yamaguchi of the Okinawa Prefectural Plant Protection Center, Miyako Branch Office for her cooperation in collecting larvae. Ikuko Hashimoto helped us handle the experiments and rear the insects at NIAS.