-
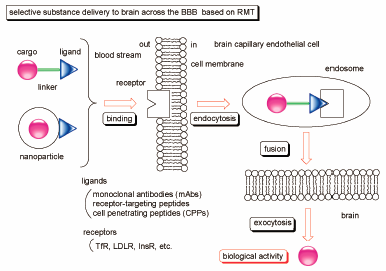
Volume 68 (2020) Issue 4 Pages 316-325Smart Strategies for Therapeutic Agent Delivery into Brain across the Blood–Brain Barrier Using Receptor-Mediated Transcytosis Read moreEditor's pick
In drug development, drug delivery into brain across the blood-brain barrier (BBB) is a serious problem. Particularly, the BBB is impermeable to large and medium-sized molecules. Accordingly, drugs for diseases of the central nervous system (CNS) are unable to elicit their activity in brain. However, receptor-mediated transcytosis can solve such impermeability. Actually, using receptors such as transferrin receptor (TfR), low-density lipoprotein receptor (LDLR), and insulin receptor (InsR), that express on the surface of brain capillary endothelial cells, delivered well-designed drugs into brain through endocytosis and exocytosis. This methodology will be a promising approach to cure patients suffering from CNS diseases.
-
Editor's pick
An efficient synthetic method for 2,5-disubstituted tetrazoles from 5-substituted tetrazoles is developed. In this paper, the authors established a cobalt-catalyzed site-selective alkylation of tetrazoles via atom-economic hydroamination reaction between tetrazoles and non-activated olefins. The authors also applied the developed reaction to an asymmetric intermolecular hydroamination of non-activated olefins, which is one of the longstanding problems in synthetic organic chemistry.
-
Volume 68 (2020) Issue 4 Pages 345-362Design and Synthesis of 2-(1-Alkylaminoalkyl)pyrazolo[1,5-a]pyrimidines as New Respiratory Syncytial Virus Fusion Protein Inhibitors Read moreEditor's pick
This report describes the design, synthesis, and evaluation of a new series of pyrazolo[1,5-a]pyrimidine derivatives for treatment against respiratory syncytial virus (RSV). The pyrazolo[1,5-a]pyrimidine series of compounds containing a piperidine ring at the 2-position of the pyrazolo[1,5-a]pyrimidine scaffold are known as candidate RSV fusion (F) protein inhibitor drugs. The piperidine ring has been revealed to facilitate the formation of an appropriate dihedral angle between the pyrazolo[1,5-a]pyrimidine scaffold and the plane of the amide bond for exertion of anti-RSV activity. A molecular-dynamic study on pyrazolo[1,5-a]pyrimidine derivatives focusing on the dihedral angles proposed and demonstrated potent anti-RSV inhibitors with an acyclic chain instead of a piperidine ring. A subsequent optimization study on pyrazolo[1,5-a]pyrimidine derivatives containing 1-methylaminopropyl group led to a highly potent anti-RSV agent with an EC50 value of less than 1 nM.
-
Volume 68 (2020) Issue 4 Pages 380-383Total Syntheses and Cytotoxic Evaluations of Cryptolactones A1, A2, B1, B2, and Their Derivatives Read moreEditor's pick
Aphids have unique polyketide pigments, which possess interesting biological activities such as cytotoxicity. In this article, the authors have focused on cryptolactone A1, A2, B1, and B2, colorless polyketide lactones, isolated from a colorless aphid, Cryptomyzus sp., and accomplished the asymmetric total syntheses of these compounds, their analogs bearing shorter carbon chains, and their antipodes. The investigation of structure-activity relationships among these compounds was carried out and revealed that both enantiomers exhibited similar cytotoxic properties towards HL-60 cell lines. However, compounds with shorter carbon chains were less cytotoxic than the others.
-
Volume 68 (2020) Issue 4 Pages 384-391Nucleophilic Addition Reaction with Dearomatization of Naphthalene Ring Read moreEditor's pick
Organic reactions using dearomatization have attracted much attention as a new approach to constructing complicated cyclic molecules. This paper describes the regioselectivity of nucleophilic addition of organolithium species (in particular, n-BuLi and sec-BuLi) to various aromatic lactones. The results of many experiments indicated that the regioselectivity varied greatly depending on various factors, such as the bulkiness of both substrates and organolithium species, and types of solvent and cosolvent. It is particularly interesting that the reactions mechanism of the addition of organolithium species differed between n-BuLi (via ionic process) and sec-BuLi (via one electron transfer process).
-
Editor's pick
Cutting-edge contributions from invited poster presentations providing significant research works in the fifth International Symposium for Medicinal Sciences (ISMS) in the 139th Chiba annual meeting in 2019 are assembled for the Current Topics section in this issue of the Chemical and Pharmaceutical Bulletin.
-
Editor's pick
The principal catalytic reaction of ferric lipoxygenases is dioxygenation of polyunsaturated fatty acids under normoxia. On the contrary, at a lower oxygen content, lipoxygenases concomitantly convert polyunsaturated fatty acids and their hydroperoxides into fatty acid allyl-radicals and fatty acid alkoxyl radicals, respectively, through one-electron redox reaction. The former radicals simultaneously react with oxygen molecule, producing fatty acid peroxyl radicals. In general, free radicals tend to abstract one electron from molecules. However, fatty acid alkoxyl radicals donate one electron to the aggressive free radicals including fatty acid peroxyl radicals, resulting in the oxo-fatty acids generation, which act as PPAR agonists.
-
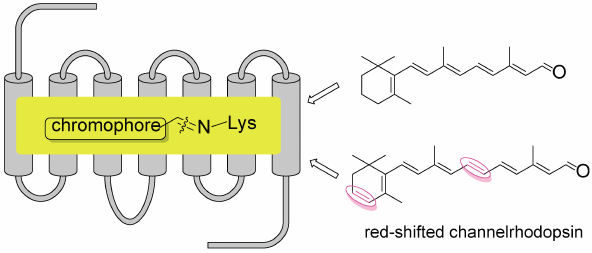
Editor's pick
Optogenetics is a new technology to control neural activity by light using ChRs. ChRs now used in optpgenitics are mostly sensitive to blue-green light (430-550 nm), and have several limitations. To overcome these problem, the development of red-shifted ChRs are eagerly required. In this paper, six kinds of new chromophores with one double bond inserted into the polyene side chain of retinal (A1) or 3,4-didehydroretinal (A2) were prepared. Among them, A2-10ex (an extra double bond was inserted at C10-C11 position of A2) bound with ChrimsonR to afford new ChR with the greatest red-shifted absorption peak at 654 nm.
-
Volume 68 (2020) Issue 3 Pages 273-287Novel Steroidal Glycosides from the Whole Plants of Helleborus foetidus Read moreEditor's pick
Phytochemical analysis of the whole Helleborus foetidus plants identified 28 steroidal glycosides, including 20 novel spirostanol glycosides and a novel furostanol glycoside. The structures of the newly identified compounds were elucidated by two-dimensional NMR spectroscopy and hydrolytic cleavage. Three isolated compounds were determined to be spirostanol trisdesmosides, which are unique in structure bearing sugar moieties at the C-1, -21, and -24 hydroxy groups of the aglycone unit. The isolated compounds were evaluated for cytotoxic activity against HL-60 human promyelocytic leukemia cells and A549 human lung carcinoma cells, and several compounds exhibited moderate cytotoxic activity.
-
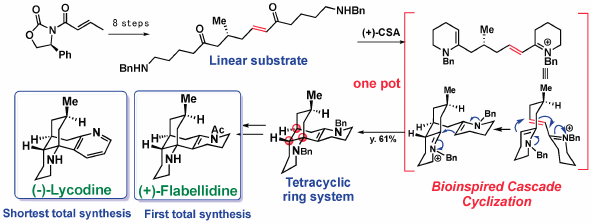
Volume 68 (2020) Issue 2 Pages 103-116Total Syntheses of Lycopodium and Monoterpenoid Indole Alkaloids Based on Biosynthesis-Inspired Strategies Read moreEditor's pick
The lessons from nature on biosynthesis of natural products might be beneficial for synthetic organic chemists to design unique synthetic approaches as well as to facilitate development of new synthetic methodologies. This review emphasized the merits of biosynthetic consideration in the chemical synthesis of complex natural products by describing the total syntheses of Lycopodium alkaloids and monoterpenoid indole alkaloids conducted in author’s laboratory.
-
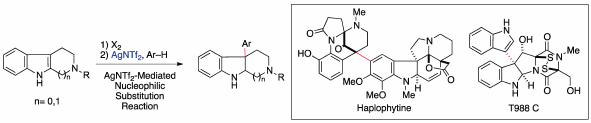
Volume 68 (2020) Issue 2 Pages 117-128Synthetic Studies toward Dimeric Indole Alkaloids Based on Convergent Synthetic Strategy Read moreEditor's pick
Various dimeric compounds comprising two structurally different indole units are ubiquitous in nature. These compounds are a pharmaceutically important class of natural products because several compounds in this class exhibit display greater potency and unique biological activities compared with the corresponding monomeric compounds. In particular, these dimeric compounds, which possess molecular weights that deviate from Lipinski’s rule, are anticipated to be useful as new drug candidates in the middle molecule drug discovery. This review presents an overview of efficient convergent syntheses of dimeric indole alkaloids, haplophytine, and T988s with the development of synthetic methodologies for linking the two indole units.
-
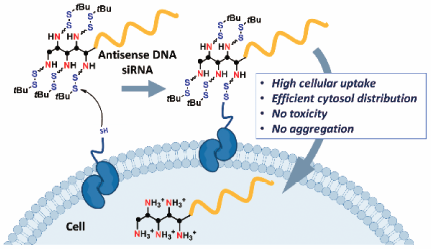
Volume 68 (2020) Issue 2 Pages 129-132Intracellular Delivery of Antisense DNA and siRNA with Amino Groups Masked with Disulfide Units Read moreEditor's pick
A new efficient delivery method of oligonucleotide (ON) therapeutics is developed. Here, antisense ON and small interfering RNA (siRNA) with disulfide-masked amino units were designed and synthesized for efficient intracellular delivery. The developed method actually enabled direct delivery of these ON into the cytosol, where these ON showed the targeted silencing effects, with minimal cytotoxicity. The molecular design and evaluation reported in this article would be very informative for further developing efficient cytosol-delivery methods of therapeutic ONs for medicinal application.
-
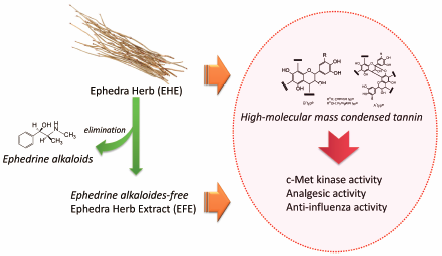
Editor's pick
The structural characteristics of the fractions from Ephedra Herb extract (EHE) having c-Met kinase inhibitory effects and the fractions from ephedrine alkaloids-free Ephedra Herb extract (EFE) having analgesic and anti-influenza activities were characterized. The fractions comprised high-molecular-mass condensed tannins, which were mainly of the procyanidin B and partly procyanidin A types, including pyrogallol- and catechol-type flavan 3-ols as the extension and terminal units. HPLC and gel permeation chromatography (GPC) analyses estimated that the ratio of pyrogallol- and catechol-type was approximately 9:2 and the weight-average molecular weight based on polystyrene standard was >45,000. The authors propose GPC-based analysis as the quality method for evaluating of high-molecular-mass condensed tannin in EHE and EFE.
-
Volume 68 (2020) Issue 1 Pages 1-33Development of New Synthetic Reactions Using Hetero-Heavy Atoms and Their Application to Synthesis of Biofunctional Molecules Read moreEditor's pick
Novel reactions using hetero-heavy atoms (P, S, Si, Se, and Sn) were developed and applied to the synthesis of biofunctional molecules and some medicine-candidates. 1) Development of introduction of C1-unit using cyanophosphates (CPs). 2) Carbene-generation from CPs and its application to organic synthesis. 3) [3,3]Sigmatropic rearrangement-ring expansion reactions of medium-sized cyclic thionocarbonates containing a sulfur atom and their application to natural product synthesis. 4) Stereoselective synthesis of novel b-imidazole C-nucleosides via diazafulvene intermediates and their application to investigating ribozyme reaction mechanism. 5) Developments of novel histamine H3- and H4-receptor ligands using new synthetic methods involving Se or Sn atoms.
-
Volume 68 (2020) Issue 1 Pages 34-45Drug Discovery Researches on Modulators of Lysine-Modifying Enzymes Based on Strategic Chemistry Approaches Read moreEditor's pick
Because lysine modifications on proteins control various cellular processes and aberrations of the modifications are associated with various diseases, chemical modulation of lysine-modifying enzymes is interesting as a method to regulate the cellular functions or as a therapeutic strategy. Therefore, the author has identified some modulators of lysine-modifying enzymes. To find the modulators, the author has combined conventional drug design with “strategic chemistry approaches,” such as drug design based on enzyme catalytic mechanism. In this review, the author’s drug discovery studies are presented.
-
Volume 68 (2020) Issue 1 Pages 77-90Discovery of 3,5-Dimethylpyridin-4(1H)-one Derivatives as Activators of AMP-Activated Protein Kinase (AMPK) Read moreEditor's pick
This paper describes that synthesis and evaluation of novel 3,5-dimethylpyridin-4(1H)-one scaffold compounds as indirect AMP-activated protein kinase (AMPK) activators. Unlike direct AMPK activators, this series of compounds inhibited cell growth of MDA-MB-453 but not that of SK-BR-3. The initial structure optimization of the lead compound 4a led to the discovery of compound 4f with potent AMPK activation activity and poor aqueous solubility. By further optimizing 4f, promising compound 25 was found out as a potent AMPK activator with good aqueous solubility.
-
Volume 68 (2020) Issue 1 Pages 100-102Morphometric Analysis of Paramylon Particles Produced by Euglena gracilis EOD-1 Using FIB/SEM Tomography Read moreEditor's pick
Euglena gracilis EOD-1, produces large quantities of paramylon, has been reported to function as a dietary fiber. However, the morphometric analysis of paramylon has not been conducted so far. The authors attempted to observe the detailed three-dimensional structure of paramylon by FIB/SEM. The inside of paramylon particles was comprised of a dense structure with no evidence of the presence of large pores and gaps. The specific surface area of paramylon particles was not as large as activated charcoal, but similar to those of plant starches, indicating that the cholesterol-lowering effect of paramylon cannot be simply attributed to its adsorption ability.
-
Volume 67 (2019) Issue 12 Pages 1259-1270Metal-Free Oxidative Cross-Coupling Reaction of Heteroaromatic and Related Compounds Read moreEditor's pick
The ubiquity of the biaryls with heteroatoms in and on the rings, are of importance in organic chemistry as building blocks of functional compounds, such as bioactive natural products, pharmaceuticals, and chemicals. Therefore, the cross-couplings can provide powerful tools for the construction of biaryls and the development of a novel coupling method has been intensively studied by synthetic chemists. In the present study, the author demonstrated the metal-free oxidative couplings of two different aromatic C-H bonds in electron-rich arenes for producing biaryls based on new strategies and concepts using hypervalent iodine reagents.
-
Volume 67 (2019) Issue 12 Pages 1301-1313Comparative Study of Pharmacopoeias in Japan, Europe, and the United States: Toward the Further Convergence of International Pharmacopoeial Standards Read moreEditor's pick
Pharmacopoeias have provided public standards to help ensure the quality of drugs. As of 2018, pharmacopoeias exist in 56 countries and 3 regions. These pharmacopoeias have contributed to the quality assurance of drug substances and products by providing common standards for users in each region/country. As drug markets and supply chain is globalizing, the independent standards in each pharmacopoeia increase users’ burden of having to perform analytical procedures in different ways and using different acceptance criteria to satisfy pharmacopoeia requirements that vary across regions. To contribute to the further convergence of pharmacopoeial standards, the authors conducted a comparative study of the pharmacopoeias in Japan, Europe, and the United States.
-
Volume 67 (2019) Issue 12 Pages 1337-1346Ebenamariosides A–D: Triterpene Glucosides and Megastigmanes from the Leaves of Diospyros maritima Read moreEditor's pick
Four new triterpene glucosides and two megastigmanes were isolated from the leaves of Diospyros maritima. The structures of triterpene glucosides were elucidated by a sequential hydrolysis of glycosidic and ester bonds using different enzymes to give partial hydrolyzed intermediates and finally aglycones. Hydrolysis of 28-O-ester bonded glucose by a crude glucosidase may give a new tool in triterpene chemistry. Two new megastigmanes were also isolated and their structures were unambiguously elucidated using the modified Mosher's method and two megastigmanes were chemically correlated by NaBH4 reduction of the ketonic one.