2016 年 39 巻 1 号 p. 114-120
2016 年 39 巻 1 号 p. 114-120
In an attempt to discover inhibitory compounds against pore-forming toxins, some of the major toxins produced by bacteria, we herein examined the effects of four kinds of indolo[3,2-b]quinoline derivatives on hemolysis induced by the aerolysin-like hemolysin (ALH) of Aeromonas sobria and also by the alpha-hemolysin of Staphylococcus aureus. The results showed that hemolysis induced by ALH was significantly reduced by every derivative, while that induced by alpha-hemolysis was significantly reduced by three out of the four derivatives. However, the degrees of reduction induced by these derivatives were not uniform. Each derivative exhibited its own activity to inhibit the respective hemolysin. Compounds 1 and 2, which possessed the amino group bonding the naphthalene moiety at the C-11 position of indolo[3,2-b]quinoline, had strong inhibitory effects on the activity of ALH. Compound 4 which consisted of benzofuran and quinoline had strong inhibitory effects on the activity of alpha-hemolysin. These results indicated that the amino group bonding the naphthalene moiety of compounds 1 and 2 assisted in their ability to inhibit ALH activity, while the oxygen atom at the 10 position of compound 4 strengthened its interaction with alpha-hemolysin. These compounds also suppressed the hemolytic activity of the supernatant of A. sobria or A. hydrophila, suggesting that these compounds were effective at the site of infection of these bacteria.
Many threats by infectious diseases had been markedly decreased by the development of various antibiotics. However, some bacteria have acquired resistance to antibiotics; therefore, the infectious diseases caused by these bacteria are a cause of concern.1,2) Moreover, multidrug-resistant bacteria, which are resistant to several types of antibiotics, have been identified, and curing the diseases caused by these pathogens has consequently become more challenging. Methicillin-resistant Staphylococcus aureus (MRSA) is the most famous multidrug-resistant bacteria in the world and causes various infections including skin and respiratory infections.3,4) Anti-MRSA drugs such as vancomycin and linezolid have been developed in an attempt to overcome the infectious diseases caused by MRSA. However, vancomycin-resistant Staphylococcus aureus (VRSA) has emerged.5) Therefore, novel approaches are needed to overcome these infections, such as the introduction of an anti-virulence strategy.
The anti-virulence strategy blocks the factor causing the pathogenicity and virulence of bacteria.6–8) Infectious diseases are established via several steps, including invasion into the human body, adhesion to the site of infection, proliferation in the infectious site, and the production of extracellular toxins. Virulent bacterial strains possess and/or produce unique factor(s) to complete these steps. Therefore, denaturing the factor(s) may change the virulent strain to an avirulent strain, and infections by these virulent pathogens may be prevented.
Pore-forming toxins (PFT) are some of the major toxins produced by several bacteria.9) Alpha-hemolysin produced by S. aureus, which is a member of PFT, has been shown to play a role in the pathogenicity of S. aureus.10) Alpha-hemolysin is extracellularly secreted as a monomer with a molecular mass of 33 kDa, and binds to target membranes.10) It subsequently constructs a pore consisting of a heptamer, the crystal structure of which has been determined.11,12) Similarly, aerolysin, which is a PFT produced by Aeromonas hydrophila, has been shown to form a heptameric pore.13,14) Aerolysin expresses enterotoxic and hemolytic activities.15) We have been studying the nature of aerolysin-like hemolysin (ALH) produced by A. sobria, which is homologous to aerolysin, and identified it as the etiological factor for diarrhea induced by A. sobria.16,17) We previously reported that ALH was secreted into the milieu as a dimer of pro-ALH, which consists of a mature ALH domain and carboxy-terminal (C-terminal) domain. After its secretion, the carboxy-terminal domain is cleaved off by proteases and mature ALH then binds to the target membranes. ALH has been suggested to form a heptameric pore and exhibits toxicity by the same mechanism as that of aerolysin.
In the present study, we examined the effects of compounds on the hemolytic activities of ALH and alpha-hemolysin and found that indolo[3,2-b]quinoline derivatives significantly weakened the activities of both hemolysins.
A. sobria 116, 118, 124, 288, and 357 and A. hydrophila 405 were isolated from patients with diarrhea. A. hydrophila 436 was isolated from a patient with septicemia. A. hydrophila 406 and 407 were isolated from environmental soil. A. hydrophila ATC C7966 was obtained from the American Type Culture Collection (Manassas, VA, U.S.A.). These bacteria were cultivated in nutrient broth medium (NB) (Eiken Compound Co., Ltd., Tokyo, Japan) with shaking at 37°C.
ReagentsAerolysin-like hemolysin was purified from a culture supernatant of A. sobria strain 357 as described previously.16) S. aureus alpha-hemolysin and baicalin hydrate were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). The indolo[3,2-b]quinolines were synthesized according to procedures described previously with minor modifications18,19) (Fig. 1). Compound 1 was synthesized with 11-chloro-10H-indolo[3,2-b]quinolone and N-(4-amino-naphthyl)metanesulfonamide, while compound 2 was synthesized with 11-chloro-10H-indolo[3,2-b]quinolone and 4-amino-2-naphthol.18) Compound 3 was synthesized in a previous study, and compound 4 was synthesized with 2-[(3-methylphenoxy)acetamido]benzoic acid via the same reaction as that for compound 3.19) As shown in Fig. 1, compounds 3 and 4 used in this study were a mixture of each isomer. The synthesized compounds were purified using column chromatography and the 1H-NMR spectra of all compounds were obtained on Varian NMR System 300.18,19)
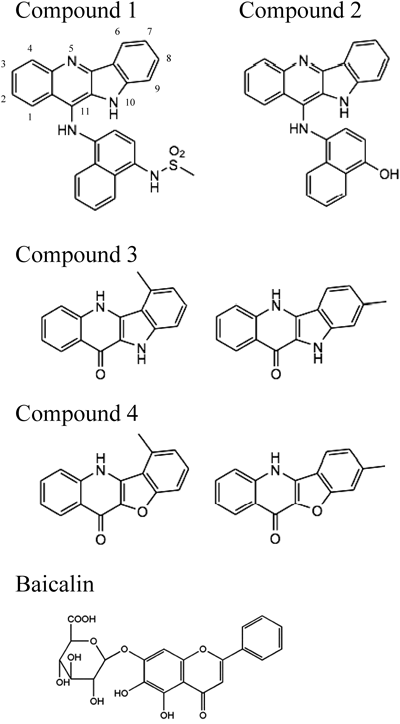
These four chemical compounds are referred to as compounds 1 to 4.
Once bacteria had been cultivated in liquid medium at 37°C overnight with rotation, 500 µL of the culture was added to 50 mL of liquid medium. After cultivation at 37°C for 6 h with shaking, the culture was centrifuged at 10000×g at 4°C for 10 min, and the culture supernatant was then recovered.
Aerolysin and ALH, which are produced by A. hydrophila and A. sobria, respectively, were extracellularly secreted as inactive preforms with the C-terminal domain, which was then cleaved off to convert the precursor form to the active form.17) In order to convert the precursor forms of aerolysin and ALH in the culture supernatant into their respective active forms, the culture supernatant was reacted with trypsin (2 µg/mL) at 37°C for 1 h, and then kept at −20°C until use.
Evaluation of Inhibitory Effects of Chemical Compounds on Hemolytic Activities of ALH and Alpha-HemolysinThe hemolytic activities of liquid samples were measured by a previously described procedure.20) The hemolytic toxin solutions used in this study, purified ALH, alpha-hemolysin of S. aureus, and culture supernatants of A. sobria and A. hydrophila, were diluted with 10 mM Tris–HCl buffer (pH 7.4) containing 0.9% NaCl. The chemical compounds used in this study were dissolved in dimethyl sulfoxide (DMSO).
Ninety-eight microliters of each toxin was mixed with 2 µL of each chemical compound. One hundred microliters of the 1% (v/v) sheep erythrocyte suspension in the same buffer was added immediately to the mixture of the toxin and compound, and incubated at 37°C for 1 h. After being incubated, the mixture was centrifuged at 1000×g for 5 min at 4°C. The supernatant was recovered and its absorbance was measured at 540 nm.
The amount of toxins used for the assay was the minimum needed for the complete hemolysis of all erythrocytes in the reaction mixture without compounds. The amounts of purified ALH and S. aureus alpha-hemolysin added to the reaction mixture were 0.06 µg/200 µL and 1 µg/200 µL, respectively. The concentrations of the culture supernatants of A. sobria and A. hydrophila were 3.125–50 µL of culture supernatant/200 µL of reaction mixture. The reaction without toxins was carried out as a blank. The residual activity (%) of the toxin in the presence of the chemical compounds was calculated as follows: 100×[(absorbance of the reaction mixture in the presence of the compounds)−(absorbance of the blank mixture in the presence of the compounds)]/[(absorbance of the reaction mixture in the absence of the compounds)−(absorbance of the blank mixture in the absence of the compounds)]. A residual activity of 100% indicated that all erythrocytes in the sample were lysed, while that of 0% indicated that erythrocytes in the sample were not lysed at all.
Statistical AnalysisExperiments were carried out in triplicate, data were analyzed using the Student’s t-test, and p-values were estimated. A p-value of <0.05 was considered significant. Data was represented as an arithmetic mean±standard deviation (S.D.).
We synthesized four indolo[3,2-b]quinoline derivatives in this study, which are presented in Fig. 1, and the synthesized compounds were subjected to 1H-NMR spectra. Compound 1 was N-[4-(10H-indolo[3,2-b]quinolin-11-yl)aminonaphtyl]methansulfonamide (1H-NMR (DMSO-d6) δ: 3.12 (3H, s), 7.36–8.66 (14H, m)). Compound 2 was 4-(10H-indolo[3,2-b]quinolin-11-yl)aminonaphthol (1H-NMR (DMSO-d6) δ: 3.12 (3H, s), 7.20–9.80 (14H, m)). Compound 3 was a mixture of isomers, 6-methyl-5H,10H-indolo[3,2-b]quinolin-11-one and 8-methyl-5H,10H-indolo[3,2-b]quinolin-11-one (1H-NMR (CD3OD) δ: 2.56 (3H*1/2, s, 8-CH3), 3.02 (3H*1/2, s, 6-CH3) 7.00–8.51 (7H, m)). Compound 4 was a mixture of isomers, 6-methylbenzofuro[3,2-b]quinolin-11-one and 8-methylbenzofuro[3,2-b]quinolin-11-one (1H-NMR (CD3OD) δ: 2.54 (3H*5/6, s, 8-CH3), 3.00 (3H*5/6, s, 6-CH3) 7.27–8.67 (7H, m)).
Inhibitory Effects of Indolo[3,2-b]quinoline Derivatives on the Hemolytic Activity of Purified ALHPFT are some of the major classes of toxins produced by several bacteria. Aerolysin produced by A. hydrophila is the representative toxin of PFT and research on aerolysin has developed extensively. We have focused on ALH, the homologous toxin of aerolysin produced by A. sobria. In the present study, we examined the effects of some chemical compounds on the hemolytic activity of ALH. The effects of these chemical compounds on the hemolytic activity of the alpha-hemolysin of S. aureus, which is a PFT, was examined in order to determine the actions of these chemical compounds in more detail.
ALH is known to induce hemolysis in sheep erythrocytes. In order to examine the effects of these chemical compounds on sheep erythrocytes, sheep erythrocytes were incubated in the presence of these chemical compounds under the conditions used to evaluate the inhibitory effects of chemical compounds (in Materials and Methods). ALH was not added to the solution. The results obtained showed that none of the chemical compounds tested induced any degree of hemolysis at a concentration of 250 µg/mL and less (data not shown). We then determined that more than 0.06 µg of ALH induced complete hemolysis under the condition used in this study (data not shown). Therefore, the effects of the chemical compounds on the activity of ALH were examined using a solution containing 0.06 µg of ALH.
We found that indolo[3,2-b]quinoline derivatives in the reaction mixture dose-dependently suppressed the hemolysis induced by ALH, as shown in Fig. 2(A). The incubation with 5 µg/mL of compounds 1 and 2 reduced the hemolysis induced by ALH to 1.2 and 11.8%, respectively. Although the inhibitory effects of compounds 3 and 4 on the activity of ALH were weak at a concentration of 5 µg/mL, hemolysis was reduced to 1.5 and 19.5% by an incubation with 25 µg/mL of compounds 3 and 4, respectively (Fig. 2(A)). These results indicated that the abilities of compounds 3 and 4 to inhibit the hemolytic activity of ALH were weaker than those of compounds 1 and 2.

(A) A. sobria ALH. (B) S. aureus alpha-hemolysin. Residual activity indicated the ratio of hemolysis after the incubation, as described in the text. A residual activity of 100% indicated that all erythrocytes in the sample were lysed, whereas that of 0% indicated that erythrocytes in the sample were not lysed at all. Experiments were performed in triplicate. Bars show the mean values of the independent experiments and error bars show the S.D. Asterisks indicated significant differences (* p<0.05, ** p<0.01), significantly different from hemolysis in the absence of compounds.
A previous study reported that baicalin, a flavonoid compound, suppressed the hemolytic activity of alpha-hemolysin.21) We tested the effect of baicalin to the hemolytic activity of ALH. However, baicalin did not inhibit the activity of ALH (Fig. 2(A)).
Inhibitory Effects of Indolo[3,2-b]quinoline Derivatives on the Hemolytic Activity of S. aureus Alpha-HemolysinWe then examined the effects of indolo[3,2-b]quinoline derivatives on the activity of S. aureus alpha-hemolysin. We used commercially-available alpha-hemolysin. Alpha-hemolysin induced complete hemolysis under the conditions used in the present study at a concentration of 1 µg/reaction mixture (200 µL) or higher (data not shown). We investigated the effects of the compounds on the hemolysis induced by 1 µg of alpha-hemolysin.
As described above, it was reported that baicalin suppressed the hemolytic activity of alpha-hemolysin and protected mice from infection by S. aureus.21) Therefore, we herein examined the effects of baicalin and compared them with those of indolo[3,2-b]quinoline derivatives. As shown in Fig. 2(B), 25 µg/mL of baicalin inhibited the activity of alpha-hemolysin to 2.0%, althought it did not inhibit the activity of ALH. This result demonstrated that the conditions used in the present study were appropriate for comparisons of the effects of chemical compounds on hemolysis induced by various toxins.
Among the indolo[3,2-b]quinoline derivatives tested, compound 4 was found to be the most effective inhibitor of alpha-hemolysin. Compound 4 significantly reduced the activity of alpha-hemolysin at a concentration of 1 µg/mL and higher in a dose-dependent manner. The inhibitory effects of compound 4 were stronger than those of baicalin (Fig. 2(B)). As shown in Fig. 2(B), compounds 1 and 3 decreased the activity of alpha-hemolysin at a concentration of 25 µg/mL. This result showed that compounds 1 and 3 exhibited inhibitory effect on the hemolytic activity of alpha-hemolysin; however, these effects were weaker than those compound 4.
As shown in Figs. 2(A) and (B), the inhibitory effects of compounds 1 and 2 were stronger on hemolysis induced by ALH than that by alpha-hemolysin, while the inhibitory effects of compound 4 were greater on hemolysis induced by alpha-hemolysin. These results suggested that the abilities of indolo[3,2-b]quinoline derivatives to inhibit the activity of PFT were dependent on their structures and also that each compound exhibited unique inhibitory effects on ALH and alpha-hemolysin; however, most indolo[3,2-b]quinoline derivatives were found to be effective inhibitors of ALH and alpha-hemolysin at a concentration of 25 µg/mL.
Inhibitory Effects on Hemolytic Activities in Culture Supernatants of Pathogenic A. sobria and A. hydrophilaIndolo[3,2-b]quinoline derivatives exhibited inhibitory effects on purified ALH and alpha-hemolysin. However, bacteria produce various extracellular enzymes. Aeromonas species have been reported to produce several enzymes outside of cells including proteases, lipase, and DNase.22,23) These extracellular enzymes, especially hydrolases, have been suggested to degrade the compounds surrounding bacteria. If the chemical compounds used in the present study are degraded, their functions to inhibit the activity of PFT are lost. Therefore, we determined whether these compounds inhibited the hemolysis induced by the culture supernatants of A. sobria and A. hydrophila, in which several extracellular enzymes were produced by the bacteria.
A. sobria and A. hydrophila were cultivated in nutrient broth for 6 h at 37°C, as described in Materials and Methods, and their culture supernatants were recovered. Before using the assay to measure the activity of the chemical compounds, ALH and aerolysin in these culture supernatants were detected and their hemolytic activities were measured. The detection of ALH and aerolysin was achieved by immunoblotting methods using an anti-ALH antibody, as described previously.20) ALH or aerolysin were detected in every sample and every sample showed sufficient hemolytic activity; however, the amount of hemolysin in these supernatants differed in every sample (data not shown). The minimum amount of hemolysin was used in order to accurately assess the inhibitory effects of the chemical compounds. To achieve this, we determined the minimum amount of the culture supernatant needed to yield complete hemolysis under the conditions used in the present study. The dosage of each culture supernatant was determined as follows; A. sobria 116 (3.125–6.25 µL, the volume was changed in every culture), A. sobria 118 (12.5 µL), A. sobria 124 (12.5–25 µL), A. sobria 288 (12.5–25 µL), A. sobria 357 (3.125–6.25 µL), A. hydrophila 405 (3.125–6.25 µL), A. hydrophila 406 (12.5 µL), A. hydrophila 407 (25–50 µL), A. hydrophila 436 (3.125–50 µL), and A. hydrophila 405 (25–50 µL).
The results of the assay for the inhibitory effects of the chemical compounds are shown in Table 1. In most assays, the effects of these compounds to inhibit the hemolytic activity of the culture supernatant were consistent with those against purified ALH. Compounds 1 and 2 significantly inhibited the hemolytic activity of culture supernatants of most of the strains tested at a concentration of 25 µg/mL, except for compound 2 in the sample from A. hydrophila 436. Similar to the results for purified ALH, the inhibitory effects of compound 4 on culture supernatants were weaker than those of compounds 1 and 2; however, these effects were detected in samples treated with 25 µg/mL of compound 4. These results indicated that the chemical compounds were stable and not easily degraded by the enzymes in the culture supernatant. However, when some of culture supernatants of A. sobria and A. hydrophila such as A. sobria 118, 124, 288 and A. hydrophila 406, 436 were treated with 5 µg/mL of compounds 1 and 2, significant decrease of the hemolytic activities could not be observed, while 5 µg/mL of compounds 1 and 2 suppressed the activity of purified ALH to 1.2 and 11.8% (Fig. 2(A)). These results suggested the possibility that these compounds are considerably degraded by these culture supernatants, or that the structure of the hemolysins produced by these strains is slightly altered from ALH and the effects of those compounds on these hemolysins is slightly weak.
| (A) Culture supernatant of A. sobria | ||||||
|---|---|---|---|---|---|---|
| Compounds | Concentrations (μg/mL) | Strains | ||||
| Residual activity (%) | ||||||
| A. sobria 116 | A. sobria 118 | A. sobria 124 | A. sobria 288 | A. sobria 357 | ||
| Compound 1 | 5 | 18.0±28.8* | 45.5±23.7 | 25.4±22.3* | 37.3±27.9 | 7.2±12.2** |
| 25 | 0.4±0.5** | 1.3±0.8** | 0.1±0.2** | 2.0±0.8** | 1.5±0.9** | |
| Compound 2 | 5 | 26.8±31.2 | 91.0±4.4 | 66.2±19.1 | 71.2±28.6 | 23.3±37.8 |
| 25 | 0.1±0.1** | 2.7±3.8** | 0.0±0.0** | 0.6±0.6** | 0.0±0.0** | |
| Compound 3 | 5 | 63.8±34.4 | 99.8±3.7 | 90.5±6.0 | 93.3±3.4 | 59.4±36.2 |
| 25 | 1.3±1.2** | 6.5±8.8** | 1.8±1.5** | 1.6±0.8** | 1.3±0.7** | |
| Compound 4 | 5 | 61.6±36.3 | 101.5±3.8 | 93.5±10.1 | 94.5±3.4 | 57.9±33.5 |
| 25 | 18.9±27.7* | 87.8±2.6* | 53.4±15.6* | 48.9±25.5 | 11.7±17.5* | |
| (B) Culture supernatant of A. hydrophila | ||||||
| Compounds | Concentrations (μg/mL) | Strains | ||||
| Residual activity (%) | ||||||
| A. hydrophila 405 | A. hydrophila 406 | A. hydrophila 407 | A. hydrophila 436 | A. hydrophila ATCC7966 | ||
| Compound 1 | 5 | 2.6±3.4** | 18.2±29.2* | 9.5±8.6** | 16.7±16.5 | 70.6±10.2* |
| 25 | 0.1±0.2** | 3.3±4.9** | 1.2±0.3** | 5.5±9.1** | 19.0±14.1** | |
| Compound 2 | 5 | 24.4±19.3* | 37.2±37.7 | 47.4±15.3* | 34.6±50.5 | 82.7±3.8* |
| 25 | 0.1±0.1** | 2.7±4.2** | 1.5±2.1** | 19.0±32.8 | 20.2±1.7** | |
| Compound 3 | 5 | 61.1±16.2 | 82.7±20.3 | 69.2±16.5 | 48.1±46.4 | 91.2±3.7 |
| 25 | 0.8±0.8** | 11.1±15.3** | 7.2±9.2** | 25.4±43.6 | 32.1±19.6* | |
| Compound 4 | 5 | 61.7±31.0 | 94.8±12.5 | 83.9±15.0 | 46.3±43.9 | 90.1±4.4 |
| 25 | 24.0±20.9* | 25.7±31.7 | 16.7±16.5* | 24.3±41.4 | 60.5±9.2* | |
The reaction mixture (200 µL) containing sheep erythrocytes and the culture supernatant of each strain was incubated for 1 h at 37°C in the presence or absence of the chemical compounds. Residual activity was then calculated as described in the text. Experiments were performed in triplicate. Data for residual activity was represented as the mean value±S.D. Asterisks indicated significant differences (* p<0.05, ** p<0.01), significantly different from hemolysis in the absence of compounds.
Indolo[3,2-b]quinolines, natural alkaloids from traditional herbal medicines, are tetracyclic compounds composed of an indole and quinoline-fused rings. Many indolo[3,2-b]quinoline derivatives have been synthesized and their biological activities, such as antibacterial, antifungal, antiprotozoal, and antitumoral activities, have been examined.24,25) In the present study, we newly synthesized indolo[3,2-b]quinoline derivatives and examined their effects on the hemolytic activity of ALH produced by A. sobria and alpha-hemolysin by S. aureus. Although both toxins are representative PFT, similarities in the amino acid sequences of the two toxins are limited. Similarities were only found in the region of the amino acid sequence from the 196th to 364th AA of ALH (GenBank accession no. AAN77507). The region is similar to the region of the amino acid sequence from the 139th to 302nd AA of alpha-hemolysin (GenBank accession no. YP_005733955) with 67.6% similarity. Nevertheless, indolo[3,2-b]quinoline derivatives broadly inhibited the activities of both toxins (Figs. 2(A), (B)). In an attempt to clarify whether the actions of indolo[3,2-b]quinoline derivatives were specific to both hemolysins, we examined the effects of these quinolines on the proteolytic activities of metalloprotease and serine protease produced by A. sobria. Proteolytic activity was measured using azocasein as a substrate.26,27) The results obtained showed that these quinolines did not affect the activities of these proteases (Supplementary Table 1), suggesting that these quinolines specifically inhibited the activities of ALH and alpha-hemolysin. These results suggest a novel function of indolo[3,2-b]quinolines.
The effects of compounds 1 and 2 were more prominent in the reaction with ALH than in that with alpha-hemolysin, while the effectiveness of compound 4 was greater in the reaction with alpha-hemolysin than in that with ALH. Based on the structures of the indolo[3,2-b]quinoline derivatives (Fig. 1), the amino group bonding the naphthalene moiety at the C-11 position of indolo[3,2-b]quinolines appeared to be beneficial for inhibiting the activity of ALH. We previously reported that the inhibitory effects of indolo[3,2-b]quinoline derivatives with amino groups bonding the benzene moiety at the C-11 position (attention: compounds 1 and 2 possess a naphthalene moiety, not a benzene moiety) was greater in the reaction with ALH than in that with alpha-hemolysin (data not shown), which supported our hypothesis that the bulky moiety at C-11 of indolo[3,2-b]quinolines enhanced the inhibitory effects of these quinolines on ALH.
On the other hand, compound 4 was very effective against alpha-hemolysin. Compound 4 is benzofuro[3,2-b]quinoline, which consisted of a benzofuran and quinoline-fused rings and contained an oxygen atom at the O-10 position, whereas the other compounds examined in the present study contained a nitrogen atom at that position. Therefore, the oxygen atom at O-10 position may have strengthened the interaction with alpha-hemolysin. Further studies are needed on the structures of compounds and their activities in order to discover more potent inhibitors of each PFT.
The flavonoids, compounds found in traditional herbal medicines, have been shown to exhibit various biological activities including anti-virulent properties.21,28–30) Qiu et al. previously reported that baicalin, a kind of flavonoid, inhibited the cytolytic activity of alpha-hemolysin by blocking the assembly of the alpha-hemolysin monomer to a heptamer and also had a protective effect against S. aureus pneumonia in a mouse model.21) In the present study, we determined whether baicalin inhibited the activity of ALH. Our results showed that baicalin did not inhibit the activity of ALH, but was effective against that of alpha-hemolysin. Therefore, indolo[3,2-b]quinoline derivatives may suppress the injuries induced by a broad range of PFT including ALH and alpha-hemolysin. However, the kinds of toxins susceptible to baicalin are more limited. These results also suggested that indolo[3,2-b]quinoline derivatives had inhibitory effects on the activities of PFTs through mechanisms that differed from those of baicalin on alpha-hemolysin. To show the mechanism of indolo[3,2-b]quinoline derivatives, we examined the effect of indolo[3,2-b]quinoline derivatives on the assembly of the alpha-hemolysin monomer to a heptamer. However, the clear result was not obtained (data not shown). In the next, we tried to examine the action of indolo[3,2-b]quinoline derivatives to inhibit the binding of ALH to cell membrane. The clear result has not been obtained, yet (data not shown). Therefore, the mechanism of indolo[3,2-b]quinoline derivatives has remained unknown. Since the ability of compound 4 to inhibit the activity of alpha-hemolysin was stronger than that of baicalin (Fig. 2(B)), indolo[3,2-b]quinoline derivatives may be critical in the development of powerful inhibitors against PFTs. Therefore, it is important to clear the mechanism of indolo[3,2-b]quinoline derivatives. Further analyses are required to reveal the mechanism of inhibition by indolo[3,2-b]quinoline derivatives against the PFTs.
Previous studies reported that some indolo[3,2-b]quinoline derivatives exhibited antibacterial activities.24,25) We tested the antibacterial activities of the indolo[3,2-b]quinoline derivatives used in the present study against S. aureus and A. sobria. The minimum inhibitory concentrations (MICs) of the compounds were determined according to the standard method described previously.31) Compounds 1, 3, and 4 did not affect the growth of bacteria, whereas compound 2 inhibited that of S. aureus and A. sobria with MICs of 4–16 µg/mL and 8–16 µg/mL, respectively (Supplementary Table 2). Since compound 2 had inhibitory effects on PFT, it may be effective in the cure of infectious diseases caused by S. aureus and A. sobria due to its bifunctional activities, antibacterial activity and anti-PFT activity. Further evaluation of these compounds including in vivo study were needed to utilize the indolo[3,2-b]quinoline derivatives as medicines.
This study highlighted new properties of indolo[3,2-b]quinoline derivatives. Our results indicate that indolo[3,2-b]quinolines are good candidates in the discovery of new anti-virulent reagents because many indolo[3,2-b]quinoline derivatives have been synthesized to date and their anti-virulent activities has not yet been examined.
This work was supported in part by Grants from Intractable Infectious Diseases Research Project Okayama (IIDTPO), Culture, Sports, Science and Technology, Japan and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.