2016 年 39 巻 4 号 p. 473-483
2016 年 39 巻 4 号 p. 473-483
Three novel series of pyridine derivatives, namely Schiff’s bases, 4-thiazolidinones and azetidin-2-ones bearing pyrazolo[3,4-b]pyridine moiety, have been synthesized. The chemical structures of the synthesized compounds were characterized. The compounds were tested for their antimicrobial activity using the agar well diffusion and broth macrodilution methods. The compounds were also evaluated for their antiproliferative activity using the sulforhodamine B (SRB) assay. The majority of the tested compounds exhibited slight to high antimicrobial activity against the test microorganisms with minimum inhibitory concentrations (MICs) of 0.12–62.5 µg/mL when compared to that of 3 standard antimicrobial agents (Ampicillin, 0.007–0.03 µg/mL; Gentamicin; 0.015–0.24 µg/mL; and Amphotericin B, 0.03–0.98 µg/mL). Compound (7b) was found to be nearly as active as the standard antimicrobial drug Amphotericin B against Fusarium oxysporum fungal strain with MIC of 0.98 µg/mL. Some of the test compounds showed remarkable cytotoxic activities against Hep G2 (hepatocellular carcinoma) cells (IC50=0.0158–71.3 µM) in comparison to the standard anticancer drug doxorubicin (IC50=0.008 µM). Among the compounds tested, (5), (6a), (6b), (7b), and (10) exhibited antiproliferative potency (IC50=0.0001–0.0211 µM) that was found to be better than that of doxorubicin (IC50=0.099 µM) against MCF7 (breast adenocarcinoma) cells. In particular, (7b) displayed the highest significant antiproliferative efficacy against both Hep G2 and MCF7 cell lines showing IC50 values of 0.0158 µM and 0.0001 µM, respectively. Our findings suggest that the synthesized compounds may be promising candidates as novel antimicrobial and antiproliferative agents.
Microbial infections remain a leading cause of mortality and morbidity worldwide. Unfortunately, a number of the current clinically efficacious antimicrobial agents are becoming less effective.1) With all of this in mind, there is an urgent need for the discovery of novel compounds endowed with antimicrobial activity.2)
Cancer is a serious health problem and the most frightening disease of mankind. Inherent and acquired resistance to treatment3) and the dose-limiting toxicity4) are recognized as major obstacles for effective cancer therapy.5) Therefore, there is a need for more innovative approaches to design anticancer drugs.6,7)
The synthesis of heterocyclic compounds has drawn the attention of medicinal chemists mainly because of their structural diversity coupled with their important therapeutic properties. A number of heterocyclic derivatives containing nitrogen atom serve as a versatile scaffolds for experimental drug design.8)
Pyridine is the parent ring system of a large number of naturally occurring products and important pharmaceuticals. Pyridine derivatives exhibit diverse pharmacological activities such as antimicrobial,9) antimycobacterial,10) antimalarial,11) antitumor,12) cytotoxic,13) antidiabetic,14) antiarrhythmic, and antidepressant.15)
The pyrazole nucleus has attracted great attention due to the use of this ring system as an important core structure in many drug substances. A large number of substituted pyrazolo[3,4-b]pyridine derivatives have been found to possess diverse biological properties such as antimicrobial,16) antiviral,17) antileishmanial,18) anti-inflammatory,19) and antitumor20) activities.
Schiff’s bases (imines or azomethines) were reported to possess a broad spectrum of potent pharmacological activities with a wide variety of biological properties including antimicrobial,21) anticancer,22) antioxidant,23) and anticonvulsant24) activities.
The 4-thiazolidinone is one of the most intensively investigated classes of five member heterocycles and forms an important class of clinically used drugs.25) The 4-thiazolidinone scaffold is very versatile and possesses various pharmacological activity profiles, namely antimicrobial,26) antitubercular,27) antiviral,28) anti-inflammatory,29) and antiproliferative30) activities.
Azetidin-2-ones are four-membered heterocyclic compounds commonly known as β-lactams. The azetidin-2-one scaffold is the common structural feature of a number of broad spectrum β-lactam antibiotics, which are the most prescribed antibiotics used in medicine.31,32) The biological activity of the β-lactam ring systems is generally believed to be associated with the chemical reactivity of their β-lactam skeleton and on the substituents especially at nitrogen of the 2-azetidinone ring.33) Azetidinones are of great biological interest, especially as antimicrobial,34) antiviral,35) anti-inflammatory,29) and anticancer36) agents.
Prompted by the various pharmacological and biological activities of pyrazolo[3,4-b]pyridine and as a part of our ongoing studies to identify novel candidates that may be of value in designing new and potent antimicrobial and antiproliferative agents, we envisioned our approach toward the synthesis of some novel heterocyclic pyridine derivatives including Schiff’s bases, 4-thiazolidinones and azetidin-2-ones bearing pyrazolo[3,4-b]pyridine moiety in order to identify compounds having better therapeutic activities. With a view to further assess the pharmacological profile of pyrazolo[3,4-b]pyridine derivatives, it was thought worthwhile to investigate the antimicrobial and antiproliferative profile of the synthesized compounds.
All reagents and solvents were of analytical grade and were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). The melting points were measured using a Gallenkamp electric melting point apparatus and are uncorrected. The IR spectra were recorded on a PyeUnicam SP-3-300 infrared spectrophotometer using potassium bromide (KBr) disks. The 1H-NMR spectra and 13C-NMR experiments were run at 300 and 400 MHz, respectively on a Varian Mercury VX-300 NMR spectrometer (Varian, U.K.) using tetramethylsilane (TMS) as internal standard (IS) in deuterated chloroform or deuterated dimethyl sulfoxide (DMSO) and the chemical shifts (δ) were reported in parts per million (ppm) and the coupling constants (J) in Hertz. The mass spectra were recorded using Shimadzu GCMS-QP-1000EX mass spectrometer (Shimadzu, Japan) at ionization energy of 70 eV with the source 200°C and an accelerative voltage of 8 kV. All the spectral measurements as well as the elemental analyses were carried out at the Microanalytical Center, Cairo University and the Main laboratories for chemical warfare. All the newly synthesized compounds gave satisfactory elemental analyses. The purity of the synthesized compounds was checked by TLC.
General Procedures for Compounds’ Synthesis5-Bromo-3-cyano-4,6-dimethyl-2-oxo-N-phenylpyridine-1(2H)-carbothioamide (5)A mixture of compound 1 (1.13 g, 0.005 mol) and phenyl isothiocyanate (0.6 mL, 0.005 mol) was refluxed in dimethyl formamide (DMF; 20 mL) containing a catalytic amount of triethylamine (TEA; 4 drops) for 5 h and then left to cool to room temperature. The reaction mixture was poured into cold water for complete precipitation, and the formed precipitate was filtered off, dried and recrystallized from ethanol to give compound 5 as orange crystals. Yield: 82%, mp: 202–205°C. Fourier transform (FT)-IR (KBr, cm−1): 3297, 3137 ν(NH), 3003 ν(CH aromatic), 2849 and 2775 ν(CH aliphatic), 2221 ν(C≡N), 1658 ν(C=O). 1H-NMR (300 MHz, DMSO-d6, δ, ppm): 8.64 (s, 1H, NH, exchangeable), 7.46–6.90 (m, 5H, Ar-H), 2.30 and 2.22 (2s, 6H, 2CH3). 13C-NMR (125 MHz, DMSO-d6): 160.54, 155.38, 150.37, 141.81, 135.97, 132.56, 129.95, 128.98, 111.55, 105.73, 26.75, 19.89. MS (electron ionization (EI), m/z, %): 361 (M·+, 00.0), 225.7 (24.1), 193.8 (57.4), 148.0 (38.9), 104.1 (27.8), 92.6 (100.0). Anal. Calcd for C15H12BrN3OS (362.24): C, 49.73; H, 3.34; Br, 22.06; N, 11.60; S, 8.85. Found: C, 49.68; H, 3.29; Br, 22.00; N, 11.51; S, 8.74.
N-(4-Chlorobenzylidene)-5-bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-amine (6a), N-((1H-Indol-3-yl)methylene)-5-bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-amine (6b), and/or 5-Bromo-N-((1,3-diphenyl-1H-pyrazol-4-yl)methylene)-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-amine (6c)A mixture of compound 4 (1.2 g, 0.005 mol) and the appropriate aromatic or heterocyclic aldehyde, namely, p-chlorobenzaldehyde, 1H-indole-3-carbaldehyde, and/or 1,3-diphenyl-1H-pyrazole-4-carbaldehyde (0.005 mol) was refluxed in absolute ethanol (20 mL) containing few drops of piperidine for 5 h. The reaction mixture was concentrated, cooled and the formed precipitate was filtered off, dried and recrystallized to give compounds 6a–c, respectively.
N-(4-Chlorobenzylidene)-5-bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-amine (6a)Orange crystals. Yield: 93%. mp: 211–212°C (EtOH). FT-IR (KBr, cm−1): 3398 ν(NH), 3045 ν(CH aromatic), 2939 ν(CH aliphatic), 1622 ν(C=N). 1H-NMR (300 MHz, DMSO-d6, δ, ppm): 13.70 (s, 1H, NH pyrazolo, exchangeable), 8.72 (s, 1H, N=CH), 7.91–7.57 (2d, 4H, Ar-H), 2.97 and 2.72 (2s, 6H, 2CH3). 13C-NMR (125 MHz, DMSO-d6): 161.55, 151.54, 148.34, 136.21, 132.42, 130.57, 129.95, 121.58, 105.65, 26.95, 19.97. MS (EI, m/z, %): 362 (M·+, 00.0), 305.2 (8.5), 215.6 (4.9), 167.1 (15.9), 127.7 (20.7), 83.8 (100.0). Anal. Calcd for C15H12BrClN4 (363.64): C, 49.54; H, 3.33; Br, 21.97; Cl, 9.75; N, 15.41. Found: C, 49.45; H, 3.24; Br, 21.89; Cl, 9.62; N, 15.35.
N-((1H-Indol-3-yl)methylene)-5-bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-amine (6b)Yellow crystals. Yield: 72%. mp: 272–275°C (dioxane). FT-IR (KBr, cm−1): 3462, 3143 ν(NH), 3064 ν(CH aromatic), 2981 ν(CH aliphatic), 1593 ν(C=N). 1H-NMR (300 MHz, DMSO-d6, δ, ppm): 13.12 (s, 1H, NH pyrazolo, exchangeable), 11.92 (s, 1H, NH indol, exchangeable), 9.22 (s, 1H, N=CH), 8.43–7.20 (m, 5H, Ar-H), 2.94 and 2.69 (2s, 6H, 2CH3). 13C-NMR (125 MHz, DMSO-d6): 155.85, 150.88, 148.29, 142.29, 137.64, 131.21, 128.76, 122.21, 120.12, 115.95, 113.61, 106.24, 26.95, 19.54. MS (EI, m/z, %): 369 (M+2]·+, 100.0), 367.2 (M·+, 75.4), 287.9 (56.1), 226.8 (52.6), 184.3 (35.1), 142.1 (49.1), 88.4 (31.6). Anal. Calcd for C17H14BrN5 (368.23): C, 55.45; H, 3.83; Br, 21.70; N, 19.02. Found: C, 55.54; H, 3.90; Br, 21.65; N, 19.10.
5-Bromo-N-((1,3-diphenyl-1H-pyrazol-4-yl)methylene)-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-amine (6c)Pale yellow crystals. Yield: 92%. mp: 289–291°C (dioxane). FT-IR (KBr, cm−1): 3133 ν(NH), 3058 ν(CH aromatic), 2983 ν(CH aliphatic), 1595 ν(C=N). 1H-NMR (300 MHz, DMSO-d6, δ, ppm): 13.32 (s, 1H, NH pyrazolo, exchangeable), 9.26 and 9.13 (each s, 1H, N=CH, two stereo-isomers), 8.05–7.42 (m, 11H, Ar-H), 2.77 and 2.68 (2s, 6H, 2CH3). 13C-NMR (125 MHz, DMSO-d6): 156.74, 153.44, 152.98, 151.01, 143.07, 139.34, 132.45, 131.19, 130.12, 129.27, 129.10, 127.79, 120.14, 119.57, 116.59, 110.02, 26.97, 19.44. MS (EI, m/z, %): 471.6 (M+2]·+, 41.6), 390.7 (M·+, 13.7), 244.8 (15.4), 230.9 (64.0), 119.1 (10.5), 104.1 (14.5), 77.1 (100.0). Anal. Calcd for C24H19BrN6 (471.35): C, 61.16; H, 4.06; Br, 16.95; N, 17.83. Found: C, 61.22; H, 4.15; Br, 16.87; N, 17.76.
3-(5-Bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-yl)-2-(1H-indol-3-yl)thiazolidin-4-one (7a) and/or 3-(5-Bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-yl)-2-(1,3-diphenyl-1H-pyrazol-4-yl)thiazolidin-4-one (7b)In 250 mL round-bottom flask, the Schiff’s bases 6b and c (0.01 mol) in dry benzene (30 mL) were taken, Dean–Stark apparatus was attached and thioglycolic acid (0.92 g, 0.01 mol) in dry benzene (20 mL) was added slowly. The reaction mixture was refluxed for 6–8 h and water was removed continuously during the reaction’s course. The solvent was evaporated and the reaction mixture was neutralized with cold diluted sodium bicarbonate solution. The formed product was filtered off and recrystallized to afford compounds 7a and b, respectively.
3-(5-Bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-yl)-2-(1H-indol-3-yl)thiazolidin-4-one (7a)Red crystals. Yield: 95%. mp: over 300°C (EtOH). FT-IR (KBr, cm−1): 3236, 3142 ν(NH), 3064 ν(CH aromatic), 2985 ν(CH aliphatic), 1657 ν(C=O), 1583 ν(C=N). 1H-NMR (300 MHz, DMSO-d6, δ, ppm): 13.45 (s, 1H, NH pyrazolo, exchangeable), 10.39 (s, 1H, NH indol, exchangeable), 8.10–7.11 (m, 5H, Ar-H), 5.09 (s, 1H, N–CH–S), 3.86 (s, 2H, CH2 of thiazolidinone ring), 2.68 and 2.54 (2s, 6H, 2CH3). 13C-NMR (125 MHz, DMSO-d6): 170.21, 161.23, 155.66, 150.88, 149.56, 137.45, 127.65, 122.98, 120.23, 119.54, 112.03, 105.45, 64.12, 34.54, 26.76, 19.75. MS (EI, m/z, %): 441 (M·+, 00.0), 348.6 (54.5), 167.1 (63.6), 141.2 (63.6), 121.6 (54.5), 95.2 (45.5), 56.0 (100.0). Anal. Calcd for C19H16BrN5OS (442.33): C, 51.59; H, 3.65; Br, 18.06; N, 15.83; S, 7.25. Found: C, 51.46; H, 3.59; Br, 18.00; N, 15.78; S, 7.17.
3-(5-Bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-yl)-2-(1,3-diphenyl-1H-pyrazol-4-yl)thiazolidin-4-one (7b)White crystals. Yield: 59%. mp: 240–242°C (EtOH). FT-IR (KBr, cm−1): 3198 ν(NH), 3068 ν(CH aromatic), 2983 ν(CH aliphatic), 1682 ν(C=O), 1595 ν(C=N). 1H-NMR (300 MHz, DMSO-d6, δ, ppm): 12.03 (s, 1H, NH pyrazolo, exchangeable), 8.80–7.34 (m, 11H, Ar-H), 5.88 (s, 1H, N–C–S), 3.75 (s, 2H, CH2 of thiazolidinone ring), 2.68 and 2.54 (2s, 6H, 2CH3). 13C-NMR (125 MHz, DMSO-d6): 170.45, 160.57, 153.75, 150.21, 149.89, 139.51, 133.23, 129.27, 129.10, 127.79, 123.14, 121.57, 116.59, 105.31, 56.75, 33.56, 26.97, 19.44. MS (EI, m/z, %): 545.8 (M+2]·+, 15.0), 468.4 (9.7), 320.0 (21.2), 218.8 (8.8), 143.7 (3.5), 76.9 (100.0). Anal. Calcd for C26H21BrN6OS (545.45): C, 57.25; H, 3.88; Br, 14.65; N, 15.41; S, 5.88. Found: C, 57.14; H, 3.79; Br, 14.57; N, 15.35; S, 5.76.
1-(5-Bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-yl)-3-chloro-4-(1H-indol-3-yl)azetidin-2-one (8a) and/or 1-(5-Bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-yl)-3-chloro-4-(1,3-diphenyl-1H-pyrazol-4-yl)azetidin-2-one (8b)To a well-stirred solution (0.01 mol) of the Schiff’s bases 6b and c and 0.02 mol of TEA in 100 mL of dry dioxane, 0.02 mol of monochloroacetyl chloride was added dropwise at room temperature. The mixture is stirred for extra 9 h, and left at room temperature for 3 d. The reaction mixture was poured onto crushed ice and the formed precipitate was filtered off, washed with 10% sodium bicarbonate solution. The residue was collected and purified to yield compounds 8a and b.
1-(5-Bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-yl)-3-chloro-4-(1H-indol-3-yl)azetidin-2-one (8a)Yellow crystals. Yield: 47.9%. mp: 212–214°C (EtOH). FT-IR (KBr, cm−1): 3216 ν(NH), 3057 ν(CH aromatic), 2927 ν(CH aliphatic), 1667 ν(C=O), 1619 ν(C=N). 1H-NMR (300 MHz, DMSO-d6, δ, ppm): 13.56 (s, 1H, NH pyrazolo, exchangeable), 10.03 (s, 1H, NH indol, exchangeable), 7.98–6.87 (m, 5H, Ar-H), 5.52 (s, 1H, Cl–CH), 5.21 (s, 1H, N–CH), 2.55 and 2.46 (2s, 6H, 2CH3). 13C-NMR (125 MHz, DMSO-d6): 160.40, 155.52, 150.71, 148.62, 137.63, 125.16, 123.05, 122.54, 120.95, 112.52, 112.37, 104.56, 72.39, 71.71, 26.97, 19.56. MS (EI, m/z, %): 387.6 (M−(CH2)4]·+, 17.8), 316.0 (44.0), 266.8 (28.9), 240.0 (82.2), 239.3 (35.6), 211.0 (15.6), 184.6 (11.1), 161.4 (13.3), 105.1 (33.3), 90.8 (26.7), 77.0 (100.0). Anal. Calcd for C19H15BrClN5O (444.71): C, 51.31; H, 3.40; Br, 17.97; Cl, 7.97; N, 15.75. Found: C, 51.25; H, 3.38; Br, 17.86; Cl, 7.96; N, 15.64.
1-(5-Bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-yl)-3-chloro-4-(1,3-diphenyl-1H-pyrazol-4-yl)azetidin-2-one (8b)Yellow crystals. Yield: 91.2%. mp: 312–314°C (toluene/ethanol). FT-IR (KBr, cm−1): 3238 ν(NH), 3137 ν(CH aromatic), 2985 ν(CH aliphatic), 1667 ν(C=O), 1585 ν(C=N). 1H-NMR (300 MHz, DMSO-d6, δ, ppm): 13.49 (s, 1H, NH pyrazolo, exchangeable), 8.32–7.45 (m, 11H, Ar-H), 5.34 (s, 1H, Cl–CH), 4.35 (s, 1H, N–CH), 2.68 and 2.54 (2s, 6H, 2CH3). 13C-NMR (125 MHz, DMSO-d6): 167.70, 157.56, 150.32, 148.75, 141.72, 131.21, 128.76, 127.89, 122.21, 121.65, 120.12, 116.78, 110.00, 74.23, 67.57, 27.09, 18.78. MS (EI, m/z, %): 546.7 (M+1]·+, 8.1), 320.6 (4.1), 282.3 (8.1), 280.8 (16.2), 266.5 (14.9), 244.9 (5.9), 239.8 (100.0), 239.5 (77.0), 162.4 (16.2). Anal. Calcd for C26H20BrClN6O (547.83): C, 57.00; H, 3.68; Br, 14.59; Cl, 6.47; N, 15.34. Found: C, 57.07; H, 3.76; Br, 14.48; Cl, 6.37; N, 15.22.
2-(2-(5-Bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-yl)hydrazono)malononitrile (10)Compound 4 (0.241 g, 0.001 mol) was dissolved in concentrated hydrochloric acid (5 mL) and the solution was then cooled to 0–5°C. Sodium nitrite (0.069 g, 0.001 mol) in water (3 mL) was then added to this solution dropwise with vigorous stirring for 1 h, while cooling at 0–5°C. The clear diazonium salt solution (compound 9) was then added dropwise to a well-cooled (0–5°C) and stirred solution of malononitrile (0.001 mol) in sodium acetate (1 g, dissolved in 5 mL of 25% aqueous ethanol). The pH of the coupling mixture, in each case, was maintained at 5–6 through the coupling process by adding sodium acetate. Stirring was continued for 4 h at 0–5°C and the precipitated product separated upon dilution with cold water (25 mL) was filtered, washed with water several times, dried, and recrystallized from benzene–ethanol to give compound 10 as orange crystals. Yield: 40%. mp: 100–102°C. FT-IR (KBr, cm−1): 3125 ν(NH), 3061 ν(CH aromatic), 2924 ν(CH aliphatic), 2229 ν(C≡N), 1597 ν(C=N). 1H-NMR (300 MHz, DMSO-d6, δ, ppm): 13.01 (s, 1H, NH pyrazolo, exchangeable), 7.23 (s, 1H, NH–N=, exchangeable), 2.68 and 2.56 (2s, 6H, 2CH3). 13C-NMR (125 MHz, DMSO-d6): 161.32, 154.43, 150.94, 149.96, 122.05, 111.55, 105.73, 85.75, 26.98, 19.74. MS (EI, m/z, %): 319 (M+2]·+, 86.4), 317.0 (M·+, 100.0), 238.6 (18.2), 238.5 (36.4), 226.2 (36.4), 210.0 (31.8), 147.7 (22.7), 117.2 (68.2). Anal. Calcd for C11H8BrN7 (318.13): C, 41.53; H, 2.53; Br, 25.12; N, 30.82. Found: C, 41.45; H, 2.47; Br, 25.01; N, 30.75.
5-((5-Bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-yl)diazenyl)quinolin-8-ol (11)The clear diazonium salt solution (9) was then added dropwise to a cold solution (0–5°C) of 8-hydroxyquinoline (0.001 mol) in 10% sodium hydroxide (25 mL). The reaction mixture was stirred at 0–5°C for 2h, and then neutralized with diluted HCl. The solid product was collected by filteration, washed with water several times, dried, and recrystallized from benzene–ethanol to afford compound 11 as orange crystals. Yield: 45%. mp: 258–259°C. FT-IR (KBr, cm−1): 3191 ν(NH), 3061, 3019 ν(CH aromatic), 2929, 2887 ν(CH aliphatic), 1598 ν(C=N). 13C-NMR (125 MHz, DMSO-d6): 160.65, 153.42, 152.21, 150.32, 149.79, 137.45, 136.02, 133.42, 130.75, 128.32, 122.54, 120.11, 112.68, 105.01, 26.89, 19.74. MS (EI, m/z, %): 371.4 (M−CN, +2H·]·+, 12.5), 175.7 (15.0), 134.0 (10.0), 128.0 (22.5), 122.2 (27.5), 104.2 (30.0), 99.0 (40.0), 55.1 (100.0). Anal. Calcd for C17H13BrN6O (397.23): C, 51.40; H, 3.30; Br, 20.12; N, 21.16. Found: C, 51.35; H, 3.24; Br, 20.00; N, 21.02.
Antimicrobial Activity TestingAll the test compounds were evaluated in vitro for their antimicrobial activity against 3 Gram-positive strains (Bacillus subtilis ATCC® 6633™, Staphylococcus aureus ATCC® 25923™ and Streptococcus pneumoniae ATCC® 49619™), 3 Gram-negative strains (Escherichia coli ATCC® 25922™, Pseudomonas aeruginosa ATCC® 27853™ and Salmonella Typhimurium ATCC® 13311™) and 3 fungal strains (Aspergillus niger ATCC® 6275™, Candida albicans ATCC® 10231™ and Fusarium oxysporum ATCC® 7601™). The strains under study were purchased from the American type culture collection (ATCC; Manassas, VA, U.S.A.). Nutrient broth/agar and Sabouraud dextrose broth/agar (Oxoid™, Basingstoke, Hampshire, U.K.) were used as cultivation media for bacterial and fungal strains, respectively.
The antimicrobial activity of the test compounds was tested using the agar well diffusion method.37) Briefly, the microbial strains under test were initially grown as cultures on agar media. The microbial inoculum used was prepared using the bacteria/fungi from a fresh culture on agar medium where the top of the microbial colonies in the Petri dish was touched with a sterile wire loop and the growth was transferred into a tube containing 5 mL of broth medium. The broth culture was incubated at 37°C (bacteria) or at 25°C (fungi) until it achieved or exceeded the turbidity of the 0.5 McFarland standard. The turbidity of the actively growing broth culture was adjusted to have an optical density of 0.1 at 600 nm with sterile broth in order to obtain a turbidity comparable to that of the 0.5 McFarland standard. This resulted in a microbial suspension containing approximately 1–2×108 colony forming units per milliliter (CFU/mL).
Nutrient/Sabouraud dextrose agar was melted, cooled to 45°C, poured into Petri dishes to get a uniform thickness of 6 mm, and allowed to solidify on a leveled surface. Once the medium had solidified, the entire surface of the agar was uniformly inoculated by streaking a sterile cotton swab that was previously dipped into the microorganism suspension. Six wells, each 6 mm in diameter, were then carefully cut out of the agar using a sterile cork borer allowing at least 30 mm between the adjacent wells.
The test compounds were dissolved in 0.25% (v/v) (DMSO; Sigma-Aldrich) at a concentration of 1000 µg/mL and 100 µL of each compound solution was introduced into the corresponding well using a sterile micro-pipette. An additional well containing an equivalent volume of the solvent (DMSO; 0.25% (v/v)) was used as a negative control in each Petri dish. Ampicillin and Gentamicin (30 µg/mL) were used as standard reference antibacterial drugs for Gram-positive and Gram-negative bacteria, respectively. Amphotericin B (30 µg/mL) was used as a standard reference antifungal drug for fungal strains.
The Petri dishes were subsequently incubated at 37°C for 24 h (bacteria) or at 25°C for 4–10 d (fungi). Following incubation of the Petri dishes, the antimicrobial activity of each test compound was determined by measuring the diameter of inhibition zone in mm, if any, surrounding the well containing that compound. In all determinations, experiments were conducted in triplicate and the results were reported as the mean of inhibition zone diameter in mm±standard deviation (S.D.).
The results were compared with those of the reference antimicrobial drugs. The diameter of inhibition zone is directly proportional to the degree of sensitivity of the microbial strain and the concentration of the compound under test. Results were interpreted according to the standards of the Clinical and Laboratory Standards Institute (CLSI, formerly the National Committee for Clinical Laboratory Standards or NCCLS)38–40) where CLSI tables were used to convert the mm zone diameters into interpretive breakpoints (inactive, mildly active, moderately active, or highly active).
Determination of Minimum Inhibitory Concentration (MIC)The MIC was defined as the lowest concentration of the assayed compound that inhibited the visible growth of the microorganism being tested. Test compounds that showed significant antimicrobial activity in the preliminary screening were selected for MIC determinations using the broth macrodilution method.41) In brief, cultures of each microorganism on agar media were prepared and used to inoculate 5 mL of sterile broth medium which were then incubated at 37°C (bacteria) or at 25°C (fungi) until an optical density of 0.1 at 600 nm was achieved to obtain an inoculum containing approximately 1–2×108 CFU/mL.
A stock solution (1000 µg/mL) of each test compound was prepared in DMSO (0.25% (v/v)) and a serial two-fold dilution of each compound was carried out to obtain the concentrations of 1000, 500, 250, 125, 62.50, 31.25, 15.63, 7.81, 3.90, 1.95, 0.98, 0.49, 0.24, 0.12, 0.06, 0.03, 0.015, 0.007, and 0.0035 µg/mL. The various concentrations of the test compounds were introduced into culture tubes containing 2 mL of the standardized microbial suspensions. The culture tubes were incubated at 37°C for 24 h (bacterial strains) or at 25°C for 4–10 d (fungal strains) and then examined for the presence or absence of visible microbial growth as evidenced by turbidity. A set of culture tubes containing only culture broth and culture broth with an equivalent volume of the solvent (DMSO; 0.25% (v/v)) were used as negative controls. Ampicillin and Gentamicin were used as standard reference antibacterial drugs for Gram-positive and Gram-negative bacteria, respectively. Amphotericin B was used as a standard reference antifungal drug for fungal strains.
The last culture tube with no growth of the microorganism under test where the test tube remained clear without turbidity was recorded to represent the MIC expressed in µg/mL, indicating that the bacterial or fungal growth was completely inhibited at this concentration. All experiments were performed in triplicate and the results were reported as the mean of MIC in µg/mL±S.D. The results were interpreted according to the NCCLS.42,43)
Assessment of Antiproliferative ActivityThe cytotoxic activity of the test compounds was determined by the sulforhodamine B (SRB) assay which is used for measuring the cellular protein content of cultures as previously described by Skehan et al.44) The growth inhibitory effect was tested in vitro against two human tumor cell lines that were purchased from The American Type Culture Collection (ATCC) (Manassas): 1) Hep G2 (ATCC® HB8065™, hepatocellular carcinoma); 2) MCF7 (ATCC® HTB22™, breast adenocarcinoma). The cell lines were cultured in Eagle’s Minimum Essential Medium (EMEM; ATCC® 302003™, Manassas, VA, U.S.A.) supplemented with 10% fetal bovine serum, 2 mM L-glutamine and 100 µg/mL penicillin–streptomycin at 37°C and in 5% CO2 atmosphere.
In brief, cells were seeded in sterile 96-well flat-bottom microtiter plates at a density of 2.5×104 cells/mL (100 µL cell suspension/well) and grown for 24 h at 37°C and in 5% CO2 atmosphere to allow attachment of cell to the wall of the plate. After 24 h, the test compounds were dissolved in DMSO (0.25% (v/v); Sigma-Aldrich) as stock solutions and diluted with culture media to furnish the concentrations of 6.25, 12.5, 25, 50 and 100 µM that were added to the cell monolayer. For the untreated cells, equivalent volume of the vehicle (DMSO, 0.25% (v/v)) was added to the negative control wells instead of the test compounds. For comparison purposes, the cytotoxicity of Adriamycin (doxorubicin; Sigma-Aldrich) as a standard anticancer drug was evaluated under the same conditions as a positive control. Monolayer cells were incubated with the compounds for 72 h at 37°C and in atmosphere of 5% CO2. After 72 h, the cells were fixed by gentle layering of cold 50% trichloroacetic acid (TCA) (Sigma-Aldrich) (50 µL/well, 10% final concentration) on the top of the culture medium in each well. The plates were incubated at 4°C for 1 h and then washed five times with distilled water. The TCA-fixed cells were stained for 30 min with 0.4% (w/v) SRB (Sigma-Aldrich) dissolved in 1% acetic acid (50 µL/well). Excess unbound dye was removed by four washes with 1% acetic acid and the protein-bound dye was extracted with 10 mM unbuffered Tris base [tris(hydroxymethyl)aminomethane] (Sigma-Aldrich) for determination of the optical density (OD) at 540 nm in a computer-interfaced, 96-well microtiter plate reader (Benchmark Plus; Bio-Rad, Hercules, CA, U.S.A.).
The experiment was performed at least three times for each cell line and each compound in a given concentration was tested in triplicates in each experiment. Cell viability was calculated using the following formula: cell viability (percentage)=(ODtreated/ODuntreated)×100. The in vitro growth inhibition effect of each compound was expressed as inhibitory concentration 50% (IC50) and defined as the concentration of the compound (µM) producing a 50% growth inhibition of treated cells as compared to the untreated control cells. The IC50 was determined by the SigmaPlot 10.0 software (Systat Software, Inc., San Jose, CA, U.S.A.) using a four-parameter logistic function for the sigmoid dose–response curves.
As a part of our ongoing studies in developing new antimicrobial and antiproliferative agents, we synthesized a series of pyridine derivatives to evaluate their antimicrobial and antiproliferative profile and to correlate the biological results with their molecular properties.
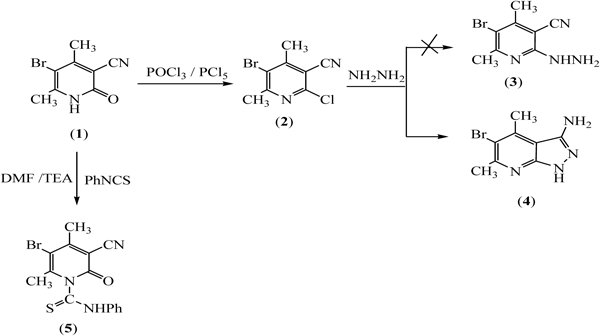
ChemistryThe synthetic procedures adopted to obtain the test compounds are depicted in Charts 1 and 2. Chlorination of 5-bromo-4,6-dimethyl-2-oxo-1,2-dihydropyridine-3-carbonitrile 1 with PCl5 in POCl3 afforded 2-chloropyridine derivative 2,45) which was cyclized under the effect of hydrazine hydrate in boiling ethanol yielded the amine derivative 4 instead of 5-bromo-2-hydrazinyl-4,6-dimethylnicotinonitrile 3.46,47) Reaction of 1 with phenyl isothiocyanate in dry dimethylformamide (DMF) containing a catalytic amount of triethylamine (TEA) gave 5-bromo-3-cyano-4,6-dimethyl-2-oxo-N-phenylpyridine-1(2H)-carbothioamide 5 (Chart 1).
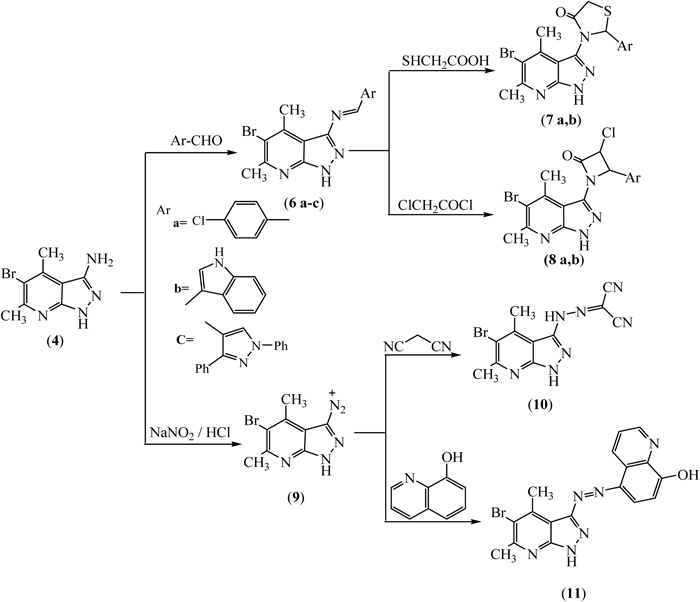
The Schiff’s bases 6a–c were formed when the amine derivative 4 was condensed with the appropriate aromatic or heterocyclic aldehyde, namely, p-chlorobenzaldehyde, 1H-indole-3-carbaldehyde and/or 1,3-diphenyl-1H-pyrazole-4-carbaldehyde in refluxing ethanol containing few drops of piperidine as a catalytic base. The IR spectra of the Schiff’s bases 6a–c revealed the disappearance of absorption bands of the amino group frequencies.. Furthermore, 1H-NMR spectra of the Schiff’s bases 6a and b displayed singlet at δ 8.72 and 9.22 ppm, respectively, due to the azomethine proton. On the other hand, 1H-NMR spectrum of the Schiff’s base 6c displayed two singlet of the azomethine proton at δ 9.26 and 9.13 ppm which were recorded in an integration of 48.86 and 51.14%, respectively, indicating the existence of the Schiff’s base 6c in syn- and anti-stereoisomers (Fig. 1).

Cyclization of Schiff’s bases 6b and c with thioglycolic acid in dry benzene using Dean–Stark apparatus afforded tetrahydrocarbazolyl thiazolidinones 7a and b. On the other hand, cyclization of Schiff’s bases 6b and c with chloroacetyl chloride in dioxane containing triethylamine afforded tetrahydrocarbazolyl azetidinones 8a and b. The IR spectra of the 2-azetidinones 8a and b showed the absorption band of the carbonyl group at 1667 cm−1, a reduced value due to hydrogen bonding between the carbonyl group and the hydrogen in the pyrazole ring.
Treatment of 4 with sodium nitrite in the presence of concentrated HCl afforded 5-bromo-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridine-3-diazoniumchloride 9, which upon coupling with malononitrile and/ or 8-hydroxyquinoline afforded hydrazonopyrimidine 10 and the corresponding azo derivatives 11, respectively (Chart 2).
Antimicrobial ActivityThe results of the in vitro antimicrobial testing against a panel of selected Gram-positive bacteria, Gram-negative bacteria and fungi are reported in Table 1 in comparison to those of the reference drugs Ampicillin, Gentamicin, and Amphotericin B, respectively. The antimicrobial effect of DMSO against the test organisms was investigated and no significant inhibitory effect was reported so that the concentration of DMSO in all the prepared tested compounds with different concentrations did not exceed 0.25% (v/v) to avoid its inhibitory effect. Data presented in Table 1 demonstrate that all the tested compounds exhibited slight to moderate antibacterial activity against the tested Gram-positive bacterial strains (Bacillus subtilis, Staphylococcus aureus, and Streptococcus pneumoniae). Moreover, all the tested compounds were found to be highly active, moderately active, or slightly active against the tested Gram-negative bacterial strains (Escherichia coli and Salmonella Typhimurium). In contrast, none of the compounds were found to be active against Pseudomonas aeruginosa. Furthermore, all the tested compounds revealed high, moderate or slight antifungal activity against the tested fungal strains (Aspergillus niger and Fusarium oxysporum). On the other hand, none of the compounds were found to be active against Candida albicans. Based on the in vitro antimicrobial testing data, the majority of the tested compounds possess promising antimicrobial activity against the test microorganisms, when compared to the standard antimicrobial drugs. Therefore, the compounds that showed significant antimicrobial activity in the preliminary screening were selected for MIC determinations. MIC values of the tested compounds for antibacterial and antifungal activities are listed in Table 2.
| Test compounds (1000 µg/mL) | Diameter of the inhibition zone (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bacterial strains | Fungal strains | ||||||||
| Gram-positive bacteria | Gram-negative bacteria | Aspergillus niger ATCC 6275 | Candida albicans ATCC 10231 | Fusarium oxysporum ATCC 7601 | |||||
| Bacillus subtilis ATCC 6633 | Staphylococcs aureus ATCC 25923 | Streptococcus pneumoniae ATCC 49619 | Escherichia coli ATCC 25922 | Pseudomonas aeruginosa ATCC 27853 | Salmonella Typhimurium ATCC 13311 | ||||
| 1 | 14.3±0.63 (+) | 13.4±0.44 (+) | 17.8±0.58 (++) | 16.3±0.37 (++) | Nil | 18.2±0.44 (+++) | 15.3±0.25 (++) | Nil | 16.2±0.44 (++) |
| 2 | 17.8±0.44 (++) | 15.8±0.53 (+) | 22.4±0.25 (++) | 18.9±0.44 (+++) | Nil | 23.6±0.58 (+++) | 18.9±0.63 (++) | Nil | 20.6±0.37 (+++) |
| 4 | 17.6±0.44 (++) | 15.9±0.37 (+) | 18.0±0.63 (++) | 13.4±0.25 (+) | Nil | 15.2±0.44 (++) | 11.9±0.58 (+) | Nil | 13.2±0.34 (+) |
| 5 | 19.6±0.53 (++) | 17.9±0.53 (++) | 22.4±0.43 (++) | 18.6±0.25 (+++) | Nil | 21.8±0.53 (+++) | 20.3±0.58 (+++) | Nil | 21.6±0.25 (+++) |
| 6a | 13.3±0.63 (+) | 12.9±0.44 (+) | 14.2±0.25 (+) | 14.3±0.44 (+) | Nil | 15.8±0.25 (++) | 12.3±0.58 (+) | Nil | 13.4±0.37 (+) |
| 6b | 18.9±0.42 (++) | 17.4±0.42 (++) | 20.2±0.15 (++) | 16.8±0.53 (++) | Nil | 18.8±0.42 (+++) | 19.8±0.58 (++) | Nil | 20.6±0.25 (+++) |
| 6c | 14.3±0.44 (+) | 13.5±0.42 (+) | 15.2±0.63 (+) | 13.6±0.37 (+) | Nil | 15.1±0.42 (++) | 16.3±0.44 (++) | Nil | 17.2±0.58 (++) |
| 7a | 18.2±0.25 (++) | 17.6±0.63 (++) | 20.3±0.44 (++) | 20.3±0.25 (+++) | Nil | 21.4±0.58 (+++) | 16.3±0.58 (++) | Nil | 18.6±0.58 (++) |
| 7b | 20.3±0.58 (++) | 18.3±0.67 (++) | 22.9±0.44 (++) | 17.9±0.63 (+++) | Nil | 20.6±0.67 (+++) | 20.9±0.44 (+++) | Nil | 22.0±0.58 (+++) |
| 8a | 21.2±0.44 (++) | 20.6±0.58 (++) | 22.9±0.44 (++) | 20.2±0.25 (+++) | Nil | 24.4±0.44 (+++) | 23.8±0.58 (+++) | Nil | 20.1±0.63 (+++) |
| 8b | 10.2±0.67 (+) | 10.0±0.67 (+) | 11.6±0.44 (+) | 12.3 ±0.46 (+) | Nil | 13.6±0.67 (+) | 10.6±0.25 (+) | Nil | 11.7±0.34 (+) |
| 10 | 11.7±0.44 (+) | 10.6±0.58 (+) | 16.5±0.37 (+) | 15.2±0.63 (++) | Nil | 16.4±0.58 (++) | 17.6±0.44 (++) | Nil | 18.2±0.25 (++) |
| 11 | 18.4±0.44 (++) | 16.3±0.58 (+) | 19.1±0.63 (++) | 16.2±0.37 (++) | Nil | 19.8±0.25 (+++) | 10.6±0.58 (+) | Nil | 11.7±0.44 (+) |
| Standard antimicrobial drug (30 µg/mL) | |||||||||
| Ampicillin | 32.4±0.3 (+++) | 26.3±0.3 (+++) | 23.8±0.2 (++) | — | — | — | — | — | — |
| Gentamicin | — | — | — | 19.9±0.3 (+++) | 17.3±0.1 (++) | 28.8±0.3 (+++) | — | — | — |
| Amphotericin B | — | — | — | — | — | — | 23.7±0.1 (+++) | 25.4±0.1 (+++) | 19.7±0.2 (++) |
| 0.25% (v/v) DMSO | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
Gram-positive bacteria: (+++) Highly active=inhibition zone diameter >25 mm; (++) Moderately active=inhibition zone diameter 17–25 mm; (+) Slightly active=inhibition zone diameter 9–17 mm. Gram-negative bacteria: (+++) Highly active=inhibition zone diameter >18 mm; (++) Moderately active=inhibition zone diameter 15–18 mm; (+) Slightly active=inhibition zone diameter 12–15 mm. Fungi: (+++) Highly active=inhibition zone diameter >20 mm; (++) Moderately active=inhibition zone diameter 15–20 mm; (+) Slightly active=inhibition zone diameter 10–15 mm.
| Test compounds | MIC (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Bacterial strains | Fungal strains | ||||||
| Gram-positive bacteria | Gram-negative bacteria | Aspergillus niger ATCC 6275 | Fusarium oxysporum ATCC 7601 | ||||
| Bacillus subtilis ATCC 6633 | Staphylococcus aureus ATCC 25923 | Streptococcus pneumoniae ATCC 49619 | Escherichia coli ATCC 25922 | Salmonella Typhimurium ATCC 13311 | |||
| 1 | 125 | 125 | 31.25 | 62.5 | 15.63 | 125 | 62.5 |
| 2 | 15.63 | 62.5 | 0.98 | 7.81 | 0.49 | 7.81 | 3.9 |
| 4 | 31.25 | 62.5 | 15.63 | 125 | 125 | 500 | 125 |
| 5 | 3.9 | 15.63 | 0.98 | 15.63 | 1.95 | 3.9 | 1.95 |
| 6a | 125 | 125 | 125 | 125 | 62.5 | 500 | 125 |
| 6b | 15.63 | 31.25 | 3.9 | 31.25 | 7.81 | 3.9 | 1.95 |
| 6c | 125 | 125 | 125 | 125 | 125 | 62.5 | 31.25 |
| 7a | 7.81 | 15.63 | 1.95 | 1.95 | 0.98 | 31.25 | 7.81 |
| 7b | 3.9 | 15.63 | 0.49 | 15.63 | 3.9 | 1.95 | 0.98 |
| 8a | 0.98 | 1.95 | 0.49 | 1.95 | 0.12 | 0.24 | 1.95 |
| 8b | 500 | 500 | 500 | 500 | 125 | 500 | 500 |
| 10 | 500 | 500 | 31.25 | 125 | 31.25 | 15.63 | 7.81 |
| 11 | 7.81 | 125 | 3.9 | 31.25 | 1.95 | 500 | 500 |
| Standard antimicrobial drug | |||||||
| Ampicillin | 0.007 | 0.015 | 0.03 | — | — | — | — |
| Gentamicin | — | — | — | 0.24 | 0.015 | — | — |
| Amphotericin B | — | — | — | — | — | 0.03 | 0.98 |
MIC values were expressed as µg/mL.
Results of the in vitro antiproliferative activity against Hep G2 (hepatocellular carcinoma) and MCF7 (breast adenocarcinoma) cells expressed as inhibitory concentration 50% (IC50) are summarized in Table 3 compared to that of the standard anticancer drug Doxorubicin. As illustrated in Table 3, the majority of the test compounds possess potential cytotoxic activities against the tested human cancer cell lines in submicromolar range.
| Test compound | HepG2 (ATCC® HB8065™, hepatocellular carcinoma) IC50 (µM) | MCF7 (ATCC® HTB22™, breast adenocarcinoma) IC50 (µM) |
|---|---|---|
| 1 | 168.54±0.379 (−) | 41.02±0.356 (+) |
| 2 | 71.3±0.098 (+) | 2.291±0.018 (++) |
| 4 | 351.9±2.647 (−) | 1.389±0.007 (++) |
| 5 | 1.65±0.011 (++) | 0.0211±0.00003 (+++) |
| 6a | 425.280±4.031 (−) | 0.0169±0.0001 (+++) |
| 6b | 3.627±0.014 (++) | 0.0198±0.00015 (+++) |
| 6c | 680.89±5.357 (−) | 2.421±0.012 (++) |
| 7a | 8.31±0.025 (++) | 54.13±0.29 (+) |
| 7b | 0.0158±0.00013 (+++) | 0.0001±0.00000036 (+++) |
| 8a | 38.69±0.174 (+) | 92.96±0.18 (+) |
| 8b | 5.47±0.032 (++) | 103.22±0.49 (−) |
| 10 | 110.131±0.76 (−) | 0.0091±0.000016 (+++) |
| 11 | 127.468±0.53 (−) | 147.23±0.865 (−) |
| Doxorubicin (standard anticancer drug) | 0.008±0.00005 (+++) | 0.099±0.00048 (+++) |
IC50: concentration of the compound (µM) producing 50% cell growth inhibition after 72 h of compound exposure, as determined by the SRB assay. Each experiment was run at least three times, and the results are presented as the mean±standard deviation (S.D.). (+++) Highly active=IC50<1 µM; (++) Moderately active=1 µM<IC50<10 µM; (+) Slightly active=10 µM<IC50<100 µM; (−) Inactive=IC50 >100 µM.
In the pyridine derivatives series (compounds 1, 2, 5), compound 2 showed improved antimicrobial activity compared to compound 1 which might be explained by the introduction of the electron-withdrawing chlorine atom at position 2 of the pyridine ring. In line with the structure–activity relationship (SAR) exploration, the presence of electron-withdrawing on the pyridine ring dramatically improved the antimicrobial activity. Furthermore, compound 5 exhibited the best antimicrobial activity among this series of compounds. One explanation is that, the introduction of carbothioamide moiety might favor the inhibitory potency.
In regards to the pyrazolo[3,4-b]pyridines series (compounds 4, 10, 11), compound 4 was recorded to be less active than compound 2. It has been known that the introduction of bulky moieties at different positions of the pyridine ring could affect the antimicrobial activity. Accordingly, the cyclization of 2 under the effect of hydrazine hydrate to yield the amine derivative 4 resulted in the introduction of the bulky aromatic pyrazolo[3,4-b]pyridine group which might be resulted in the loss of antimicrobial activity. These results suggest that the addition of bulky substituents might deteriorate the antimicrobial activity of this derivative in this series of compounds, suggesting that this bicyclic system plays a negative role on the antimicrobial effectiveness. In contrast, compound 10 exhibited better antimicrobial activity than 4 against Salmonella Typhimurium, Aspergillus niger and Fusarium oxysporum, an observation that could be explained by the introduction of the aliphatic malononitrile moiety, suggesting that pyrazolo[3,4-b]pyridine system bearing malononitrile moiety might enhance the antimicrobial efficacy against these microbial strains. Similarly, compound 11 was proved to be more active than 4 against Bacillus subtilis, Streptococcus pneumonia, Escherichia coli, and Salmonella Typhimurium, possibly due to the introduction of the aromatic hydroxyquinoline moieties, indicating that pyrazolo[3,4-b]pyridine system bearing hydroxyquinoline moiety might enhance the antimicrobial efficacy against these microbial strains. Moreover, compound 11 appeared to be more active than compound 10 against four of the test microorganisms. It is evident that the presence of a hydroxyl group endowed with an electron-donating and hydrophilic character in 11 could potentially contribute to the enhanced antimicrobial activity with respect to introducing a malononitrile moiety in 10.
Concerning the Schiff’s bases series (compounds 6a–c), compound 6a was found to be less active than 4. Additionally, compounds 6b and c were proved to be more active than 6a. Furthermore, compound 6b appeared to be more active than compound 6c. The varying degree of antimicrobial activity of these derivatives seems to be governed in part by the physicochemical properties of the aromatic or heterocyclic aldehyde that has been condensed with 4 to afford the different Schiff’s bases.
In the 4-thiazolidinones series (compounds 7a, b), compound 7a was proved to be more active as antibacterial agent than compound 6b, whereas compound 6b was found to be more active than compound 7a as antifungal agent, suggesting that the cyclization of the Schiff’s base 6b with thioglycolic acid to yield the 4-thiazolidinone 7a resulted in improved antibacterial activity and diminished antifungal activity. On the other hand, compound 7b was found to be more active than compound 6c against most of the test microorganisms, indicating that the cyclization of the Schiff’s base 6c with thioglycolic acid to yield the 4-thiazolidinone 7b encourages the antimicrobial activity. Interestingly, compounds 7a and b were observed to have comparable antimicrobial activity against most of the test microorganisms, suggesting that their antimicrobial activities were not significantly affected by the position or physicochemical properties of the different substituents and functional groups.
Noteworthy, the azetidin-2-one 8a was found to be more active than compound 6b against most of the test microorganisms, indicating that the cyclization of the Schiff’s base 6b with chloroacetyl chloride to afford the azetidin-2-one 8a was in favor of enhanced antimicrobial activity. In contrast, the Schiff’s base 6c was proved to be more active than the azetidin-2-one 8b particularly at the antifungal activity level, suggesting that the cyclization of the Schiff’s base 6c with chloroacetyl chloride to afford the azetidin-2-one 8b resulted in a diminished antimicrobial activity. Unexpectedly, although both 8a and b belong to the azetidin-2-one series, 8a was proved to be the most active, whereas 8b was found to be the least active among the tested compounds against most of the test microorganisms. Perhaps their antimicrobial activities were significantly affected by the physicochemical properties of the different substituents on the azetidin-2-one nucleus.
In most cases, the inhibitory potency exhibited by the tested compounds is lower than that of the standard antimicrobial agents. Noteworthy exception arises with compound 7b which is nearly as active as the standard antimicrobial drug Amphotericin B against Fusarium oxysporum. Notably, the inhibitory potency of the tested compounds against Gram-positive bacterial strains was somewhat superior when compared to Gram-negative bacterial strains and fungal strains.
Antiproliferative ActivityAmong the pyridine derivatives (compounds 1, 2, 5), compound 5 was proved to be the most active among this series against HepG2 and MCF7 cells, confirming that the introduction of carbothioamide moiety might favor the cytotoxic potency. The cytotoxic activity against both HepG2 and MCF7 cells was enhanced in compound 2 when compared to compound 1, possibly due to the introduction of an electron-withdrawing chlorine atom at position 2 of the pyridine ring, suggesting that the electronic influence of the substituents in the pyridine ring appears to play an important role in the antiproliferative activity.
In the pyrazolo[3,4-b]pyridines series (compounds 4, 10, 11), the cyclization of 2 under the effect of hydrazine hydrate to yield the amine derivative 4 resulted in the introduction of the bulky aromatic pyrazolo[3,4-b]pyridine group, causing a deterioration of cytotoxic potency against HepG2 cells. Accordingly, compounds 10 and 11 were found to be inactive against the HepG2 cells. Unexpectedly, the introduction of the pyrazolo[3,4-b]pyridine group resulted in improved antiproliferative potency of compounds 4 and 10 against MCF7 cells, demonstrating that compound 10 is more potent than compound 4 against the MCF7 cells, an observation that might be attributed to the introduction of the small aliphatic malononitrile moiety. In contrast, the introduction of the bulky aromatic hydroxyquinoline moiety to yield 11 resulted in the loss of the antiproliferative activity against both HepG2 and MCF7 cells. The real reason for this discrepancy is not clear. It seemed quite probable that this behavior depends on the cancer cells to which the compounds were subjected to.
In regards to the Schiff’s bases series (compounds 6a–c), it has been observed that compound 6b was found to be the only active among this series of compounds against HepG2 cells. One possibility is that the condensation of 4 with the heterocyclic aldehyde, 1H-indole-3-carbaldehyde to afford 6b was favorable for increasing antiproliferative potency. In contrast, the condensation of 4 with p-chlorobenzaldehyde or 1,3-diphenyl-1H-pyrazole-4-carbaldehyde to yield 6a and c, respectively, led to diminished cytotoxicity against the HepG2 cells. In contrast, the condensation of 4 with p-chlorobenzaldehyde or 1H-indole-3-carbaldehyde to afford the corresponding Schiff’s bases 6a and b, respectively, might be favorable for improving cytotoxic activity against MCF7 cells. However, the condensation of 4 with 1,3-diphenyl-1H-pyrazole-4-carbaldehyde to yield 6c was unfavorable for antiproliferative efficacy against the MCF7 cells.
Concerning the 4-thiazolidinones series (compounds 7a, b), compound 7a was found to be less potent than compound 6b, indicating that the cyclization of the Schiff’s base 6b with thioglycolic acid to yield the 4-thiazolidinone 7a resulted in decreased antiproliferative activity against both HepG2 and MCF7 cells. In contrast, compound 7b was found to be more potent than compound 6c against both HepG2 and MCF7 cells, suggesting that the cyclization of the Schiff’s base 6c with thioglycolic acid to yield the 4-thiazolidinone 7b resulted in enhanced cytotoxic potency. It has been shown that compound 7b was proved to be more active than compound 7a against both HepG2 and MCF7 cells. One explanation might be that the presence of the 1,3-diphenyl-1H-pyrazol-4-yl moiety in 7b could enhance its cytotoxicity.
Regarding the azetidin-2-ones series (compounds 8a, b), compound 8a was found to be less active than the Schiff’s base 6b against both HepG2 and MCF7 cells, suggesting that the cyclization of 6b with chloroacetyl chloride to yield the azetidin-2-one 8a was not in favor of enhanced antiproliferative activity. In contrast, the azetidin-2-one 8b was proved to be more active than the Schiff’s base 6c against HepG2 cells, indicating that the cyclization of 6c with chloroacetyl chloride to afford 8b resulted in improved cytotoxic efficacy. Surprisingly, this cyclization led to a substantial decrease in antiproliferative potency against the MCF7 cells.
The test compounds showed comparatively much weaker cytotoxic profiles when compared to the standard anticancer drug against HepG2 cells. On the other hand, the cytotoxic effect revealed by the test compounds (5, 6a, b, 7b, 10) was found to be better than that of Doxorubicin against MCF7 cells. In particular, compound 7b displayed the highest antiproliferative efficacy against both HepG2 and MCF7 cells, demonstrating that this compound could be an interesting lead antiproliferative candidate. Noteworthy, the antiproliferative efficacy of the tested compounds against MCF7 cells was somewhat superior when compared to HepG2 cells.
We report herein the synthesis of three series of compounds, including Schiff’s bases, 4-thiazolidinones and azetidin-2-ones. The synthesized compounds were evaluated for their antimicrobial and antiproliferative efficacy. Our results revealed that some of the tested compounds exhibited a significant antimicrobial potency against a wide spectrum of bacteria and fungi. Moreover, some of the tested compounds have been found to possess a promising antiproliferative activity against HepG2 and MCF7 cell lines. The synthesized compounds certainly hold great promise towards pursuit to discover novel antimicrobial and antiproliferative agents. Our study is a preliminary step toward developing newly synthesized antimicrobial and antiproliferative agents. Further studies are being conducted to acquire more information about SAR of these novel synthesized compounds in order to clarify their mode of action as antimicrobial and antiproliferative substances. Modifications to improve the potency of the synthesized derivatives by structural diversification are currently under progress in our laboratory.
The authors declare no conflict of interest.