抄録
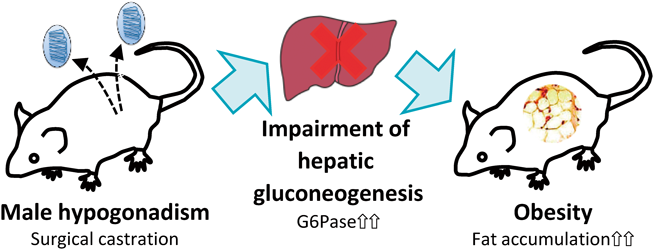
The steroid hormones synthesized by the male gonads play diverse roles in biological processes. Androgens, the primary hormones produced by the male gonads, are key regulators of fat homeostasis, hence androgen-deprivation therapies often induce obesity. However, the molecular mechanism by which male gonadal dysfunction leads to obesity remains unclear, because results from animal studies regarding fat accumulation in the context of gonadal defects do not reflect clinical findings. Here, we investigated the mechanism underlying the development of obesity in animals with male gonadal dysfunction by analyzing the long-term physiological changes in adult male mice with surgical castration. Nine weeks after surgery, white adipose tissue (WAT) mass was higher in the castrated (Cas) mice than in sham-operated (Sham) mice. In addition, castration induced hyperlipidemia and hyperglycemia. However, genes involved in lipid metabolism, including hormone-sensitive lipase, were unchanged in the adipose tissue of the Cas mice, despite the increase in WAT. In contrast, a hepatic gluconeogenesis gene, glucose-6-phosphatase, was significantly upregulated in the Cas mice than in Sham mice. Our findings suggest that long-term hypogonadism in mice mimics the effects in humans, and a potential molecular basis for the induction of obesity in this model is impairment of hepatic gluconeogenesis.