2016 年 39 巻 6 号 p. 927-934
2016 年 39 巻 6 号 p. 927-934
During the past two decades, it has been reported that treatment with all-trans-retinoic acid (ATRA) induces alveolar regeneration in rodent emphysema models. In the present study, we investigated the regeneration by ATRA at various exposure conditions in two strains of mice with induced emphysema. The emphysema model was created by postnatal administration of dexamethasone to ICR and FVB mice, which were then treated with ATRA from postnatal day 42. The regeneration was observed in ICR mice but not in FVB mice given 10 and 40 mg/kg/d ATRA for 10 d. The concentration–time profiles of ATRA in plasma and lung were similar in both strains. These results suggest that the strain difference in the regeneration by ATRA was not caused by differences in the exposure to ATRA. On the other hand, the regeneration in ICR mice was enhanced by an increase of the intraperitoneal dose in the range of 10–40 mg/kg/d for 10 d. At an intraperitoneal dose of 40 mg/kg/d, the regeneration was observed after 10 and 20 d of treatment but not after 1 to 5 d of treatment. Meanwhile, the regeneration by intraperitoneal administration of ATRA was enhanced more than that by oral administration. Exposure to ATRA during repeated intraperitoneal administration to ICR mice was higher than that in oral administration. The results suggest that the regeneration in ICR mice requires at least 10 d of treatment with ATRA and its effects depend on the exposure to ATRA in plasma, which parallels that in lung.
Emphysema is characterized by enlargement of alveoli and reduction of alveolar surface area with dysfunction of gas-exchange. In general, once alveoli are damaged, it is extremely difficult to repair. It has been reported that alveoli regeneration can be induced in rodents by treatment with some compounds, such as adrenomedullin,1) simvastatin,2) granulocyte-colony stimulating factor,3) tamibarotene4) and all-trans-retinoic acid (ATRA).5) However, a basic remedy for emphysema by alveoli regeneration has not been established in human using such compounds. At the present time, only symptomatic therapies such as bronchodilator agents are available. Thus development of an effective basic remedy for restructuring alveoli is highly desired.
ATRA, one of the active forms of vitamin A, is a morphogen that has important effects on cell growth, differentiation, and organogenesis. Also it has been used as a treatment for acute promyelocytic leukemia. It was reported that vitamin A was necessary for the growth of the lungs in rodents in the perinatal and postnatal period6–9) and for maintenance of tissue homeostasis in the lungs in adult rodents.10–12) Massaro G.D. and Massaro D.12) reported that regeneration of alveoli with enlargement of alveolar surface area was observed in emphysema-model rats treated with ATRA, suggesting that ATRA induces alveolar regeneration and improves lung function.
Various investigations of the alveolar regeneration by ATRA using animal models of emphysema have been reported. Regeneration was observed in about half of these studies5,12–18) but not in the others.19–24) Especially in mice, regeneration was observed in the reports using three strains, ICR (CD-1), NIHS8) and TO15,17) but not in those using three other strains, FVB,20) A/J and B63F1.24) In C57BL/6 mice with induced emphysema, successful regeneration of alveoli by ATRA treatment was reported by Ishizawa et al.16) but failure by Fujita et al.22) In addition to such strain or species differences, the divergent result in C57BL6 might come from the differences in the following experimental conditions; the kind of emphysema models and the ATRA dosing conditions. Stinchcombe and Maden reported that the dose of ATRA required to induce regeneration was different between NIHS and ICR mice.18) However, there have been no studies examining these issues concurrently, and thus the reason for the divergent results remains unclear.
In the present study, we investigated the alveolar regeneration by ATRA at various exposure conditions in two strains of mice with induced emphysema.
ATRA and dexamethasone (DEX) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Acitretin was purchased from Sigma-Aldrich Inc. (St. Louis, MO, U.S.A.). All other reagents and solvents in this study were commercially available and were either extra pure, molecular biology or biochemical grade.
AnimalsPregnant ICR and FVB mice (day 14 of gestation) were obtained from CLEA Japan, Inc. (Tokyo, Japan). These strains were selected since it was reported that the regeneration was induced by ATRA in the ICR strain18) but not in the FVB strain.20) Their male offspring were used for alveolar regeneration experiments. Postnatal day 1 (P1) was defined as the first day after birth. Male ICR and FVB mice (6 weeks old) were obtained also from the same breeder and used for pharmacokinetic (PK) experiments after 5 or more days of acclimatization. All mice were housed in a temperature- (23±1°C) and humidity- (55±5%) controlled room with 12-h light/dark cycle. Water and food were available ad libitum except for the single oral administration in PK experiment as described below. The protocols were approved by the institutional review committee in the Tokyo University of Science as animal experiment protocols No. Y12004, Y13031 and Y14036. All experimental animals were handled in accordance with the institutional and national guidelines for the care and use of laboratory animals.
Preparation of the Drug Solutions and SuspensionsTwenty micrograms per milliliter of DEX solution for intraperitoneal administration was prepared as described previously (Kamei et al., 2014). Briefly DEX was dissolved in dimethylsulfoxide and diluted 200-fold with phosphate buffered saline (pH 7.4). ATRA suspension for intraperitoneal and oral administration was prepared as follows; ATRA was suspended in peanut oil at a concentration of 5, 10 or 20 mg/mL. As described by Saadeddin et al.,25) ATRA solutions for intravenous administration were prepared as follows. One hundred fifty milligrams of ATRA was dissolved in 0.7 mL of 0.1 mM sodium hydrate, 3.5 mL of ethanol and 500 mg of Tween 20. After 3 min of sonication, the solution was diluted to 5 mL with ultrapure water. All of the bottles and tubes containing ATRA were covered by aluminum foil to prevent exposure to light.
Alveolar Regeneration ExperimentsEffect of Dose in FVB MiceTo evaluate the effect of ATRA on the alveolar regeneration the following experiments were conducted according to the method reported by Maden17) and Stinchcombe and Maden18) with modifications. Newborn mice from 8 dams were divided equally on P3 to 4 groups, RA10, RA40, DEX and control (Cont) groups. To create the emphysema model, 0.4 µg per animal (20-µL injection) DEX was administered subcutaneously to newborn mice in the RA and DEX groups from P3 to P14 daily with a 2-d break on P8 and P9 according to our previous reports.26) Phosphate buffered saline (pH 7.4) containing 0.5% dimethylsulfoxide as a vehicle was administered to mice in the Cont group. At P28, 4 male mice were randomly selected and the others were excluded in each group. Then, 10 and 40 mg/kg/d ATRA (suspension; 2 mL/kg/d) were administered intraperitoneally to mice in the RA10 and RA40 groups from P42 to P53 daily with a 2-d break on P47 and P48 according to the previous report by Maden.17) Peanut oil as a vehicle was administered to the other mice in the DEX and Cont groups. At P90 the lungs were isolated from the mice after euthanasia by exsanguination under isoflurane anesthesia, and were used for the evaluation of alveolar regeneration.
Effect of Dose in ICR MiceFour groups of mice with induced emphysema (RA10, RA20, RA40 and DEX groups) and one group of control mice (Cont group) were prepared from newborn ICR mice as described for FVB mice (n=4 in each group). Ten, 20 and 40 mg/kg/d ATRA (suspension) were administered intraperitoneally to mice in the RA10, RA20 and RA40 groups, respectively, and peanut oil as a vehicle was administered to the mice in the DEX and Cont groups. At P90 the lungs were isolated from the mice as described before.
Effect of Duration of Dosing in ICR MiceSix groups of mice with induced emphysema (RA-1d, RA-3d, RA-5d, RA-10d, RA-20d and DEX groups) and one group of control mice (Cont group) were prepared as described before (n=4 in each group). Forty milligram/kilogram/day ATRA (suspension) was administered intraperitoneally to the mice in RA-1d, RA-3d, RA-5d, RA-10d and RA-20d groups for 1, 3, 5, 10 and 20 d, respectively, from P42 to P67 with a break on P47, P48, P54, P55, P61 and P62. Peanut oil as a vehicle was administered to the mice after the last day of ATRA administration in the treatment groups and in the DEX and Cont groups at the same time as the other groups until P67. At P90 the lungs were isolated from the mice as described before.
Effect of Administration Route in ICR MiceThree groups of mice with induced emphysema (RA-per os (p.o.), RA-intraperitoneally (i.p.) and DEX groups) and one group of control mice (Cont group) were prepared as described before (n=4 in each group). Forty milligram/kilogram/day ATRA (suspension) was administered orally and intraperitoneally to the mice in RA-p.o. and RA-i.p. groups, respectively, from P42 to P53 daily with a 2-d break on P47 and P48. The mice in the DEX and Cont groups were not treated. At P90 the lungs were isolated as described before.
PK ExperimentsSingle Administration to FVB MiceTen or 40 mg/kg ATRA (suspension) was administered intraperitoneally to 64 male FVB mice (6 weeks old). At 0 (predose), 30, 60, 120, 240, 300, 360 and 420 min after the administration, blood was collected from the inferior vena cava and the lungs were isolated under isoflurane anesthesia (n=4 at each time point).
Single Administration to ICR MiceNinety-six male (6 weeks old) mice were divided equally to 3 groups, and then 10, 20 and 40 mg/kg ATRA (suspension) was administered intraperitoneally to the mice in each group. In a separate group, 40 mg/kg ATRA (suspension) was administered orally to 32 male mice (6 weeks old), which were fasted overnight from the day before the administration. Blood and lungs were collected as described above (n=4 at each time point). Also 40 mg/kg ATRA (solution) was administered intravenously to 27 male mice (6 weeks old), and then blood and lungs were collected at 0 (predose), 5, 15, 30, 60, 120, 240, 300 and 360 min after the administration as described above (n=3 at each time point).
Repeated Administration to ICR MiceEight male ICR mice (6 weeks old) were divided into two equal groups and 10 mg/kg/day ATRA (suspension) was administered intraperitoneally and orally to the mice in each group, respectively, from day 1 (1st administration day) to 12 with a 2-d break at days 6 and 7. On days 1, 5, 8 and 12, about 50 µL of blood was collected from the tail vein using a heparinized hematocrit tube (Drummond Scientific Co., PA, U.S.A.) at 0 (predose), 30, 120, 240 and 420 min after the administration (n=4 at each time point).
Blood samples were centrifuged at 3000×g at 4°C for 10 min to obtain plasma. The plasma and lungs were frozen at −80°C until the measurement of ATRA concentration, which was conducted within a week after sample collection.
Histopathological AnalysesLung sections were prepared and the alveolar mean chord length (Lm) was calculated according to our previous report (Kamei et al., 2014). Briefly, the isolated lungs were inflated to standard pressure of 20 cm H2O with 1/2-Karnovsky’s solution (2% paraformaldehyde and 2.5% glutaraldehyde). After fixation, the samples were embedded in paraffin and sliced at 5 µm thickness. The sections were stained with the Elastica-van Gieson method and were observed with a light microscope (100×). The Lm was calculated from the image of the section using image analyses software, Image J (National Institutes of Health, U.S.A.), as an index of the distance between alveolar walls. The % Lm recovery was calculated as an index of alveolar regeneration by ATRA using the following equation reported by Maden17); % of Lm recovery=[(Lm,DEX−Lm,RA)/(Lm,DEX−Lm,Control)]×100. The subscript notation of Lm means the group name.
Determination of ATRA Concentration in Plasma and LungAccording to the method of Saadeddin et al. (2014), plasma samples were pretreated as described below. A four-fold volume of acetonitrile containing 1 µg/mL of acitretin (internal standard) was added to thawed plasma, and the mixture was centrifuged at 2000×g at 4°C for 10 min) to precipitate the proteins. Then, the supernatant was applied to the HPLC system. Meanwhile according to the method of Parthasarathy et al.,27) lungs were pretreated as described below. One hundred milligram of thawed lung was added to 400 µL of homogenizing solution (ascorbic acid–ethylenediaminetetraacetic acid–ultrapure water–acetic acid, 50 : 0.15 : 50 : 0.5, w/w/v/v) and homogenized using a homogenizer, Physcotron (Microtec Co., Ltd., Chiba, Japan). A three-fold volume of acetonitrile containing 10 µg/mL of acitretin was added to the lung homogenate, the mixture was centrifuged at 9000×g at 4°C for 10 min to precipitate the proteins and its supernatant was applied to the HPLC system.
HPLC was performed on a model 20-A Prominence system (Shimadzu Corp., Kyoto, Japan, U.S.A.) equipped with a Betasil C18 column (3.9×150 mm, 5 µm, Thermo Fisher Scientific, Waltham, MA, U.S.A.). The column temperature was set at 35°C. The mobile phase consisted of 100 mM acetic acid buffer (pH 5.0)–acetonitrile (20 : 80, v/v), and was delivered at a flow rate of 1.0 mL/min. The eluent from the column was analyzed by SPD-20 A UV detector at a wavelength of 340 nm.
Data AnalysesPharmacokinetics of ATRA in plasma and lung were characterized by determining the peak concentration (Cmax), time to Cmax (Tmax) and area under the concentration–time curve (AUC) from time zero to last sample (AUC0–last) and to infinity (AUC0–∞) after single administration of ATRA. The Cmax and Tmax values were taken directly from the data. The elimination rate constant (ke) was determined by log-linear regression of the last three measurable time points on the terminal phase of the logarithmic concentration–time curve. AUC0–last was calculated by the linear trapezoidal rule. AUC0–∞ was determined by the equation; AUC0–∞=AUC0–last + Clast/ke, where Clast means the ATRA concentration at the last measurable sampling point. Bioavailability (BA) was calculated by the equation; BA (%)=[(AUCp.o./Dosep.o.)/(AUCi.v./Dosei.v.)]×100. The subscript notation of Dose and AUC mean the administration routes; i.v., intravenous administration; p.o., oral administration.
As statistical analyses, Tukey’s and Dunnett’s multiple comparison tests and Student’s t-tests were used to determine the statistical significance using SPSS 17.0 (IBM, Armonk, NY, U.S.A.). Differences were considered statistically significant when p<0.05.
DEX was administered to ICR and FVB mice in the postnatal period to prepare the emphysema model. Larger alveoli were observed in the DEX groups of both strains compared to each respective Cont group (Fig. 1). Furthermore, the Lm calculated from the lung sections was also significantly larger compared to that for the Cont group (p<0.05, Figs. 2, 3). From these results, the emphysema model was confirmed in each strain. However, the Lm in the DEX group was 3.6- and 1.9-times longer than that in the Cont group in ICR mice and FVB mice, respectively, indicating that alveoli in FVB mice were less sensitive to DEX treatment compared to those in ICR mice. On the other hand, in ICR mice, smaller alveoli were observed in the RA group compared to those of the DEX group (Fig. 1). Furthermore, Lm of the RA group was significantly smaller compared to that of the DEX group (p<0.05, Fig. 3). Meanwhile the Lm of FVB mice were not statistically significantly different among DEX, RA10 and RA40 groups (Fig. 2).

Panels A, C, E and G are lung sections from ICR mice, while panels B, D, F and H are from FVB mice. Peanut oil as a vehicle was administered intraperitoneally to the mice in the Cont. groups (A, B) and the mice with induced emphysema in the DEX groups (C, D). Ten and forty mg/kg/d ATRA was administered for 10 d to the mice with induced emphysema in the RA10 groups (E, F) and RA40 groups (G, H), respectively. The sections were stained with Elastica-van Gieson. Original magnification, 100×. Bars, 200 µm.

Zero (only peanut oil as a vehicle), 10 and 40 mg/kg/d ATRA was administered intraperitoneally for 10 d to FVB mice with induced emphysema in the DEX, RA10 and RA40 groups, respectively. Then the Lm was calculated from lung sections from each mouse as an index of the alveolar size. Data are shown as the mean±S.D., n=4. Superscript letters over the bars indicate results of the pairwise comparison. The means of different letters are significantly different (p<0.05, Tukey’s multiple comparison test) for pairs of bars with different characters.
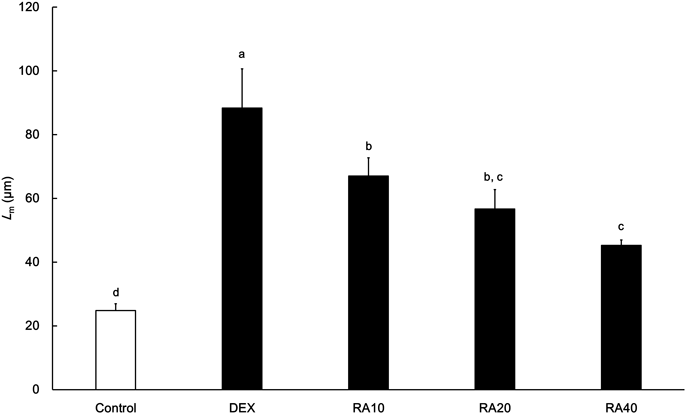
Zero (only peanut oil as a vehicle), 10, 20 and 40 mg/kg/d ATRA was administered intraperitoneally to ICR mice with induced emphysema in DEX, RA10, RA20 and RA40 groups for 10 d, respectively. Then the Lm was calculated from lung sections from each mouse as an index of the alveolar size. Data are shown as the mean±S.D., n=4. Superscript letters over the bars indicate results of the pairwise comparison. The means of different letters are significantly different (p<0.05, Tukey’s multiple comparison test).
The effect of dose, duration of dosing and administration route of ATRA on alveolar regeneration in ICR mice was evaluated by using the Lm values at P90 after each treatment. For the evaluation of the dose, 10, 20 or 40 mg/kg/d ATRA was administered intraperitoneally to ICR mice for 10 d. Shorter Lm was observed in the mice to which higher doses of ATRA were administered (Fig. 3). The % of Lm recovery at the doses of 10, 20 and 40 mg/kg/d were 34, 50 and 68%, respectively, indicating that the alveolar regeneration was dose-proportional at least until 40 mg/kg/d. For the evaluation of duration of dosing, 40 mg/kg/d ATRA was administered intraperitoneally to ICR mice for 1, 3, 5, 10 or 20 d. The Lm after 1, 3 and 5 d administration were not significantly different compared to that in DEX group, while those after 10 and 20 d administration were significantly shorter (p<0.05, Fig. 4). However the Lm after 10 d administration was comparable to that after 20 d administration. For the evaluation of administration route, 40 mg/kg/d ATRA was administered intraperitoneally or orally to ICR mice for 10 d. The Lm after intraperitoneal administration was significantly shorter than that after oral administration (p<0.05, Fig. 5).

Forty mg/kg/d ATRA was administered intraperitoneally to ICR mice with induced emphysema in RA-1d, RA-3d, RA-5d, RA-10d and RA-20d groups, for 1, 3, 5, 10 and 20 d, respectively. Only peanut oil as a vehicle was administered to the mice with induced emphysema in DEX group. Then the Lm was calculated from lung sections from each mouse as an index of the alveolar size. Data are shown as the mean±S.D., n=4. Superscript letters over the bars indicate results of the pairwise comparison. The means of different letters are significantly different (p<0.05, Tukey’s multiple comparison test).

Forty mg/kg/d ATRA was administered orally and intraperitoneally to ICR mice with induced emphysema in RA-p.o. and RA-i.p. groups for 10 d, respectively. The mice with induced emphysema in DEX group were not treated. Then the Lm was calculated from lung sections from each mouse as an index of the alveolar size. Data are shown as the mean±S.D., n=4. Superscript letters over the bars indicate results of the pairwise comparison. The means of different letters are significantly different (p<0.05, Tukey’s multiple comparison test).
The ATRA concentrations in plasma and lung were determined after single administration to ICR and FVB mice. After intraperitoneal administration of 10 or 40 mg/kg ATRA to FVB mice, the plasma concentration reached a maximum at 120 min, then decreased (Fig. 6A). Moreover the concentration profiles in lung were almost parallel to those in plasma (Figs. 6A, B). When ATRA was administered to ICR mice at the same doses as given to the FVB mice as described above, similar concentration profiles were observed and similar PK values were obtained in the two strains (Figs. 6A, B, Table 1).

A and B; 10 mg/kg ATRA was administered intraperitoneally to ICR and FVB mice. C and D; 40 mg/kg ATRA was administered intraperitoneally (i.p.) and per os (p.o.) to ICR mice. Data are shown as the mean±S.D., n=4. * p<0.05 for comparison between i.p and p.o. groups or between ICR and FVB mice (Student’s t-test).
| Strain | Dose (mg/kg) | Route | Plasma | Lung | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cmax (µg/mL) | Tmax (min) | AUC0–∞ (µg·min/mL) | BA (%) | Cmax (µg/g) | Tmax (min) | AUC0–∞ (µg·min/g) | |||
| FVB | 10 | i.p. | 2.7±0.5 | 120 | 543 | — | 11.4±1.9 | 60 | 1968 |
| 40 | i.p. | 5.8±0.9 | 120 | 1882 | — | 29.7±6.0 | 120 | 8185 | |
| ICR | 10 | i.p. | 2.7±0.3 | 120 | 492 | 93 | 10.0±1.9 | 60 | 1572 |
| 20 | i.p. | 4.5±0.5 | 60 | 903 | 95 | 22.6±1.7 | 120 | 4809 | |
| 40 | i.p. | 6.0±1.3 | 120 | 1606 | 76 | 27.7±7.2 | 120 | 7423 | |
| 40 | p.o. | 7.9±1.3 | 120 | 2053 | 95 | 35.2±9.8 | 120 | 9214 | |
| 40 | i.v. | — | — | 1621 | — | — | — | 7706 | |
All values are expressed as the mean±S.D., n=4. i.p., intraperitoneal administration; p.o., oral administration; i.v., intravenous administration; BA, bioavailability.
The AUC0–∞ and Cmax in plasma after intraperitoneal administration were increased with the increase of doses in the range of 10–40 mg/kg in ICR mice (Table 1). Also AUC0–∞ and Cmax in lung were positively correlated with those in plasma (data not shown). On the other hand AUC0–∞ and Cmax in plasma after oral administration of 40 mg/kg ATRA were comparable to those after intraperitoneal administration (Fig. 6C, Table 1).
BioavailabilityAfter intravenous administration of 40 mg/kg ATRA to ICR mice, the AUC0–∞ in plasma was calculated for determining bioavailability. The bioavailability was about 100% after intraperitoneal administration at the dosing range of 10–40 mg/kg and after oral administration of 40 mg/kg ATRA (Table 1).
Effect of Repeated Administration on the Exposure in PlasmaThe AUC0–420 min was determined during repeated administration of 40 mg/kg/d ATRA to ICR mice (Fig. 7). After oral administration, the AUC0–420 min on day 12 was significantly lower than that on day 1, and a tendency to lower AUC on day 8 was also observed. Meanwhile after intraperitoneal administration the AUC0–420 min on all sampling days were comparable. On days 8 and 12, the AUC0–420 min was significantly lower after oral administration than that after intraperitoneal administration (p<0.05, Fig. 7).

Forty mg/kg/d ATRA was administered orally and intraperitoneally to ICR mice for 10 d (including 2-d break on day 6 and day 7). Data are shown as the mean±S.D., n=4. * p<0.05 for comparison between i.p and p.o. groups (Student’s t-test) and † p<0.05 for comparison vs. day 1 (Dunnett’s multiple comparison test).
We evaluated the effects of ATRA on alveolar regeneration in ICR and FVB mice given DEX in the postnatal period, which is a model for emphysema. The regeneration was clearly observed in ICR mice but less in FVB mice (Figs. 1–3). These results are consistent with the previous reports by Lucey et al.20) and Stinchcombe and Maden,18) and suggest that the regeneration depends on the strain of mice. On the other hand, however, there was no difference in ATRA exposure in plasma and lung between these strains (Figs. 6A, B). These results suggest that the strain difference in alveolar regeneration by ATRA was not caused by differences in the exposure of ATRA, but probably by the differences in pharmacological sensitivity to ATRA in those mice.
It was thought that the sensitivity was affected not only by the strains but also by some other factors, e.g., severity of alveolar damage in the emphysema models. In the present study, the alveoli of FVB mice were less damaged by DEX compared to those of ICR mice. This difference might cause the strain difference of the alveolar regeneration by ATRA. Previously, emphysema models for experiments have been created by different methods; DEX treatment in the neonatal period,15,17,18) elastase treatment in adult animals5–7,11,12,14,16) and exposure to cigarette smoke in adults.19,21,24) The divergent results about the regeneration of alveoli in many reports might be caused by those differences in methodology and the strain and species differences of the sensitivity to those methods.
The mechanism of alveolar regeneration by ATRA has not been clarified completely so far. Recently, however, Horiguchi et al.28) reported that ATRA induced the differentiation of alveolar epithelial stem cells to type-I and type-II alveolar epithelial cells in vitro. Thus ATRA might promote development of undifferentiated cells such as stem cells in the lung and make the cells regenerate extracellular matrices, and this would result in the regeneration of alveoli. If this is the main mechanism, the number of stem cells present in the lung would be a very important factor for the sensitivity. Furthermore, since in general the number of stem cells is lower in the elderly compared to the young, ATRA might be more effective in the young than in the elderly.
The relationship between pulmonary exposure to ATRA and alveolar regeneration was investigated in ICR mice under various administration conditions. In the PK experiments the ATRA concentration profile in lung was parallel to that in plasma, suggesting that the plasma concentration is a good index of the pulmonary exposure to ATRA. The alveolar regeneration by ATRA was dependent on the level of exposure in the dose range of 10–40 mg/kg/d (Fig. 3, Table 1).
In the examination of administration route, the AUC0–420 min after oral administration at days 8 and 12 was lower than that after intraperitoneal administration. Accordingly the total exposure to ATRA after repeated administration was lower after oral administration than that after intraperitoneal administration. The difference in the total exposure coincided with the degree of regeneration (Figs. 3, 5). Furthermore, considering the effect of duration of dosing at a dose of 40 mg/kg/d on the regeneration, the treatment for 10 d was almost comparable to that for 20 d, but the treatment for 5 d or less was not effective (Fig. 4). These results suggest that 10 d treatment is necessary and sufficient for the regeneration. Thus the efficacy might depend on both concentration and treatment duration of ATRA exposure.
In general, exposure to ATRA decreased after repeated administration. As a reason for the phenomenon, induction of CYP26,29–31) an enzyme responsible for metabolism of ATRA in humans was reported by Ozpolat et al.32) Since CYP26 is also expressed in mice,33) the decrease of AUC0–420 min during repeated oral administration would be caused by the induction of CYP26 in the present study. During intraperitoneal administration, however, the decrease of AUC was not observed (Fig. 7). The reason for this difference might be that ATRA can avoid first pass metabolism in the intestine after intraperitoneal administration but not after oral administration. Enzyme induction in the intestine might affect the pharmacokinetics of ATRA.
Roth et al.34) and Muindi et al.35) reported that emphysema patients showed no improvement in lung function during treatment with 2 mg/kg/d ATRA for six months with a 3-d break in each week. The AUC from the time 0 to 12 h after the 1st administration in that clinical study was 63.4 µg·min/mL, which was clearly lower than 482 µg·min/mL, the AUC0–420 min (from the time 0 to 7 h) in ICR mice after administration of 10 mg/kg/d ATRA in the present study. Furthermore the AUC decreased to about 1/3 on the last day in that clinical study. These facts imply the possibility that total exposure to ATRA was too low to allow recovery from emphysema in humans. Thus, if the sensitivity to ATRA in humans is similar to that in ICR mice, a higher dose of ATRA, one which would yield the same exposure as in mice, could be effective in patients with emphysema. Unfortunately, however, a higher dose of ATRA could lead to a higher risk of retinoid syndrome such as hepatic dysfunction. Also in the rats with cigarette smoke-induced lung injuries, retinoic acid supplementation reported to decrease the bone mineral content and bone mineral density.36) To achieve the same exposure to ATRA in humans by oral medication, the calculated dose would be more than 50 mg/kg/d. This dose would be extremely higher than the conventional dose for acute promyelocytic leukemia, 45 mg/m2, which is about 1.3 mg/kg for a human (170 cm height and 60 kg weight). Thus, new formulations for transpulmonary dosage are anticipated in order to get enough therapeutic effects and a minimum risk of systemic exposure, of course based on a careful consideration of the species differences.
In conclusion, we demonstrated strain differences in alveolar regeneration by ATRA between ICR and FVB mice with emphysema induced by postnatal administration of DEX. The results suggest that the strain dependence cannot be accounted for by any difference in PK. Furthermore the present study also suggests that the regeneration requires at least 10 d of treatment, and this effect depends on the level of exposure to ATRA at least in ICR mice with emphysema induced by DEX.
We would like to thank Dr. Donald Hinman for scientific advice and editing the manuscript.
The authors declare no conflict of interest.