2016 年 39 巻 6 号 p. 1022-1028
2016 年 39 巻 6 号 p. 1022-1028
Diethylnitrosamine (DEN) is a potent toxic material that can cause necrosis and subsequent fibrosis in the liver. Based on the previously reported hepatoprotective effect of Limonium tetragonum against the proliferation of hepatic stellate cells, we tested the EtOAc soluble fraction of L. tetragonum extract (EALT) in a DEN-induced hepatotoxic rat model. The development of hepatotoxicity including mononuclear cell infiltration and fibrosis induced by intraperitoneal injections of DEN (70 mg/2 mL/kg body weight (b.w.) per week) was observed at 4, 6 and 8 weeks after the first DEN treatment. Administration of EALT (200 mg/kg body weight, per os (p.o.)) induced significant reductions in serum alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), and triglycerides (TG) in DEN-injected rats. Increased oxidative stress in DEN-induced liver fibrosis rats was diminished by EALT treatment through a decrease in malondialdehyde (MDA) and increase in superoxide dismutase (SOD). Histologic findings that included markedly attenuated mononuclear cell infiltration and fibrosis could be observed in liver samples from the EALT-treated groups. An extract of Hovenia dulcis fruit and Sylimarin were used as positive controls. The present study provides direct experimental evidence for EALT attenuated hepatic injury and fibrosis in DEN-treated mice. The L. tetragonum EtOAc fraction might be useful in treating fibrotic liver diseases.
Hepatic fibrosis is a reversible wound-healing process resulting from chronic liver injury including hepatitis virus B and C, alcoholic liver disease, and non-alcoholic steatohepatitis.1) A key discovery in understanding fibrosis has been that collagen-producing cells like hepatic stellate cells (HSCs) play critical role in the development of liver fibrosis.2) Oxidative stress stimulates proliferation and invasiveness of HSCs and plant-derived antioxidants such as curcumin and resveratrol reduce HSC activation, thus suppressing liver fibrosis.3–5) Animal models to investigate the potential hepatoprotective drugs that suppress liver fibrosis have been well defined.6) Carbon tetrachloride (CCl4), thioacetamide (TAA), dimethylnitrosamine or diethylnitrosamine (DMN or DEN) are considered as the most common hepatotoxic agents in experimental models.6,7) According to the previous research, histological and genetic features of DEN-induced hepatocarcinogenesis were similar to those of human hepatocellular carcinoma.8) The development of antifibrotic drugs is important since patients infected with viral hepatitis usually develop liver cirrhosis and hepatocellular carcinoma, the main form of liver cancer, through the stage of fibrosis. In this reason, DEN-induced animal models might be useful as a tool for searching antifibrotic agents.
Limonium tetragonum (THUNB.) BULLOCK (Plumbaginaceae) is a biennial herbaceous halophyte that is mainly distributed in the southwestern costal area of South Korea.9) It has been widely used in treating uterine hemorrhage, oligomenorrhea, dysgalactia, and tinnitus.10) The edible buds and shoots of L. tetragonum contains several active compounds like myricetin glycosides and quercetin glycosides.11) Crude extracts and fractions from halophyte L. tetragonum exhibited biological properties such as anticancer and antioxidant activities.11,12) Although salt-tolerant plants including L. tetragonum have been recognized as one of the most important ecosystems in a tidal zone, biological and phytochemical studies on halophyte vegetation have been little reported yet.
In our recent study, the methanolic extract of L. tetragonum (MELT) was shown to protect the liver by suppression of the hepatic stellate cell line, HSC-T6 proliferation in vitro.10) Besides, the hepatoprotective effect of EtOAc soluble fraction of L. tetragonum extract (EALT) had been detected against liver injury induced by acute alcohol in Sprague-Dawley (SD) rats.13) The species of Limonium have been used for a long time in the treatment of human hepatitis and the hepatoprotective action of Limonium sinense (GIRARD) KTZE, a traditional Chinese folk medicine, has been well-documented.14,15) Here, based on the previous in vitro results of L. tetragonum, we examined the direct effect of EALT on liver fibrosis induced by hepatotoxin DEN in animal model. Changes in serum serum alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT) and triglyceride (TG) as well as oxidative stress parameters in the liver tissue were evaluated to identify the potential therapeutic effects of L. tetragonum extract on an experimental model of fibrosis.
The whole plants of L. tetragonum were collected from the foreshore in Sinan-gun, Korea in July 2013 and identified by Prof. Min Hye Yang of Pusan National University. A voucher specimen (GNP-70) has been deposited in the laboratory of pharmacognosy, college of life sciences and natural resources, Gyeongnam National University of Science and Technology. The EtOAc fraction of L. tetragonum extract (EALT) were prepared as previously described.13) Briefly, the aerial parts of L. tetragonum (2 kg) were dried using freezing-dryer, and then extracted with methanol. Removal of the solvent in vacuo yielded a methanolic extract (560.90 g), which was further suspended in distilled water and partitioned successively with CHCl3, EtOAc, and n-BuOH. EtOAc soluble fraction (42.69 g) of EALT was filtered and evaporated in vacuum. The dried Hovenia dulcis fruits (200 g) were extracted with hot water for 3 h, and then the solvent was removed in vacuo followed by freeze dried yielded H. dulcis extract (HDE) (41.1 g). EALT and HDE was accurately weighed and dissolved in 0.5%-carboxymethyl cellulose (CMC) for the treatment to experimental animals. For the usage of sylimarin as positive control, Legalon® which contains the patented extract of milk thistle ETHIS-094™ (Euromed) has been used.
Experimental Animals and TreatmentSix-weeks-old male SD rats weighing 160–180 g were purchased from Orient Bio (Seongnam, Korea). Rats were housed in a pathogen-free animal facility under a 12 h light/dark cycle at constant temperature and humidity throughout the experiment. After a period of adaptation for 1 week, the rats were randomly assigned to five groups (n=12 for each group) based on sample treatment. One hour after the sample (or saline solution) was administered orally, DEN was injected. Each rats subsequently received an intraperitoneal (i.p.) injection of DEN once a week for 8 weeks. The amount of saline, DEN and samples administered was adjusted for body weight (b.w.) each week (0.3 mL/100 g b.w.) and prepared just before use. All of the animal experiments were carried out according to the guidelines of the Gyeongnam National University of Science and Technology’s Committee on the Care and Use of Laboratory Animals.
Five groups are as follows.
After 4 and 6 weeks after the first DEN injection, the blood sample was collected from the caudal vein of experimental rat. After leaving a serum separation tube in room temperature for 30 min, blood samples were centrifuged at 3000 rpm for 10 min. The supernatant consisted of blood serum was stored at −70°C before use, and was used in the analysis of serum levels of ALT, AST, ALP, GGT and TG by spectrophotometry using commercially available kits.
Histological ExaminationAfter 6 weeks after the first DEN injection, 6 rats of each treatment group were randomly selected. Immediately after euthanasia, the whole liver was excised and washed in 0.05 M phosphate buffer saline (PBS) (pH 7.4) and weighed. Liver tissues were cut into pieces and immersed in 10% buffered formaldehyde for histological analyses. Additional liver samples for antioxidant enzyme or thiobarbituric acid-reactive substance (TBARS) assay were homogenized with 0.1 M PBS (pH 7.4). Fixed liver samples were embedded in paraffin blocks and sections of 3 µm were prepared. Serial slices were stained with haematoxylin–eosin (H&E) and examined under a Nikon Labophot microscope (Nikon Corporation, Tokyo, Japan). The remained rats of each treatment group were further incubated for additional 2 weeks and the whole liver was excised and prepared in the same way. Slices of 6 µm-thick were prepared and used for picrosirius red staining. Histological analysis was performed with Nikon Eclipse Ni (Nikon Instruments Inc., Melville, NY, U.S.A.), and polarized images was obtained by Olympus BX50 and U-AN360P/ U-TP 530 filters (Olympus Optical Co., Tokyo, Japan).
Antioxidant Enzyme ActivitiesThe liver was homogenated with 0.1 M PBS (pH 7.4) under standard conditions at 4°C, and centrifuged at 3000×g for 30 min and the supernatant (cytosolic and mitochondrial fractions) was collected for assessing antioxidative enzyme activity and glutathione (GSH) content. The activity of superoxide dismutase (SOD) was determined according to the method of McCord and Fridovich (1969) by the xanthine–xanthine oxidase reaction.
TBARS AssayThe liver was homogenized with 0.1 M PBS (pH 7.4) at a concentration of 10% (w/v). The homogenate was diluted to 5% (w/v) and resuspended with a hand homogenizer. The homogenate was incubated at 37°C. Four-milliliters of homogenate was taken for malondialdehyde (MDA) measurement by the thiobarbituric acid reaction. Two milliliters of trichloroacetic acid (28% (w/v) in 0.25 N HCl) was added to 4 mL of the homogenate followed by centrifugation. Then, 4 mL of supernatant was combined with 1 mL of thiobarbituric acid (1% (w/v) in 0.25 N HCl) and boiled for 15 min to allow for chromophore development. The absorbance was read at 535 nm using a spectrophotometer. The MDA content was calculated using a molar extinction coefficient of 1.56×105 M−1 cm−1.
It is evident that DEN produced a marked increase in the activities of serum fibrosis indicators such as ALT, AST, ALP, GGT, and TG in rat model (Table 1). Plasma concentrations of ALT and AST in the EALT-treated group were lower than in the negative control group throughout the experiment. Especially, the levels of ALT and AST in the 6 weeks model group markedly decreased in comparison to that in the 4 weeks rats group. The DEN plus EALT group showed a significant decrease in the enzyme levels of ALP and GGT, but the levels were still higher than those of normal control groups. At the same time, administration of EALT lowered serum TG concentration as Table 1 showed. After 6 weeks of treatment, TG level increased 2.5-fold in fibrotic rat liver induced by DEN, whereas EALT treatment attenuated this TG increase by 34.1%. When sylimarin and Hovenia extract were given as positive materials, the enzyme levels were lower than those of the DEN-treated rats, but differences were not significant in some cases.
| Treatment groups | AST (U/L) | ALT (U/L) | ALP (U/L) | GGT (U/L) | TG |
|---|---|---|---|---|---|
| (A) | |||||
| Group I | 70.0±7.8 | 68.2±7.8 | 482.3±40.2 | 0.2±0.0 | 45.7±5.2 |
| Group II | 538.1±37.4# | 537.1±22.8# | 1277.4±98.2# | 11.5±1.9# | 120.5±11.9# |
| Group III | 508.4±37.2 | 483.0±44.3 | 1326.5±87.4 | 5.6±0.6* | 119.3±15.4 |
| Group VI | 516.5±46.1 | 525.6±31.9 | 1215.4±99.5 | 6.4±0.5* | 98.5±9.6* |
| Group V | 488.5±23.9 | 495.4±45.4 | 905.0±46.5* | 3.5±0.4* | 78.5±8.8* |
| (B) | |||||
| Group I | 81.0±7.8 | 72.3±5.6 | 443.9±33.6 | 0.3±0.0 | 44.5±3.9 |
| Group II | 441.2±30.9# | 410.4±41.1# | 2080.5±120.9# | 48.5±5.4# | 111.0±12.3# |
| Group III | 444.7±41.5 | 401.5±29.9 | 2093.1±101.4 | 38.3±2.9 | 125.2±17.0 |
| Group VI | 481.6±33.7 | 442.6±50.0 | 1836.5±166.2 | 26.5±2.1* | 92.4±9.9 |
| Group V | 372.1±30.1 | 376.8±36.4 | 1777.5±98.4* | 26.1±3.3* | 88.3±7.4* |
# p<0.01. * p<0.1.
The enhancement of oxidative stress is associated with liver fibrogenesis. Reactive oxygen species (ROS) are known to cause oxidative stress, affecting both onset and progression of fibrosis in hepatic tissue.16) The liver total anti-oxidant capacity of EALT was determined by measuring the levels of hepatic MDA and superoxide dismutase (SOD). The content of MDA was significantly increased in DEN-induced liver fibrotic rats, while the level of SOD was decreased (Fig. 1). EALT (200 mg/kg b.w. per day) attenuated the increase of MDA by 88.2% of control. Conversely, the level of SOD was increased in the livers of rats receiving EALT as compared to non-treated fibrosis group. Positives including sylimarin and HDE (200 mg/kg b.w. per day) significantly inhibited MDA formation and recovered SOD activity in DEN-induced rat liver.

Values are the mean±S.D. of 10 rats. # p<0.01 compared to Group I; * p<0.1 compared to Group II; ** p<0.01 compared to Group II.
After treated with DEN in period of 4 and 6 weeks, the body weight rats were decreased as compared to that of normal control group. As shown in Table 2, rats liver weight of DEN treated group were also markedly decreased by 20.7 and 21.3% of control, respectively, at the time points of 4 and 6 weeks. The relative liver weight, represented as body weight/liver weight ratio, was elevated in EALT and sylimarin-treated rats after treatment of 6 weeks. Despite comparable final body weight, the liver of the animals in the sylimarin group was heavier than that of DEN-treated group, hence the relative liver weight was markedly increased in rats receiving sylimarin. Both body and liver weights were greatly increased in EALT administered group upto 63.6 and 19.4%, respectively, compared to that of negative control.
| Treatment groups | Parameters | ||
|---|---|---|---|
| Body weight (g) | Liver weight (g) | Liver weight/Body weight ratio (%) | |
| (A) | |||
| Group I | 398.3±17.4 | 15.16±2.4 | 3.0 |
| Group II | 362.5±23.4 | 12.01±1.0 | 3.3 |
| Group III | 441.0±35.1 | 12.68±1.6 | 2.8 |
| Group VI | 414.7±25.0 | 12.31±1.8 | 2.9 |
| Group V | 388.5±30.0 | 11.86±1.3 | 3.0 |
| (B) | |||
| Group I | 399.3±33.6 | 15.20±1.9 | 3.8 |
| Group II | 377.5±29.8 | 11.96±0.8 | 3.1 |
| Group III | 379.6±35.4 | 14.56±1.5 | 3.8 |
| Group VI | 397.5±30.9 | 12.11±1.0 | 3.0 |
| Group V | 392.0±27.7 | 12.59±1.1 | 3.2 |
| (C) | |||
| Group I | 398.1±33.6 | 15.16±1.5 | 3.8 |
| Group II | 341.7±21.8 | 12.05±0.9 | 3.5 |
| Group III | 347.5±37.4 | 13.03±1.4 | 3.7 |
| Group VI | 386.0±32.9 | 14.22±1.1 | 3.6 |
| Group V | 395.0±25.9 | 15.90±1.0 | 4.0 |
Six weeks after DEN injection, histopathological examination with H&E staining slides revealed that livers of the sample treatment Groups III, IV, and V showed relatively minor necrosis and mononuclear cell infiltration than those of non-treated Group II (Fig. 2A). For statistical analysis, infiltrated mononuclear cells were counted in 10 fields of each slide at ×400 magnification (Fig. 3). Specifically, administration of silymarin and Limonium extract significantly reduced mononuclear cell infiltration in the livers. Hovenia extract treatment also alleviated those cells accumulation, though statistically insignificant (p=0.066).
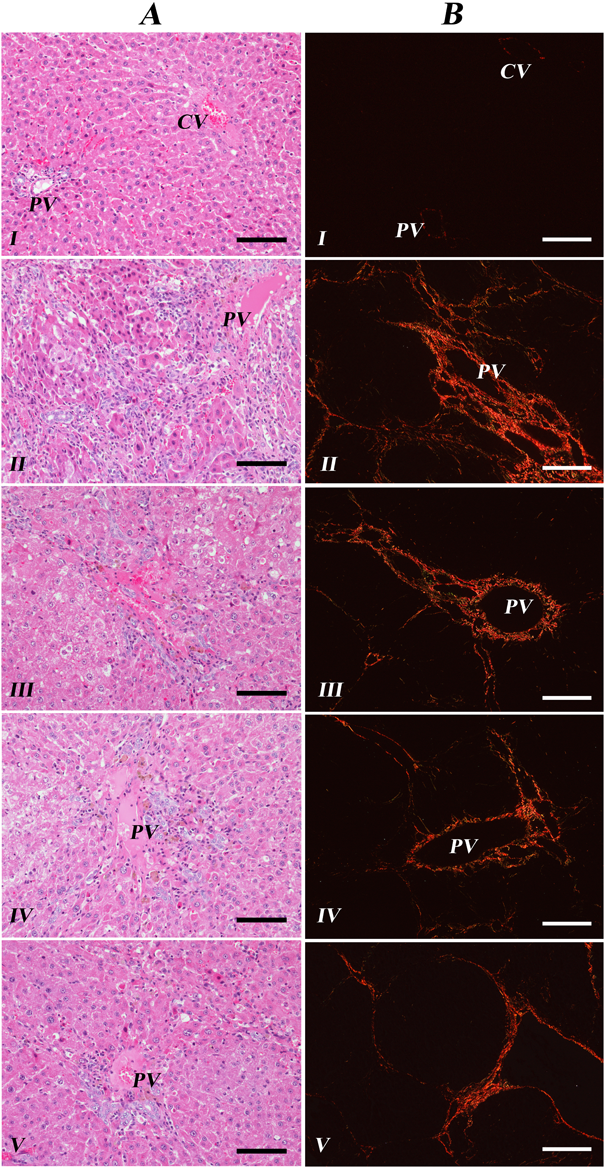
H&E staining of 6 weeks after DEN injection (A); sample treated Groups III, IV, and V were decreased mononuclear cell infiltration than non-treated Group II. Picrosirius red staining under polarized light of 8 weeks after DEN injection (B); sample treated Groups III, IV, and V showed less fibrotic lesions than non-treated group II. Bar=200 µm. CV: central vein. PV: portal vein.

The results were expressed as the mean±S.D. for 10 fields of each slide. # p<0.01 compared to Group I; * p<0.001 compared to Group II.
As for the protective effect of silymarin, Hovenia, and Limonium extract from the liver fibrosis (Groups III, IV, V), all of them could ameliorate DEN-induced injuries (Group II) during 8 weeks. Fibrotic tissues were stained picrosirius red solution and visualized with polarizing microscopy (Fig. 2B) and areas of fibrosis in 10 fields of each slide at ×100 magnification were measured (Fig. 3). Among them, administration of Limonium and Hovenia extract showed significant protective effects on the liver fibrosis. The livers of silymarin treated group were diminished in fibrotic area on average, but not significant.
Chronic liver diseases represent a major public health problem worldwide. Hepatic fibrosis is a classical outcome of many chronic liver diseases, ultimately leading to liver cirrhosis and hepatocellular carcinoma.17) Herbal medicines including herbs, herbal materials, and herbal preparations have become increasingly popular as an alternative therapeutic approach. Treating liver diseases with herbal drugs has a long tradition, but scientific evidence to establish the safety and efficacy of most herbal medicines is still sparse. Despite this limitation, reports are accumulating about promising effects of herbal products including silymarin attenuating liver fibrosis progression,18) glycyrrhizin for antiviral activity against chronic viral hepatitis B and C,19,20) and Phyllanthus preparations to treat hepatitis B virus infection.21) Thus, herbal and alternative medicine use is providing a new approach to the treatment of liver fibrosis.
The extract of L. tetragonum has been reported to be effective for reducing cell proliferation in the HSCs as the major fibrogenic cell type in the injured liver.10) To confirm the antifibrotic effect of EtOAc fraction of L. tetragonum methanolic extract, we examined the activities of liver associated enzymes, ALT, AST, ALP, GGT, and antioxidant enzymes SOD using DEN-induced liver fibrosis model.
In the previous study that reported hepatoprotection of L. tetragonum against acute alcohol intoxication,13) the effective concentration of the extract was selected as 200 mg/kg b.w. through dose–response experiment for both EALT and HDE. For the comparison of hepatoprotective efficacy of EALT in two different liver disease models, the same concentration of EALT and HDE (200 mg/kg b.w.) was applied in the present study. Diverse nitrosamines like DMN and DEN are well known hepatotoxins and hepatocarcinogens, inducing hepatic necrosis and subsequent fibrosis in animal species.7,8) In this study, DEN-injected rats exhibited elevated levels of serum ALT, AST, ALP, and GGT. Serum amino transferases such as AST and ALT indicate the leaked concentration of hepatic intracellular enzymes into the circulation that is a sensitive marker for hepatocellular injury. The increase in ALT and AST levels is also well described in hepatic fibrosis progression. Especially, out of several serum marker enzymes, ALT is considered to be an enzyme that is more specific for liver cell injury.22) The serum concentration of ALT in the 200 mg/kg EALT-treated group was significantly lower than that of negative DEN-treated group after 4 and 6 weeks of treatment. The administration of EALT appears to suppress liver inflammation and fibrosis in vivo. GGT presents in the cell membranes of many tissues, the most prominent being of hepatobiliary system. The increase of serum GGT level is commonly known as extremely sensitive marker to identify bile duct or liver diseases including cholestatis. The serum GGT level is reported to increase up to 10–20 times of upper limits in viral hepatitis or alcoholic liver disease, and persistence elevation of GGT may be an indicator of Cirrhosis.23,24) In this study, the most remarkable effect of EALT is the decreased concentration of GGT in serum. Treatment with EALT preserved the increased GGT induced by DEN, and the increased concentration of cholesterol was also significantly reduced by EALT.
The use of sylimarin as well as HDE in the treatment of liver diseases including chronic hepatitis and alcohol-induced liver diseases has been already reported, thus showing hepatoprotective effects in this study as positive controls.25,26) H. dulcis has a long history of usage in Korean and Chinese traditional medicine to relieve intoxication due to alcohol poisoning by stimulating alcohol metabolism and lowering alcohol free radical scavenging.27) H. dulcis contains a variety of biologically active compounds including saponin derivatives, dammarane-type triterpene saponins and flavonoids. Recently, four bioactive flavonoids, ampelopsin, taxifolin, myricetin and quercetin in the extract of H. dulcis fruits has been determined by HPLC experiment.28)
The body and liver weight were decreased after 4 weeks of weekly DEN-injections. Previous research has indicated that experimental rats have serial progression of hepatocarcinogenesis following a period of DEN administration. Animal models have been divided into three different stages including the inflammation stage (weeks 4–8), the cirrhosis stage (weeks 10–14), and the hepatocellular carcinoma stage (weeks 16–20) after treatment of DEN. The characteristic histological change of the hepatitis has been detected at time point of “week 6.”29) In our present study, both body and liver weights were significantly diminished after 6–8 weeks of injection with DEN according to the onset of liver fibrosis. The diminished ratios of liver weight to body weight of EALT-treated groups were significantly recovered corresponding that of control group in weeks 8. Histological examination of liver samples also showed that the loss of architecture, fibrosis, and fatty infiltration in the DEN-treated rats. After 8 weeks of EALT treatment, the extent of hepatic fibrosis was significantly weaker when compared to animals treated with DEN alone. These results obviously indicated that the administration of EALT has a beneficial effect against the liver injury and chemically induced liver fibrosis.
DEN is a strong hepatotoxin, carcinogen and mutagen (Haggerty and Holsapple, 1990). The toxicity by DMN is known to be mediated by its reactive metabolites, formaldehyde and methanol not by the parent compound. In the rat model, DEN-induced hepatic injury is reported to be closely related to increased generation of ROS in cytochrome P450 system, especially by CYP2E1 in the liver.31–33) Reactive metabolites transformed from DEN generate reactive electrophiles that cause oxidative stress leading to cytotoxicity and carcinogenicity. Based on these observations, parameters of antioxidation in the liver such as MDA and SOD were estimated in EALT-treated liver fibrosis rats. MDA has been identified as one of end products formed via the radical induced decomposition of polyunsaturated fatty acids.34) It has been reported that certain lipid peroxidation products such as MDA as well as free radicals stimulate collagen gene expression by initiating the activation of HSCs.35,36) Furthermore, a strong correlation between the MDA index or the lipid peroxidation index and the fibrosis score in chronic hepatitis C patients demonstrated that MDA adducts play a significant role in the pathogenesis of liver fibrosis.37) Daily administration of EALT (200 mg/kg b.w.) in the present study dramatically decreased the level of the lipid peroxidation product MDA, resulting in a potential preventive effect against DEN-induced hepatic fibrogenesis in rats. In regards to antioxidative enzymes, our results exhibited a significant increase of SOD activity in the liver of EALT-treated group compared with negative DEN-treated control group. Lipid peroxidation is usually caused by free radicals and chemical scavengers of oxygen radicals can reduce oxidative stress-induced lipid peroxidation and tissue damage.38) In fact, oxidative stress is often associated with fibrogenesis occurring in various organs like liver, lung, arteries, and nervous system.36,39) Therefore, induction of free radical scavenging enzyme activity by receiving EALT might prevent the cell damage from oxidative stress and benefit for reducing hepatic fibrosis progression.
Through phytochemical study using HPLC, it was identified that myricitrin and isomyricitrin were contained as constituents of EALT (Supplementary Materials). Myricitrin (Myricetin-3-O-α-rhamnoside) is a naturally occurring flavonoid glycoside that has been shown to possess a strong antioxidant activity, with stronger free radical scavenging activity than other flavonol rhamnosides or quercetin.40) Also, myricitrin exhibited a significant hepatoprotective and antifibrotic activities in carbon tetrachloride-intoxicated mice demonstrating that myricitrin provided better hepatoprotection than silymarin, a positive control.41) Myricitrin contained in EALT might contribute to hepatoprotection of L. tetragonum.
In brief, L. tetragonum EtOAc fraction showed potent antioxidant activity and prevented the development of DEN-induced liver fibrosis in the animal model in the present study. This finding was consistent with our previous research revealing attenuation of HSCs proliferation and collagen deposition after treatment of L. tetragonum extract in vitro. Assessment of liver functions revealed that hepatic enzyme activities of ALT, AST, ALP, and GGT were elevated by treatment of EALT, which could contribute to promote liver fibrogenesis. Diethylnitrosamine-induced oxidative stress was strikingly attenuated with EALT supplementation by down-regulation of MDA and up-regulation of SOD.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A1008069) and partially supported by Gyeongnam National University of Science and Technology Grant (2015).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.