2016 年 39 巻 6 号 p. 898-902
2016 年 39 巻 6 号 p. 898-902
The gas phase of cigarette smoke is important from the viewpoint of human health, because it can pass through alveolar epithelium and enter the circulation. There is no standard method for the preparation of a gas phase extract of cigarette smoke (CSE), although CSE is widely used for research instead of whole cigarette smoke. We have established a standard method for the preparation of CSE. One cigarette per trial is continuously combusted under a reduced pressure generated by an aspiration pump with a velocity of 1.050 L/min: the main stream of the smoke is passed through a Cambridge filter to remove tar, and subsequently, bubbled through a glass ball filter (pore size, 20–30 µm) into 15 mL of phosphate-buffered saline (PBS). To express the concentration of CSE, a virtual tar concentration is introduced, which is calculated assuming that tar trapped on the Cambridge filter is dissolved in the PBS. CSEs prepared from smaller numbers of cigarettes (original virtual tar concentration≤15 mg/mL) show similar concentration–response curves for cytotoxicity versus virtual tar concentrations. CSEs prepared from various brands of cigarettes and by different smoking regimes (continuous and puff smoking) show similar cytotoxic potency if the virtual tar concentrations are the same. In conclusion, using the standardized method for CSE preparation in combination with the virtual tar concentration, it becomes possible to simply and rapidly prepare standard CSEs with defined concentrations from any brand of cigarettes, which are toxicologically equivalent to CSE prepared by puff smoking.
The mainstream of cigarette smoke consists of a tar (particle) phase containing nicotine and a remaining gas phase.1,2) In view of human health, the gas phase is more important, because it can pass through the lung alveolar epithelium and induce injury in tissues remote from the lung.3,4) The gas phase is comprised of 400–500 chemical compounds.5) The stable cytotoxic compounds in the gas phase of cigarette smoke induce various cytotoxic effects in various types of cells.6–8) Recently, we have reported that the gas phase extract of cigarette smoke (nicotine- and tar-free cigarette smoke extract; CSE) induces cell death and cell membrane injury through reactive oxygen species generation mediated by protein kinase C (PKC) and reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX).9–11) The gas phase extract can also oxidize low density lipoprotein in vitro and promote atherosclerosis in the aorta in vivo.3,12,13) Importantly, we have identified acrolein (ACR), methyl vinyl ketone (MVK), and 2-cyclopentene-1-one (CPO) as the major cytotoxic components in the CSE using LC/MS and GC/MS in combination with functional assay.14) Furthermore, we have demonstrated that ACR and MVK-induced cell damage is PKC- and NOX-dependent like that by CSE, although CPO-induced cell damage is independent of PKC and NOX.14)
In general, a Cambridge filter is used for preparation of the gas phase of cigarette smoke.15–17) The Cambridge filter is a glass fiber filter and can retain 99.9% of the particulate matters larger than 0.1 µm.1) The fraction trapped on the Cambridge filter is defined as the tar phase, while the fraction which passes through the filter is the gas phase. The CSE is prepared by bubbling the gas phase in an aqueous solution such as phosphate buffered saline (PBS) and culture medium. However, because no standard methods for the preparation of CSE have been established in spite of the importance for human health, researchers prepared CSE according to their own methods. One of the main differences in preparation methods consists in the way of combustion of cigarettes (called smoking regime): puff smoking vs. continuous smoking. Another difference is cigarette brand. Finally, the most critical difference is the concentration of CSE preparation, owing to the absence of a clear definition for the concentration of CSE. Therefore, comparison of experimental results published from various laboratories has been impossible.
In this review, we will briefly review smoking regimes (puff smoking vs. continuous smoking) and the expression of concentrations of CSE. After that, we will introduce a newly established standardized method for the preparation of a gas phase extract of cigarette smoke.
For research and development purposes, cigarettes are smoked mechanically using specialized machines. There are two methods of combustion of cigarettes: puff smoking vs. continuous smoking. In “puff smoking” which superficially simulates the act of smoking by humans, cigarettes are actively and periodically smoked through aspiration by smoking machines for a short duration called “puff,” while they are left to combust spontaneously without aspiration for the remaining time. In so-called “continuous smoking” which is frequently used for the efficient collection of smoke, cigarettes are actively and continuously smoked by smoking machines.
Regarding puff smoking, there are several smoking regimes which define puff volume, puff frequency, puff duration, and ventilation blocks (Table 1). Among these, the smoking regime defined by the International Organization for Standardization (ISO) has become the standard regime.18–21) Notably, in many countries, the yield of tar and nicotine of cigarettes combusted according to ISO smoking regime is printed on cigarette packages.20,22,23) In the ISO smoking regime, smoking conditions are strictly defined: puff duration, 2.00±0.02 s; puff volume, 35.0±0.3 mL; puff interval, 60±0.5 s; puff profile, bell-shaped with a maximum between 0.8 s and 1.2 s from the start of the puff.24) To prepare smoke according to this regime requires specialized smoking machines which are usually too expensive for many researchers.
| Puff | Ventilation | |||
|---|---|---|---|---|
| Volume (mL) | Duration (s) | Interval (s) | Block (%) | |
| ISO | 35 | 2 | 60 | 0 |
| FTC | 35 | 2 | 60 | 0 |
| MDPH | 45 | 2 | 30 | 50 |
| HCI | 55 | 2 | 30 | 100 |
ISO: International Organization for Standardization; FTC: US Federal Trade Commission; MDPH: Massachusetts Department of Public Health; HCI: Canadian Health Ministry. Cigarette filter ventilation consists of several holes of filter paper to dilute mainstream of cigarette smoke. The smoke yield of mainstream is increased by the block of ventilation. Modified from ref. 20.
In contrast, in so-called “continuous smoking,” there is no definite smoking regime, hence researchers arbitrarily combust cigarettes according to their own smoking regimes at various aspiration rates. This method is used by many researchers, because it does not require specialized smoking machines.
Regarding the chemical composition of the two types of smoke prepared according to either the puff or continuous smoking regime, some researchers suspect the smoke may be different, since different combustion temperatures resulting from differences in the speed and/or profile of combustion25,26) could generate different combustion products. However, there has been no clear evidence suggesting the presence of such a difference, thus, at present, it is unknown whether the chemical composition of the two types of smoke is similar to each other or not.
In the case of whole cigarette smoke extract and the tar phase extract, concentrations have been expressed in terms of the concentrations of endogenous substances such as nicotine27–29): the amount of these substances is relatively easy to determine using gas chromatography or the weight trapped on the Cambridge filter, respectively. However, in the case of nicotine- and tar-free CSE, it is difficult to express its concentration, because it does not contain appropriate endogenous substances like nicotine and tar. In this context, most researchers represent the concentration by the number of cigarettes used for CSE preparation16,30–32) or the number of puffs.33–36) Other researchers represent the concentration in terms of the optical density at 302 nm (OD).4) However, these values do not necessarily represent actual concentrations of CSE, because they do not reflect parameters such as the efficiency of extraction of chemical compounds in the gas phase into the aqueous phase and saturation of aqueous phase with chemical compounds. Therefore, it is an urgent matter to develop a reliable expression system for the concentration of gas phase extract.
We have employed continuous smoking for combusting cigarettes to establish a method which is simple and rapid. The schematic diagram of an apparatus for CSE preparation by continuous smoking is shown in Fig. 1. One cigarette per trial is continuously combusted by reduced pressure generated using an aspiration pump, with the flow rate set at 1.050 L/min. The main stream of the cigarette smoke is passed through a Cambridge filter to remove the tar phase and nicotine, and subsequently, bubbled into 15 mL of PBS in a 100-mL graduated cylinder kept at 25°C. For increasing the bubbling efficiency, a glass ball filter with a pore size of 20–30 µm is used. The CSE is aliquoted and stored at −80°C until use. The CSE prepared by continuous smoking regime is designated as cCSE.

Detailed methods for cCSE preparation are in the text. The Cambridge filters were purchased from Heinr Borgwaldt GmbH (Hamburg, Germany). Modified from ref. 10.
The flow rate is based on ISO3308 in which the volume and duration of a puff should be 35 mL and 2 s, i.e. 1.050 L/min. According to ISO4387, cigarettes with and without filters are combusted up to 3 mm from the tipping paper or 23 mm from the end of the cigarette, respectively.
To express the concentration of CSE, we have introduced a virtual tar concentration, which is calculated on the assumption that the tar phase (dry weight) trapped on the Cambridge filter is dissolved in PBS used for CSE preparation.10) Because the tar phase contains water,37) the Cambridge filter is air-dried at 25°C for 12 h in order to vaporize the water before measuring the dry weight of tar. Drying at higher temperatures should be avoided because chemical compounds with low vaporizing temperatures may vaporize.
The relationship between cCSE cytotoxicity and the numbers of cigarette used for cCSE is examined.10) The concentration–response curves for the inhibition of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) reduction activity are comparable among cCSEs prepared from 2–6 Hi-Lite brand cigarettes (equivalent to the original virtual tar concentrations of 5–15 mg/mL; as described later, cigarette brands do not affect the cytotoxic activities of CSEs, as long as the virtual tar concentrations of the CSEs are similar). However, concentration–response curves shift to the right, when more than 8 Hi-Lite brand cigarettes (equivalent to the original virtual tar concentrations ≥20 mg/mL) are used for cCSE preparation (Fig. 2). The rightward shift of the curves means that the cytotoxic activities of cCSEs prepared from more than 8 Hi-Lite cigarettes are lower than expected at a given tar concentration. These results suggest that with the virtual tar concentrations of up to 15 mg/mL, the cytotoxic activity of cigarette smoke gas phase is extracted into PBS at a constant efficiency, but when concentrations exceed 20 mg/mL, some part of the cytotoxic activity leaks without extraction. In fact, significant leakage of cytotoxic activity is detected in the second reservoir incorporated downstream of the first reservoir in the smoking apparatus, at a high virtual tar concentration (35 mg/mL) but not a low concentration (10 mg/mL).10) Therefore, cCSE should be prepared at the original tar concentrations of ≤15 mg/mL.

The CSEs were prepared from different numbers (2–40) of Hi-Lite brand cigarettes by the continuous smoking regime. Rat C6 glioma cells were treated with various concentrations of CSE for 4 h. The cytotoxicity of CSEs was assessed by MTS reduction assay. Modified from ref. 10.
Many researchers use commercially available cigarette brands for their research, although the Center for Tobacco Reference Products, University of Kentucky (U.S.A.) provides reference cigarettes (Kentucky reference cigarettes) for research use. It is possible that cigarette brands affect the cytotoxicity of cCSE, because different brands of cigarettes, which are made from different tobacco leaves and contain different additives, can generate smoke with varying chemical composition. To clarify this point, we have examined the cytotoxic activities of cCSEs prepared from 8 types of representative cigarette brands (5 brands from JT, Japan; 3 brands from other countries) with nominally different tar yields.10) Notably, the concentration–response curves of the cCSEs prepared from all 8 brands of cigarettes are comparable, when the corresponding cCSEs at the original tar concentration of 10 mg/mL are used (Fig. 3). These results demonstrate that the cytotoxic activities of cCSEs depend on the virtual tar concentration but not on the brand or nominal tar yield of cigarettes: hence, the virtual tar concentration can be used as a universal unit of cCSE concentration.
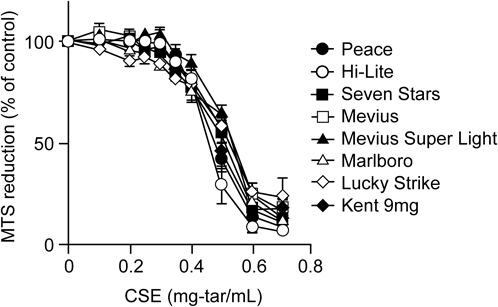
CSEs at the original virtual tar concentration of 10 mg/mL were prepared from various brands of cigarettes by the continuous smoking regime. The cytotoxicity of cCSEs against rat C6 glioma cells was assessed by MTS reduction activity assay. Cigarette used in this study: Peace (JT, Japan; 28 mg tar, 2.3 mg nicotine), Hi-Lite (JT, Japan; 17 mg tar, 1.4 mg nicotine), Seven Stars (JT, Japan; 14 mg tar, 1.2 mg nicotine), Mevius (JT, Japan; 10 mg tar, 0.8 mg nicotine), Mevius Super Light (JT, Japan; 6 mg tar, 0.5 mg nicotine), Marlboro (Phillip Morris, U.S.A; 12 mg tar, 1.0 mg nicotine), Lucky Strike (British American Tobacco, U.K.; 11 mg tar, 0.9 mg nicotine), Kent 9 mg (British American Tobacco, U.K.; 9 mg tar, 0.8 mg nicotine). The amounts of tar and nicotine described above were defined by the ISO smoking regime. Modified from ref. 10.
Finally, the properties of cCSE are compared with those of CSE prepared by the puff smoking regime (pCSE) in terms of cytotoxic activities and chemical constituents. The concentration–response curve for the inhibition of MTS reduction activity of cCSE is comparable to that of pCSE (Fig. 4), and the cytotoxic activities of both CSEs are PKC- and NOX-dependent.10) Furthermore, the concentrations of the major cytotoxic compounds such as ACR and MVK are also comparable in both CSEs (Table 2). These results show that cCSE is identical to pCSE from the viewpoint of toxicology and pharmacology.

The CSEs were prepared from Hi-Lite brand cigarettes by either the continuous smoking regime (cCSE) or ISO smoking regime (pCSE). The cCSE was prepared by a method described in the text. The pCSE was prepared using a mechanical smoking machine (RM200, Heinr Borgwaldt GmbH) according to the ISO3308 smoking regime. The original virtual tar concentration of the CSEs were 10 mg/mL. The cytotoxicity of CSEs against rat C6 glioma cells were assessed by MTS reduction activity assay. Modified from ref. 10.
| Carbonyl compounds | cCSE | pCSE |
|---|---|---|
| Acetone (µM) | 287.9±29.2 | 326.1±20.3 |
| Acrolein (µM) | 36.7±1.3 | 41.7±2.8 |
| Propionaldehyde (µM) | 24.4±3.5 | 28.7±6.9 |
| Methyl vinyl ketone (µM) | 17.5±3.0 | 13.8±0.5 |
The concentrations of carbonyl compounds in 10 mg/mL cCSE and pCSE were analyzed by GC/MS. Values represent the mean±S.D. of three experiments. Modified from ref. 10.
Understanding the toxicological properties of CSE is important for research on smoking science, since the gas phase of cigarette smoke is mainly responsible for various disorders in organs remote from the lung. As the first step toward the development of smoking science, we have established a standard method for practical CSE preparation and also introduced a new representation method for CSE concentration, i.e. the virtual tar concentration. Using these methods, we have shown that the virtual tar concentration can be used as a reference value to normalize the cytotoxic activities of cCSE, irrespective of the number of combusted cigarettes, cigarette brands or smoking protocols (continuous smoking vs. puff smoking), as long as the tar concentrations in the original cCSEs are ≤15 mg/mL of PBS. The standardized method for CSE preparation will contribute to the development of smoking science.
This work was supported by a Grant from Smoking Research Foundation to S.M., by a Grant-in-Aid for Young Scientists (B) (26860166) from the Japan Society for the Promotion of Science to T. Higashi, by a Grant from Akiyama Life Science Foundation to T. Higashi, and by a Grant from NISHINOMIYA Basic Research Fund (Japan) to T. Higashi.
The authors declare no conflict of interest.