2017 年 40 巻 1 号 p. 43-48
2017 年 40 巻 1 号 p. 43-48
The Ephedra herb, which has been used in Kampo medicines, originates from terrestrial stems of Ephedra species. It is important to establish cultivation methods and cultivars to secure a stable supply of the Ephedra herb that would meet the quality standards for the ephedrine alkaloids content. In this study, we first grew Ephedra sinica plants derived from seeds in the field for 5 years. Then, for selective breeding of cultivars that could meet the quality standards for the ephedrine alkaloids content, we measured the content of total alkaloids (TAs), ephedrine (Eph), and pseudoephedrine (PEph) in individual plants derived from seedlings and grown for 4 years in the field. The range of the TA content in each individual plant was narrower than that among individual plants grown in the field. Therefore, individual plants were selected according to their TA content, Eph/PEph ratio, and stolon-formation capability. The selected individuals were propagated using stolons, and their TA content was studied for 2 years. In the second year, the TA content in terrestrial stems derived from stolons of the selected individuals was as high as that of their parents. Therefore, it was confirmed that the selected individuals that were propagated using stolons could produce TA reproducibly. This study suggested that selective breeding using stolon propagation is effective for stabilizing Ephedra herb TA content.
The Ephedra herb has been used in traditional Japanese medicines and Kampo medicines. The Japanese Pharmacopeia describes the Ephedra herb as terrestrial stems of Ephedra sinica STAPF, E. intermedia SCHRENK et C. A. MEYER, or E. equisetina BUNGE (Ephedraceae). When stems are dried, they must contain ≥0.7% of total alkaloids (TAs), ephedrine (Eph), and pseudoephedrine (PEph).1) In Japan, the Ephedra herb has been frequently included in Kampo prescriptions, such as Kakkonto, Maoto, and Makyokansekito. However, for obtaining the Ephedra herb, Japan relies on China, which has had export restrictions for the prevention of desertification since 1999.2) Thus, it is necessary to sustainably cultivate Ephedra plants in Japan.
Recent studies on ephedrine alkaloids in Ephedraceae have shown that the range of the TA content is wide in each species.3) The Eph content is higher than that of PEph in E. sinica,4) and the ephedrine alkaloid composition ratio of the Ephedra herb depends on genetic factors.5) However, other studies have shown that the content of ephedrine alkaloids is affected by environmental factors.6,7) Even though numerous studies have been conducted on Ephedra plants, there remain questions about ephedrine alkaloids in cultivated Ephedra plants and about cultivation methods for their stabilization. For example, it is not clear how the content of ephedrine alkaloids varies from year to year in individual plants growing in the field, how long cultivated plants maintain their ephedrine alkaloid content in the field, which methods of cultivation meet the quality standards for the alkaloid content, and how to establish an easier propagation method.
Thus, selective breeding of Ephedra plants that would satisfy the quality standards for the alkaloid content in the field under the same environmental conditions seems like an efficient strategy. Hence, it is important to research ephedrine alkaloids in cultivated individuals over years in the field and to establish cultivation methods and cultivars so that a stable supply of the Ephedra herb meeting the quality standards for the alkaloid content is secured. Vegetative propagation of E. sinica is performed using stolons, and this is one of effective methods to propagate selected lines.
In this study, we first measured, over a period of 4 years, the TA content in individual E. sinica plants derived from seeds and grown in the field. Individual plants were selected for generating selected lines according to the TA content and Eph/PEph ratio. Then, we propagated the selected individuals using stolon propagation. Finally, we confirmed the reproducibility of the TA content in the selected lines.
In this study, we used seeds of E. sinica, which were derived from cultivation field in Mongolia. Plants grown from these seeds were identified based on morphological characteristics.8) Voucher specimens (THS 94868, THS 94870 and THS 94875) were deposited in the Herbarium of TSUMURA Botanical Raw Materials Research Laboratories, Tsumura & Co., Ibaraki, Japan (THS). E. sinica plants were cultivated in the field in Ibaraki prefecture, Japan.
Cultivation of SeedlingsE. sinica plants were raised from seeds and grown in the field for a year, from June 22, 2011 to May 11, 2012. Seeds were directly sown with 1.0-m row spacing in the field. As a basal fertilizer, 2000 g·m−2 of farmyard manure was used. A chemical fertilizer was applied in July to supply 2 g·m−2 of N, 2 g·m−2 of P2O5, 2 g·m−2 of K2O, and 0.2 g·m−2 of Mg.
Selection and Cultivation of Individual PlantsFifty of 30000 seedlings were selected uniformly over the entire field, transplanted with 1.0-m row spacing and 0.3-m spacing between plants in each row, and have been cultivated since May 11, 2012. As a basal fertilizer, 2000 g·m−2 of farmyard manure was added. A chemical fertilizer was applied in April, May, and June of each year to supply 2 g·m−2 of N, 2 g·m−2 of P2O5, 2 g·m−2 of K2O, and 0.2 g·m−2 of Mg.
Sampling of Individuals Derived from Seedlings to Measure TA ContentTerrestrial stems of individual plants were cut at a height of about 5 cm from the ground. The sampling dates in each year were as follows: (1) October 11, 2012 (2-year-old), (2) August 21, 2013 (3-year-old), (3) September 19, 2014 (4-year-old), and (4) October 27, 2015 (5-year-old).
ProcessingThese samples were dried at 30°C for 3 d and at 50°C for 4 h in a drying oven.
Determination of AlkaloidsThe quantitative analysis of the ephedrine alkaloids (Eph and PEph) was performed as described below.
Ephedra herb samples were powdered using a rod mill. The powder of an Ephedra herb sample was accurately weighted to obtain about 0.25 g, then 20 mL of diluted MeOH (1 in 2) was added, and the mixture was shaken for 30 min on a shaker (Taitec Recipro Shaker, model SR-1) and centrifuged at 3000 rpm for 10 min. The supernatant was taken into a new tube. This process was repeated twice, then the extracts were combined, and diluted MeOH (1 in 2) was added to 50 mL. The supernatant was filtered using a 0.45-µm membrane filter (Advantec 13HP045AN) and used as a sample solution. Samples were analyzed using HPLC system (LC-10ADvp, Shimadzu, Kyoto, Japan). As a mobile phase, H2O–CH3CN–H3PO4 (650 : 350 : 1) with 0.5% sodium lauryl sulfate was used. The HPLC conditions were as follows: the column, Inertsil ODS-3 (4.6 mm×150 mm i.d.; GL Sciences, Inc., Tokyo, Japan); the column temperature, 40°C; the flow rate, 1.1 mL/min.
Selection of IndividualsThe criteria for the selection of individual plants were as follows: (1) the TA content of ≥1.0% after 3 years of cultivation; (2) the Eph/PEph ratio of >1 after 3 years of cultivation; (3) the difference between the maximum and minimum values of the TA content of <0.3 after 3 years of cultivation; and (4) individuals showed stolon-formation capability.
Cultivation of Individuals Derived from StolonsThe stolon is shown in Fig. 1, and the process of stolon propagation is shown in Fig. 2. Stolons were collected on June 19, 2014 from three individuals as follows: nine stolons from #2, 14 stolons from #19, and nine stolons from #26. The collected stolons were transplanted with 1.0-m row spacing and 0.3-m spacing between plants in each row for the propagation of the breeding material in the field.

The white bar corresponds to 10 cm. The double-headed arrows indicate parts of the stolon.
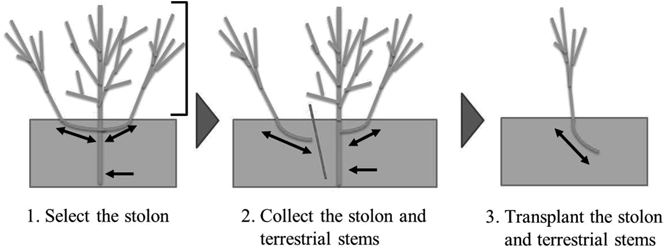
The double-headed arrows indicate stolons, and the single-headed arrow indicates the root. The right square bracket indicates terrestrial stems.
The number of survived stolons was counted on November 14, 2014 and on August 4, 2015. The survival rate was defined as follows: Survival rate (%)=[(number of survivors)/(number of stolons planted)]×100.
Sampling of Individuals Derived from Stolons for Measuring TA ContentThe sampling dates in each year for the plants derived from stolons were as follows: (1) November 14, 2014 (1-year-old) and (2) August 4, 2015 (2-year-old).
Statistical AnalysisThe coefficient of variation (CV) was calculated using Microsoft Excel 2010 as follows: CV=standard deviation (S.D.)/average.
We measured the TA content of the 50 individuals over 4 years, from 2012 to 2015. The data are shown in Table 1.
| Individuals | 2-Year-old (2012) | 3-Year-old (2013) | 4-Year-old (2014) | 5-Year-old (2015) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eph (%) | PEph (%) | TA (%) | Eph/PEph | Eph (%) | PEph (%) | TA (%) | Eph/PEph | Eph (%) | PEph (%) | TA (%) | Eph/PEph | Eph (%) | PEph (%) | TA (%) | Eph/PEph | |
| #1 | 0.13 | 0.42 | 0.55 | 0.30 | 0.10 | 0.30 | 0.40 | 0.34 | 0.09 | 0.33 | 0.42 | 0.26 | 0.08 | 0.26 | 0.34 | 0.29 |
| #2 | 0.94 | 0.02 | 0.96 | 47.6 | 1.07 | 0.02 | 1.09 | 50.0 | 1.03 | 0.02 | 1.05 | 50.9 | 1.03 | 0.00 | 1.03 | * |
| #3 | 0.63 | 0.45 | 1.08 | 1.39 | 0.51 | 0.42 | 0.93 | 1.22 | 0.55 | 0.53 | 1.08 | 1.04 | 0.37 | 0.31 | 0.68 | 1.18 |
| #4 | 0.61 | 0.01 | 0.62 | 45.0 | 0.48 | 0.02 | 0.50 | 26.2 | 0.41 | 0.01 | 0.42 | 35.7 | 0.25 | 0.00 | 0.25 | * |
| #5 | 0.71 | 0.21 | 0.92 | 3.38 | 0.58 | 0.17 | 0.75 | 3.38 | 0.60 | 0.19 | 0.79 | 3.15 | 0.59 | 0.19 | 0.78 | 3.10 |
| #6 | 0.23 | 0.38 | 0.61 | 0.61 | 0.21 | 0.39 | 0.60 | 0.55 | 0.20 | 0.41 | 0.61 | 0.49 | 0.17 | 0.34 | 0.51 | 0.50 |
| #7 | 0.30 | 0.43 | 0.73 | 0.70 | 0.31 | 0.44 | 0.75 | 0.71 | 0.25 | 0.41 | 0.66 | 0.60 | — | — | — | — |
| #8 | 0.26 | 0.11 | 0.37 | 2.41 | 0.48 | 0.18 | 0.66 | 2.67 | 0.47 | 0.19 | 0.66 | 2.44 | 0.41 | 0.17 | 0.58 | 2.39 |
| #9 | 0.51 | 0.54 | 1.05 | 0.94 | 0.58 | 0.68 | 1.26 | 0.86 | 0.64 | 0.73 | 1.37 | 0.87 | 0.52 | 0.64 | 1.16 | 0.81 |
| #10 | 0.41 | 0.31 | 0.72 | 1.32 | 0.37 | 0.29 | 0.66 | 1.27 | 0.35 | 0.29 | 0.64 | 1.20 | 0.23 | 0.18 | 0.41 | 1.26 |
| #11 | 0.42 | 0.32 | 0.74 | 1.32 | 0.27 | 0.27 | 0.54 | 1.00 | 0.19 | 0.17 | 0.36 | 1.11 | 0.25 | 0.20 | 0.45 | 1.20 |
| #12 | 0.19 | 0.35 | 0.54 | 0.56 | 0.22 | 0.38 | 0.60 | 0.57 | 0.20 | 0.37 | 0.57 | 0.55 | 0.20 | 0.41 | 0.61 | 0.49 |
| #13 | 0.32 | 0.31 | 0.63 | 1.01 | 0.36 | 0.40 | 0.76 | 0.90 | 0.34 | 0.40 | 0.74 | 0.84 | 0.22 | 0.26 | 0.48 | 0.86 |
| #14 | 0.40 | 0.03 | 0.43 | 15.4 | 0.61 | 0.06 | 0.67 | 10.5 | 0.70 | 0.05 | 0.75 | 13.1 | 0.62 | 0.04 | 0.66 | 15.2 |
| #15 | 0.74 | 0.12 | 0.86 | 6.31 | 0.58 | 0.00 | 0.58 | * | 0.65 | 0.03 | 0.68 | 20.3 | 0.47 | 0.00 | 0.47 | * |
| #16 | 0.30 | 0.33 | 0.63 | 0.92 | 0.35 | 0.35 | 0.70 | 1.01 | 0.28 | 0.30 | 0.58 | 0.93 | 0.30 | 0.32 | 0.62 | 0.93 |
| #17 | 0.16 | 0.24 | 0.40 | 0.69 | 0.22 | 0.30 | 0.52 | 0.73 | 0.21 | 0.32 | 0.53 | 0.65 | 0.19 | 0.31 | 0.50 | 0.63 |
| #18 | 0.69 | 0.12 | 0.81 | 5.97 | 0.90 | 0.19 | 1.09 | 4.73 | 0.73 | 0.17 | 0.90 | 4.34 | 0.80 | 0.18 | 0.98 | 4.40 |
| #19 | 1.04 | 0.14 | 1.18 | 7.50 | 1.36 | 0.20 | 1.56 | 6.94 | 1.26 | 0.19 | 1.45 | 6.81 | 1.30 | 0.22 | 1.52 | 5.90 |
| #20 | 0.14 | 0.22 | 0.36 | 0.65 | 0.20 | 0.45 | 0.65 | 0.46 | 0.18 | 0.45 | 0.63 | 0.40 | 0.15 | 0.37 | 0.52 | 0.41 |
| #21 | 0.83 | 0.01 | 0.84 | 58.5 | 0.81 | 0.02 | 0.83 | 43.7 | 0.70 | 0.01 | 0.71 | 52.8 | 0.65 | 0.04 | 0.69 | 17.6 |
| #22 | 0.25 | 0.26 | 0.51 | 0.98 | 0.20 | 0.27 | 0.47 | 0.74 | 0.17 | 0.26 | 0.43 | 0.65 | 0.10 | 0.23 | 0.33 | 0.45 |
| #23 | 0.07 | 0.31 | 0.38 | 0.24 | 0.11 | 0.55 | 0.66 | 0.20 | 0.10 | 0.51 | 0.61 | 0.19 | 0.11 | 0.59 | 0.70 | 0.19 |
| #24 | 0.54 | 0.09 | 0.63 | 6.26 | 0.60 | 0.16 | 0.76 | 3.65 | 0.57 | 0.16 | 0.73 | 3.47 | 0.54 | 0.21 | 0.75 | 2.64 |
| #25 | 0.52 | 0.09 | 0.61 | 5.99 | 0.53 | 0.17 | 0.70 | 3.05 | 0.47 | 0.18 | 0.65 | 2.61 | 0.64 | 0.27 | 0.91 | 2.38 |
| #26 | 0.46 | 0.13 | 0.59 | 3.71 | 0.96 | 0.44 | 1.40 | 2.21 | 0.81 | 0.42 | 1.23 | 1.94 | 0.79 | 0.39 | 1.18 | 2.03 |
| #27 | 0.13 | 0.01 | 0.14 | 20.7 | 0.25 | 0.01 | 0.26 | 22.8 | 0.20 | 0.02 | 0.22 | 10.2 | 0.16 | 0.00 | 0.16 | * |
| #28 | 0.14 | 0.04 | 0.18 | 3.98 | 0.82 | 0.51 | 1.33 | 1.62 | 0.61 | 0.39 | 1.00 | 1.57 | 0.70 | 0.51 | 1.21 | 1.38 |
| #29 | 0.36 | 0.36 | 0.72 | 1.00 | 0.34 | 0.42 | 0.76 | 0.81 | 0.38 | 0.46 | 0.84 | 0.83 | 0.38 | 0.50 | 0.88 | 0.75 |
| #30 | 0.37 | 0.24 | 0.61 | 1.53 | 0.39 | 0.41 | 0.80 | 0.95 | 0.35 | 0.41 | 0.76 | 0.84 | 0.41 | 0.46 | 0.87 | 0.89 |
| #31 | 0.41 | 0.02 | 0.43 | 21.2 | 0.84 | 0.05 | 0.89 | 16.7 | 0.79 | 0.05 | 0.84 | 17.2 | 0.83 | 0.05 | 0.88 | 18.1 |
| #32 | 0.32 | 0.05 | 0.37 | 6.05 | 0.48 | 0.07 | 0.55 | 6.50 | 0.40 | 0.06 | 0.46 | 6.78 | 0.39 | 0.05 | 0.44 | 7.66 |
| #33 | 0.38 | 0.10 | 0.48 | 3.71 | 0.39 | 0.19 | 0.58 | 2.12 | 0.36 | 0.19 | 0.55 | 1.94 | 0.30 | 0.16 | 0.46 | 1.89 |
| #34 | 0.72 | 0.66 | 1.38 | 1.10 | 0.65 | 0.73 | 1.38 | 0.90 | 0.68 | 0.74 | 1.42 | 0.92 | 0.75 | 0.85 | 1.60 | 0.88 |
| #35 | 0.41 | 0.03 | 0.45 | 12.0 | 0.53 | 0.04 | 0.57 | 14.1 | 0.44 | 0.03 | 0.47 | 15.7 | 0.54 | 0.03 | 0.57 | 20.1 |
| #36 | 0.52 | 0.36 | 0.88 | 1.44 | 0.57 | 0.37 | 0.94 | 1.55 | 0.62 | 0.38 | 1.00 | 1.64 | 0.63 | 0.38 | 1.01 | 1.64 |
| #37 | 0.46 | 0.33 | 0.79 | 1.39 | 0.38 | 0.39 | 0.77 | 0.97 | 0.30 | 0.46 | 0.76 | 0.64 | 0.32 | 0.41 | 0.73 | 0.78 |
| #38 | 0.59 | 0.30 | 0.89 | 2.01 | 0.55 | 0.53 | 1.08 | 1.03 | 0.58 | 0.60 | 1.18 | 0.97 | 0.57 | 0.61 | 1.18 | 0.94 |
| #39 | 0.20 | 0.14 | 0.34 | 1.39 | 0.49 | 0.53 | 1.02 | 0.92 | 0.45 | 0.47 | 0.92 | 0.96 | — | — | — | — |
| #40 | 1.02 | 0.03 | 1.05 | 33.2 | 0.75 | 0.01 | 0.76 | 52.5 | 0.83 | 0.01 | 0.84 | 72.0 | 0.75 | 0.15 | 0.91 | 4.99 |
| #41 | 0.14 | 0.11 | 0.25 | 1.27 | 0.20 | 0.22 | 0.42 | 0.91 | 0.09 | 0.10 | 0.19 | 0.85 | 0.06 | 0.07 | 0.13 | 0.81 |
| #42 | 0.52 | 0.10 | 0.62 | 5.05 | 0.80 | 0.23 | 1.03 | 3.43 | 0.75 | 0.21 | 0.96 | 3.66 | 0.62 | 0.16 | 0.78 | 3.82 |
| #43 | 0.18 | 0.28 | 0.46 | 0.63 | 0.19 | 0.32 | 0.51 | 0.59 | 0.17 | 0.31 | 0.48 | 0.54 | 0.13 | 0.26 | 0.39 | 0.50 |
| #44 | 0.46 | 0.28 | 0.74 | 1.67 | 0.45 | 0.34 | 0.79 | 1.32 | 0.34 | 0.32 | 0.66 | 1.06 | 0.25 | 0.26 | 0.51 | 0.97 |
| #45 | 0.23 | 0.00 | 0.23 | 73.0 | 0.82 | 0.03 | 0.85 | 27.1 | 0.75 | 0.02 | 0.77 | 33.4 | 0.80 | 0.02 | 0.82 | 35.9 |
| #46 | 0.53 | 0.01 | 0.54 | 36.1 | 0.62 | 0.02 | 0.64 | 27.3 | 0.73 | 0.03 | 0.76 | 23.9 | 0.63 | 0.04 | 0.67 | 17.8 |
| #47 | 0.86 | 0.08 | 0.94 | 11.3 | 0.55 | 0.05 | 0.60 | 11.1 | 0.50 | 0.04 | 0.54 | 11.7 | — | — | — | — |
| #48 | 0.72 | 0.06 | 0.78 | 12.3 | 0.79 | 0.07 | 0.86 | 11.5 | 0.75 | 0.06 | 0.81 | 12.1 | — | — | — | — |
| #49 | 0.24 | 0.34 | 0.58 | 0.71 | 0.21 | 0.28 | 0.49 | 0.74 | — | — | — | — | — | — | — | — |
| #50 | 0.18 | 0.07 | 0.25 | 2.48 | 0.14 | 0.10 | 0.24 | 1.48 | 0.15 | 0.09 | 0.24 | 1.68 | 0.11 | 0.06 | 0.17 | 1.78 |
Eph, ephedrine; PEph, pseudoephedrine; TA, total content of Eph and PEph; Eph/PEph, Eph/PEph ratio; —, no data (terrestrial stem was too small to collect). * The value could not be calculated.
The average TA content in each year was 0.64±0.27% (2012; n=50), 0.76±0.26% (2013; n=50), 0.73±0.30% (2014; n=49), and 0.70±0.38% (2015; n=45) (Fig. 3). The respective CV values were 0.42 (2012), 0.37 (2013), 0.41 (2014), and 0.54 (2015). The average TA content satisfied the criteria of JP161) after 3 years of cultivation (in 2013) (Fig. 3). The TA content of the individual plants were as follows: 0.14–1.38% (2012), 0.24–1.56% (2013), 0.19–1.45% (2014), and 0.13–1.60% (2015) (Table 1, Fig. 4). The difference between the maximum and minimum values of the TA content among the individuals in each year was 1.24 (2012), 1.32 (2013), 1.26 (2014), and 1.47 (2015), whereas the difference for each individual was from 0.03 to 0.40 after 3 years of cultivation (Fig. 4) and from 0.00 to 1.15 between 2012 and 2013 (Table 1). Accordingly, the range of the TA content among the individuals was larger than that for each individual over 4 years (Fig. 4). The range of the TA content for each individual after 3 years of cultivation was smaller than that before 3 years of cultivation (Table 1). Therefore, under the same field conditions used to cultivate E. sinica, the TA content depended on the individual plants.

Contents are indicated in mean±S.D. The bars represent the S.D.

The bars show the range for the TA content in terrestrial stems of each individual plant derived from seeds after 3 years of cultivation and that of the content for each year. The squares indicate selected individuals.
We selected three individual plants, #2, #19, and #26, for breeding. The numbers of stolons collected from the plants were nine (#2), 14 (#19), and nine (#26).
We examined the survival rates of stolons to evaluate the efficiency of stolon propagation. The data are shown in Fig. 5. In the first year (2014), the survival rates of the cultivated individuals derived from stolons were 55.6% (#2), 50.0% (#19), and 88.9% (#26). The survival rates in the second year (2015) were the same as those in the first year (2014) (Fig. 5).

The survival rate was defined as follows: Survival rate (%)=[(number of survivors)/(number of stolons planted)]×100.
We measured the content of the ephedrine alkaloids in terrestrial stems of the selected lines derived from stolons over a period of 2 years. The data are shown in Table 2.
| Individuals | 1-Year-old (2014) | 2-Year-old (2015) | Parent (2013–2015) | ||||
|---|---|---|---|---|---|---|---|
| Eph (%) | PEph (%) | TA (%) | Eph (%) | PEph (%) | TA (%) | TA (%) | |
| #2-1 | 0.84 | 0.01 | 0.85 | 1.22 | 0.01 | 1.23 | 1.06±0.02 |
| #2-2 | 0.74 | 0.01 | 0.75 | 1.21 | 0.01 | 1.22 | |
| #2-3 | 0.55 | 0.01 | 0.56 | 1.14 | 0.01 | 1.15 | |
| #2-4 | 0.51 | 0.01 | 0.52 | 1.24 | 0.00 | 1.24 | |
| #2-5 | 0.68 | 0.04 | 0.71 | 0.92 | 0.06 | 0.98 | |
| #19-1 | 0.68 | 0.07 | 0.75 | 1.22 | 0.14 | 1.36 | 1.51±0.04 |
| #19-2 | 0.42 | 0.04 | 0.46 | 1.26 | 0.14 | 1.40 | |
| #19-3 | 0.45 | 0.04 | 0.49 | 0.97 | 0.09 | 1.06 | |
| #19-4 | 0.55 | 0.05 | 0.60 | 0.73 | 0.07 | 0.80 | |
| #19-5 | 0.65 | 0.07 | 0.72 | 0.82 | 0.08 | 0.90 | |
| #19-6 | 0.46 | 0.05 | 0.51 | 0.97 | 0.10 | 1.07 | |
| #19-7 | 0.68 | 0.07 | 0.75 | 0.95 | 0.10 | 1.05 | |
| #26-1 | 0.24 | 0.09 | 0.33 | 0.95 | 0.45 | 1.40 | 1.27±0.09 |
| #26-2 | 0.57 | 0.24 | 0.81 | 1.06 | 0.47 | 1.53 | |
| #26-3 | 0.63 | 0.26 | 0.89 | 1.43 | 0.57 | 2.00 | |
| #26-4 | — | — | — | 0.95 | 0.38 | 1.33 | |
| #26-5 | 0.48 | 0.16 | 0.64 | 1.14 | 0.57 | 1.71 | |
| #26-6 | 0.36 | 0.07 | 0.43 | 0.56 | 0.16 | 0.72 | |
| #26-7 | 0.40 | 0.14 | 0.54 | 1.03 | 0.48 | 1.51 | |
| #26-8 | 0.45 | 0.11 | 0.56 | 0.63 | 0.23 | 0.86 | |
Eph, ephedrine; PEph, pseudoephedrine; TA, total content of Eph and PEph; —, no data (terrestrial stem was too small to collect).
In the first year (2014), the average TA content was 0.68±0.13% (#2), 0.61±0.12% (#19), and 0.60±0.26% (#26) (Fig. 6), i.e., lower than that in their parents (Table 2, Fig. 6). The TA content of individual plants were as follows: 0.52–0.85% in line #2, 0.46–0.75% in line #19, and 0.33–0.89% in line #26 (Table 2, Fig. 7). The CV values were 0.18 (#2, n=5), 0.19 (#19, n=7), and 0.44 (#26, n=7). The difference between the maximum and minimum values of the TA content in each line was 0.33 (#2), 0.29 (#18), and 0.56 (#26) (Fig. 7).

Contents are indicated in mean±S.D. The bars represent the S.D. For the parents, an average TA content is shown after 3 years of cultivation.

The bars show the range of the TA content in terrestrial stems of the selected lines derived from stolons and that of the content for each year.
In the second year (2015), the average TA content was 1.16±0.10% (#2), 1.09±0.20% (#19), and 1.38±0.39% (#26) (Fig. 6). The TA content of individual plants were as follows: 0.98–1.24% in line #2, 0.80–1.40% in line #19, and 0.72–2.00% in line #26 (Table 2, Fig. 7). The CV values were 0.08 (#2, n=5), 0.19 (#19, n=7), and 0.29 (#26, n=8). The difference between the maximum and minimum values of the TA content in each line was 0.26 (#2), 0.60 (#18), and 1.28 (#26) (Fig. 7).
Compared to the first year, the average TA content increased and the CV values decreased in the second year. The TA content of stolon-derived terrestrial stems from the 2-year-old selected lines was also as high as that of their parents (Table 2, Fig. 6). In addition, the difference in the TA content of the selected individuals derived from stolons was smaller than that of the unselected individuals derived from seeds, except #26 (Fig. 7). Moreover, the CV values for the selected individuals derived from stolons were smaller than those for the unselected individuals derived from seeds.
Previous studies have researched ephedrine alkaloids in Ephedra plants in their habitats,3,6,7) but no study of annual variation of ephedrine alkaloids in individual plants has been conducted until now. Therefore, data on ephedrine alkaloids of Ephedra plants cultivated in the field are needed for sustainable cultivation of the plants.
We first grew E. sinica plants derived from seeds in the field for 5 years and measured the TA content of each individual plant over 4 years. Sampling time was from August to November. Kajimura et al.9) has demonstrated that the ephedrine type alkaloid contents were low in the early stages of the stem growth in Ephedra plants, but the ephedrine type alkaloid contents were stable when growth of terrestrial stems was stopped. So, terrestrial stems were collected when growth of terrestrial stems was stopped in each year. Therefore, sampling time was different in each year. In this study, the average TA content was >0.7% after 3 years of cultivation (in 2013). The TA content was widely distributed among the individuals (Table 1, Fig. 3): 0.14–1.38% (2012), 0.24–1.56% (2013), 0.19–1.45% (2014), and 0.13–1.60% (2015) (Fig. 4). Hong et al.3) has also demonstrated a wide range of the TA content. On the other hand, the difference in the TA content among the individuals in each year was 1.24 (2012), 1.32 (2013), 1.26 (2014), and 1.47 (2015), whereas the difference in the TA content in each individual was from 0.03 to 0.40 after 3 years of cultivation (Fig. 4) and from 0.00 to 1.15 between 2012 and 2013 (Table 1). Therefore, in each 3-year-old individual, the TA content was stable and its range was narrower than that among the individuals grown for 4 years (Table 1, Fig. 4). Our results suggest that the TA content of cultivated E. sinica plants derived from seeds and grown in the field depends on the individual and that the variation of the TA content among individuals is wide. For these reasons, individuals were selected according to the following criteria: (1) the TA content of ≥1.0% after 3 years of cultivation; (2) the Eph/PEph ratio of >1 after 3 years of cultivation; (3) the difference between the maximum and minimum values of the TA content of <0.3 after 3 years of cultivation; and (4) individuals showed stolon-formation capability.
Second, we examined a vegetative propagation method using stolons to propagate selected individuals (Figs. 1, 2). Ephedra plants are dioecious gymnosperms, and therefore, it is difficult to produce seeds in Ephedra plants. The propagation has been performed using methods such as cutting10–12) until now. However, these methods require difficult skills to propagate selected individuals. On the other hand, when using stolon propagation, it is easy to collect stolons, and the survival rate for stolon propagation is >50% (Fig. 5). Consequently, it can be suggested that stolon propagation is an effective method for propagation of selected lines.
Finally, we propagated selected individuals using stolons and then measured their TA content for 2 years (Table 2). In the first year, the TA content in terrestrial stems of the selected lines derived from stolons was lower than that of their parents (Table 2), but in the second year, the TA content was almost as high as those of parents in the selected individuals (Table 2, Fig. 6). Hence, it is assumed that several years are needed for E. sinica plants to start producing sufficient amounts of ephedrine alkaloids. Also, the range of the TA content in selected individuals derived from stolons of the selected lines was narrower than that in the unselected individuals derived from seeds (Tables 1, 2). Thus, the TA content of the selected individuals derived from stolons was more stable and higher than that of the unselected individuals derived from seeds (Figs. 3, 6). Our results support the hypothesis that in the same cultivation environment, the TA content of E. sinica plants cultivated in the field is determined by genetic factors.
Thus, we obtained selected lines that had a stable TA content using stolon propagation. Taken together, we conclude that the TA content of E. sinica is determined by genetic factors, and stolon propagation is an effective approach to selective breeding for securing a stable supply of the Ephedra herb. Previous studies have shown that the content of ephedrine alkaloids is affected by environmental factors.6,7) Therefore, it is necessary to confirm that the TA content of the selected lines can be reproduced when they are cultivated in various fields.
Future studies are needed to establish methods for increasing the number of stolons in individual plants, to investigate the variation of selected lines, and to improve the efficiency of selective breeding. In particular, there is room for improvement of the methods of selective breeding. Recent studies have revealed biosynthetic enzyme genes for ephedrine alkaloids.13–15) The data obtained in this study and the selected lines may provide the advantage for developing approaches to genetic analysis of biosynthetic enzyme genes for ephedrine alkaloids.
The authors are grateful to Dr. K. Hashimoto, Mr. K. Kondo and Dr. M. Nagasawa, for helpful suggestions. We also thank Dr. Y. Nakamura for discussions concerning chemical analyses. We appreciate Ms. K. Nishidate and Ms. Y. Ohko to perform sample processing.
The authors are employees of Tsumura & Co.