2017 年 40 巻 1 号 p. 61-67
2017 年 40 巻 1 号 p. 61-67
Pneumolysin (PLY) is a devastating bacterial protein toxin of Streptococcus pneumoniae that punctures the cytomembrane, leading to pathological reactions, such as cell disruption and inflammation. Drugs capable of closely impacting the toxin are considered advantageous in the treatment of bacterial infections. Amentoflavone (AMF) is a chemical substance extracted from traditional Chinese herbs. Previous studies have demonstrated that AMF has multiple pharmacological effects and mentioned without attenuating pneumolysin-mediated cytotoxicity. This work focuses on the influence of AMF on inhibitory hemolytic mechanisms. AMF interacts with the toxin at Ser254, Glu277, Arg359, and effectively weakens the oligomerization of wild-type PLY and provides considerable protection against pneumolysin-mediated human alveolar epithelial (A549) cell damage. The results of our study demonstrate that AMF could be a candidate against pneumolysin-related injury.
Antibiotic abuse is a growing problem worldwide, referenced a recent WHO report as supposing that the problem is so severe and may lead to 1 million early deaths annually by 2050. Furthermore, antibiotics are used more frequently in livestock breeding industry for the purpose of reducing costs and pursuing profits. The outcome of this is bacteria are developing greater resistance to antibiotics. To curb the drug-resistance and improve the cure rate, government should strengthen the supervision of the application of existing antibiotics and novel agents must be developed, at the same time. As thus, advancements in the research of anti-virulence strategies demonstrate its importance as a potential drug target.1)
Streptococcus pneumoniae is a widespread human bacterial pathogen. It is the primary cause of otitis media, sinusitis, meningitis, pneumonia and even bacteremia.2) The rates of morbidity and mortality caused by S. pneumoniae remain high around the world and are a dominant factor of community-acquired pneumonia (CAP) and hospitalization. Presently, conventional antibiotics are the first choice for clinical treatment of S. pneumoniae-infection. However, the overuse of antibiotics around the globe has led to the emergence of antibiotic-resistant bacteria. S. pneumoniae is among this new class of resistant bacteria that has developed resistance to β-lactam antibiotics and even vancomycin.3,4) The bacterial toxin pneumolysin is a notable virulence factor of S. pneumoniae. Pneumolysin (PLY, 53-kDa) is a member of the cholesterol-dependent cytolysin (CDC) family5,6) and is released as a soluble monomer. PLY ruptures target cells by locating cholesterol-containing cell membranes and gathering them into oligomeric rings, forming large pores. Members of the family include Streptococcus, Clostridium, Listeria, Bacillus and others. The crystal structure reveals that perfringolysin (secreted by C. perfringens) shares 48% sequence identity and 60% sequence similarity with PLY. It is not hard to see that these two toxins are both structurally and functionally homologous.7) In this case, the most conserved regions, the tryptophan (Trp)-rich loop at the base of domain 4 and the three hydrophobic loops (L1, L2, L3), insert themselves into cholesterol-rich cell membranes and begin the oligomerization process. This oligomerization process is a crucial mechanism of the lytic action of PLY.
Amentoflavone (AMF; 4′,4‴,5,5″,7,7″-hexahydroxy-3‴,8-biflavone), is a naturally occurring compound that is widely used in traditional Chinese medicine. AMF belongs to the flavonoid class and is extracted from Selaginella tamariscina and other plants. It has many beneficial biological properties, including enhancement of osteogenesis, protection against radiation, as well as anti-human immunodeficiency virus (HIV), anti-inflammatory, anti-oxidative, and anti-apoptotic effects.8–11) However, as far as we know the antivirulence effects of AMF on PLY-mediated cytotoxicity have not been examined. To determine the molecular mechanisms of AMF antivirulence function, homology modeling and molecular docking studies were carried out on protein mutants of the PLY–AMF complex. We predicted that AMF would block the PLY oligomerization process and inhibit its cytolytic activity by binding to PLY at the cleft between domains 3 and 4. Compounds that target virulence factors instead of the basic bacterial physiology are considered to be ideal adjuvant antibacterial therapies.
AMF (98% purity) was purchased from Herbpurify (lot number S-044-150423, Chengdu, China), and the specimens are deposited in faculty key laboratory of zoonosis, Jilin University, Changchun, China. Phosphate buffered saline (PBS) powder was supplied by Boster (Wuhan, China). Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). An anti-pneumolysin antibody [PLY-4] and rabbit anti-mouse immunoglobulin G (IgG) H&L (HRP) were obtained from Abcam (Cambridge, U.K.). The blotting reagent kit was purchased from Beyotime (Shanghai, China). Alveolar epidermal cells (A549) were supplied by ATC C (Manassas, VA, U.S.A.). Dulbecco’s modified Eagle’s medium (DMEM) and fetal calf serum (Invitrogen, CA, U.S.A.) were used for procedures carried out in this study.
Cell CultureA549 human lung epithelial cells were cultured adherently in DMEM containing 10% fetal bovine serum (an expression vector according to Chemical Abstracts of the United States), 100 U/mL penicillin and 100 U/mL streptomycin at 37°C in a humid environment of 5% CO2. The culture medium was displaced every 24 h.
Construction, Expression, and Purification of Native PLYProcesses of vector constructing and recombinant protein overexpressing were carried out by employing methods described previously.12) The supernatant of centrifuged cell lysate was loaded onto a Ni-NTA agarose column. The recombinant protein was then rinsed with washing buffer (PBS consisting of 20 mM imidazole, pH 7.4). The target protein was flushed with elution buffer (PBS containing 200 mM imidazole, pH 7.4). The eluted solution with His-tagged protein was concentrated using a 30-kDa molecular weight cutoff filter (Millipore, Bedford, MA, U.S.A.) for desalting. The purified protein in PBS was identified using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Purification of pneumolysin mutants were conducted using similar methods.
Hemolysis AssayDetails regarding the examination of hemolytic activity used in the current study have been described previously.13) Briefly, 1 µL of purified PLY (33 µg/mL) was preincubated in tubes with a sequence of concentrations of AMF (ranging from 2 to 64 µg/mL) at 37°C for 10 min. Twenty-five microliters of defibrinated rabbit blood erythrocytes was added to each tube, yielding a final volume of 1 mL with PBS. Next, the tubes were incubated at 37°C for 15 min. After centrifugation at 10000×g for 1 min, supernatants were transferred into a quartz colorimetric utensil to indicate the absorption of each sample at 543 nm. Simultaneously, in the control group erythrocytes was only incubated with AMF as a reference.
Oligomerization AnalysisWild-type PLY (20 mg/mL) incubated with various concentrations of AMF were cultured at 37°C for 1 h in a thermostat water bath. A group without the compound served as the control. Equal volumes were heated in Laemmli sample buffer at 50°C for 10 min. Afterwards, the prepared samples were loaded onto a 6% SDS-PAGE gel and transferred to a polyvinylidene fluoride (PVDF) membrane through a semi-dry transfer cell. The PVDF membrane was then shorn and sealed off overnight with 5% skim milk at 4°C. Mouse monoclonal to PLY (primary antibody) with a dilution ratio at 1 : 1000, was incubated with the PVDF membrane for 2 h at room temperature. Rabbit polyclonal secondary antibody to mouse IgG-H&L (HRP) was added at a 1 : 2000 dilution. The blotting reagent kit for enhanced chemiluminescence (ECL) was applied to develop the blots.
Site-Directed Mutagenesis of PLYThe primers used to introduce these three mutants (S254A, E277A, R359A) are listed in Table 1. ply gene mutations in targeted residues were created with the pET28a-PLY plasmid using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, U.S.A.).
| Primer | Sequence | Amino acid change |
|---|---|---|
| 1fwd | 5′-CAAGTTGGAAACCACGGCGAAGAGTGATGAAGTAG-3′ | S254A |
| 1red | 5′-CTACTTCATCACTCTTCGCCGTGGTTTCCAACTTG-3′ | |
| 2fwd | 5′-GTAGCTCCTCAGACAGCGTGGAAGCAGATTTTG-3′ | E277A |
| 2red | 5′-CAAAATCTGCTTCCACGCTGTCTGAGGAGCTAC-3′ | |
| 3fwd | 5′-GTTACAGCTTACGCCAACGGAGATTTAC-3′ | R359A |
| 3red | 5′-GTAAATCTCCGTTGGCGTAAGCTGTAAC-3′ |
Circular dichroism (CD) spectra were measured on a MOS-500 Spectrometer (Bio-logic, Claix, France) at 25°C and a fentes bandwidth of 2 nm; the protein solution (0.33 mg/mL) was placed in a 1-mm-pathlength quartz cuvette. All spectra were collected in the far-UV (190–250 nm) region.
Live/Dead and Cytotoxic TestingThe experimental cells (A549) were digested by trypsin and delivered to a 96-well plate, 1.5×104 cells in each well. The A549 cells were treated to isolated incubation under the assistance of WT-PLY, S254A, E277A, and R359A (0.825 µg/mL, at the same concentration) and increasing concentrations of AMF. These test samples as well as the positive control (DMEM) were settled in a temperature-controlled box for 6 h at 37°C. A Cytotoxic Detection Kit (Roche, Mannheim, Germany) can be applied to indicate the releasing capacity of lactate dehydrogenase (LDH), thus the cell viability could be evaluated. Live/dead assays were implemented using the methods proposed by the manufacturer (Invitrogen, Carlsbad, CA, U.S.A.). LDH activity was determined at 490 nm on a multimode reader (TECAN, Salzburg, Austria). In the control group A549 cells were only incubated with AMF as a reference. Microscopic images of stained cells were obtained using a confocal laser scanning microscope (Olympus, Tokyo, Japan). The results are representative of a minimum of three independent experiments.
Homology Modeling Study and Molecular Docking CalculationThe monomeric three-dimensional (3D) structure of soluble wild-type PLY has not yet been published. Generally, a WT-PLY model was proposed based on a homology simulation study using the reported structure of perfringolysin O (PFO) as the template (Protein Data Bank code1PFO). The Align Sequence to Templates tool in Discovery Studio 2.5 (BIOVIA, San Diego, CA, U.S.A.) was used to carry out sequence alignment between PLY and the template.14) Computational modeling of PLY was performed using the Build Homology Models protocol implemented in Discovery Studio 2.5. To acquire the equilibrium structure, a 500-ns molecular dynamics simulation was implemented with the 3D structure of PLY using the Gromacs 4.5.5 software package.15) To also attain docking and dynamic analogy of compounds, the Gaussian 09 program was utilized to optimize the structure of AMF at the B3LYP/6-31G* level.
The original structure of PLY was achieved from the homology modeling. In this part of the work, AMF, considered to be a flexible ligand, was docked into PLY using the docking program AutoDock 4.0 (The Scripps Research Institute, La Jolla, CA, U.S.A.).16,17) The particular docking method was described previously.18) The docking model of AMF with PLY has been provided in the Supplementary materials.
Mouse Model of Intranasal Lung InfectionFor animal infection studies, C57BL/6J male mice aged 8 weeks and weigh 20±2 g were obtained from the Experimental Animal Center of Jilin University, and testing S. pneumoniae strain D39 (NCTC 7466) were cultured in Todd–Hewitt Broth at 37°C to the mid-exponential phase (OD600=0.8). AMF was dissolved in DMSO to make stock solution.
The mice were anesthetized intraperitoneally with ketamine and xylazine, and then, 20 µL of strain D39 (5×107 colony-forming units) suspension was dropped into the left nare. The animals were kept upright to revive and were then observed for 72 h. To investigate the effects of AMF treatment, mice were administered 100 µL of AMF subcutaneously 2 h after infection with S. pneumoniae and then at 12-h intervals. The control group mice were treated with 100 µL of sterile DMSO on the same schedule.
The pictures of histopathologic slides were captured by an electron microscope (Olympus, Tokyo, Japan).
Statistical AnalysisData statistics were analyzed using the independent samples t-test. SPSS 13.0 statistical software was used for these analyses (SPSS, Inc., Chicago, IL, U.S.A.). Differences were considered statistically significant when p<0.05 (*) and p<0.01 (**).
AMF has numerous pharmacological effects.8–11) Drug screening verified this natural flavonoid compound. A hemolysis assay determined its effectiveness in inhibiting the hemolytic activity of pneumolysin. The molecular structure of AMF is shown in Fig. 1A. The hemolysis test revealed that the hemolytic activity of cytotoxin was significantly suppressed when the AMF molecule intervened. The A543 nm values were sharply reduced with increasing concentrations of AMF, indicating that more intact red blood cells survived (Fig. 1B). The above outcomes suggest that AMF may directly interact with PLY.
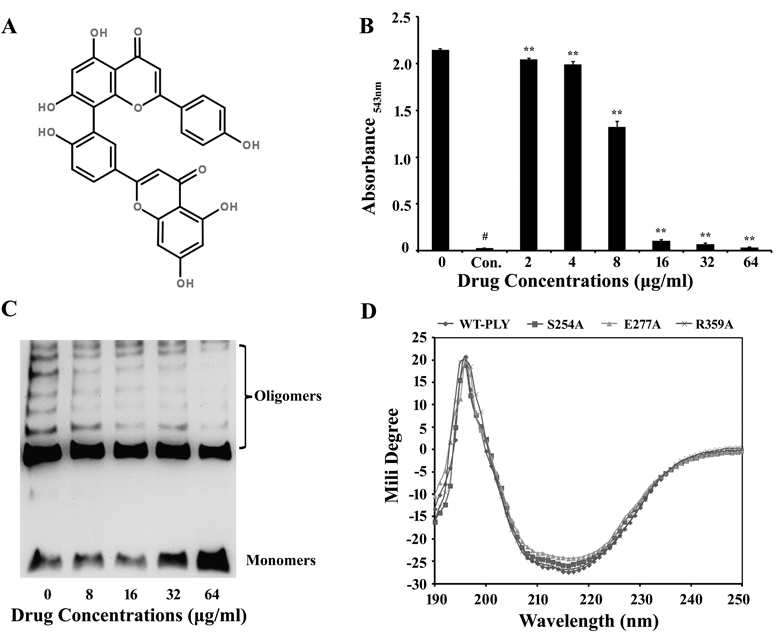
B, Reactions associated with PLY and AMF using purified rPLY and rabbit blood cells in PBS. Hemolysis was indicated by centrifugal supernatant values at absorbance543 nm. The addition of AMF decreased hemoglobin release. Data shown are from 3 independent experiments; ** p<0.01, by 2-tailed Student t-tests. C, The formation of oligomers was explored using Western blot after SDS-PAGE. AMF inhibits the oligomerization of WT-PLY in a dose dependent manner. D, Signals were recorded in the far-UV (190–250 nm) region. No significant wave changes occurred when these proteins were compared, indicating conformations of the mutations remained stable.
Oligomerization analysis determined if AMF alleviated PLY-induced hemolytic activity by affecting oligomerization. As far as we know, in solution, PLY can self-associate from monomers to form oligomers in the absence of cholesterol and cytomembranes.19) A series of drug concentration increases were mixed into the reaction system. An rPLY sample without AMF served as the control. The experimental results are presented in Fig. 1C. Increasing concentrations of AMF hindered rPLY oligomerization. As shown in Fig. 1C, the blots of oligomers became shallower, thinner, and sometimes disappeared, while the monomer blots remained dark and thick. Taken together, these results suggest that AMF molecules interfere with PLY oligomerization.
Conformations of Mutations Remain StableProtein mutants were created, and homology modeling and molecular docking analyses were carried out for the PLY-AMF complex. The outcomes predicted that AMF binds to the cleft between domains 3 and 4 of PLY, consequently blocking oligomerization.
Circular dichroism (CD) analyses were used to verify any noticeable conformational changes in the three mutations (S254A, E277A, R359A). In contrast to WT-PLY’s, the curves of the mutations maintained a consistent trend. There was no significant amplitude or position changes within the 190–250 nm region (Fig. 1D). The results shown above demonstrate that site-directed mutagenesis of PLY only caused mutatations at a certain site of the functional amino acid residue and did not cause any conformational changes. Further experiments were conducted to demonstrate the functionality of the residues.
AMF Weakens PLY-Mediated Human Alveolar Epithelial (A549) Cell InjuryIt is known that PLY is a pore-forming cytotoxin of Streptococcus pneumoniae (S. pneumoniae) and is a direct cause of lung cell damage. It has been demonstrated that PLY plays an important role in apoptosis and necrosis.2) Previous studies have used the human alveolar cell line A549 to explore PLY-related issues.20) As shown in Fig. 2, intact A549 cells cultured in normal DMEM exhibited green fluorescence (Fig. 2A) indicating the presence of healthy viable cells. On the other hand, A549 cells incubated with 0.33 mg/mL WT-PLY exhibited red fluorescence, suggesting the presence of necrosis (Fig. 2B). In contrast with the WT-PLY exposed group, the number of green fluorescent cells increased when treated with 8 µg/mL AMF, suggesting that AMF provided effective protection (Fig. 2C). When the drug concentration was increased to 32 µg/mL, most of the cells maintained green fluorescence (Fig. 2D), though the whole cell morphology could not be compared across groups.
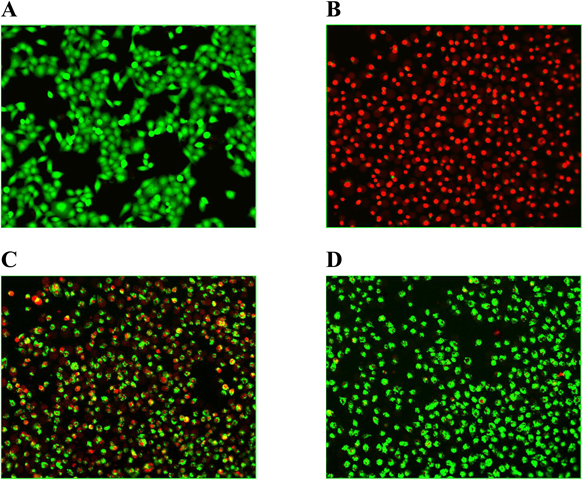
Live/dead stained A549 cells were photographed by confocal laser scanning microscopy after incubated with PLY. A, Normal A549 cells. B, Cells incubated with PLY in the absence of AMF. C, Co-cultured cells treated with 8 µg/mL of AMF. D, Co-cultured cells treated with 32 µg/mL of AMF.
The effect of AMF on the PLY-mediated cytotoxicity was evaluated using LDH release tests. As expected, AMF hindered WT-PLY virulence in a concentration dependent manner. Using low AMF concentrations (2 µg/mL), AMF showed significant differences (p<0.01). When the AMF concentration was increased to 64 µg/mL, it provided nearly 70% A549 cells (Fig. 3A). LDH release assays were performed to examine the influence of Ser254, Glu277, Arg359 on lytic activity. On the question of virulence of the mutants, this test found that nearly there were no differences in the LDH release activity between WT-PLY and its mutants, also manifested that one single amino acid mutated did not influence the activity. Relative to WT-PLY, lysin mutations slightly altered cytotoxicity. Mutant virulence was not as strongly affected as the WT-PLY by increased drug concentrations (Figs. 3B–D). These results support our claim that the aforementioned residues play an essential role in PLY’s virulence. Additionally, AMF may be an effective therapy for future applications.
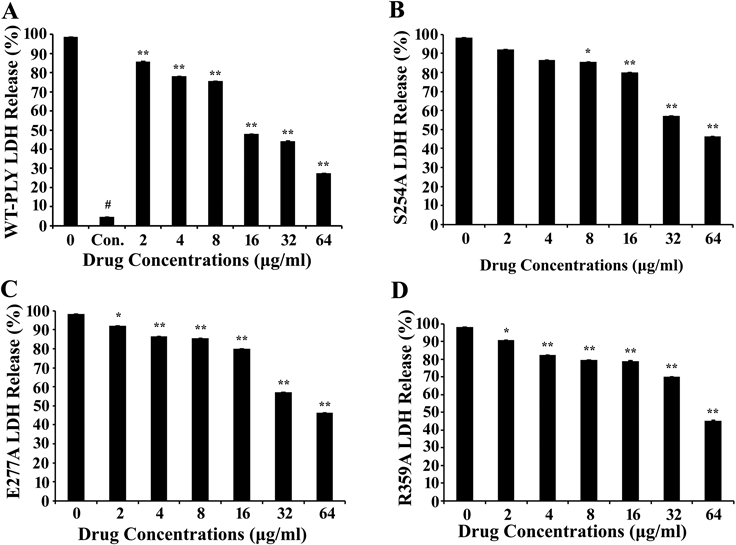
Degrees of LDH release were measured to detect the variation of mutant inhibition in the presence of various concentrations of AMF in each group. A–D, WT-PLY group, S254A group, E277A group, R359A group.
The information from live/dead and cytotoxicity assays indicated that AMF could protect alveolar epithelial cells against necrocytosis caused by PLY. Based on these in vitro evidences, we further evaluated the protective effects of AMF in vivo on mice S. pneumoniae-related pneumonia. Previous studies have indicated that when administered in purified PLY to lung tissues, PLY perturbs lung function and induces severe lethality, similarly to wild type S. pneumoniae21) and PLY is essential for development of severe pneumonia and bacterial survival in the blood.22) To discover the effects of AMF treatment on lung injury in S. pneumoniae pneumonia, the mice were administered 100 µL of AMF (50 mg/kg) subcutaneously 2 h after infection with D39 and then at 12-h intervals.
We performed histopathologic analysis of lungs 72 h after infection from mice that treated with either 50 mg/kg of AMF or DMSO as a control. The untreated mice had crimson lungs which exhibited severe hyperemia (Fig. 4A), fully distinguishable from the normal lung tissue (Fig. 4B), and the lung tissue of mice treated with AMF had a tight texture and only showed mild hyperemia (Fig. 4C). As shown in Fig. 4D, there were accumulations of inflammatory cells in alveolar space and significant damaged alveolar structures in the group infected with strain D39. Treatment of mice with AMF clearly improved the histopathologic features of pneumonia, as indicated by less inflammatory exudates within the pulmonary alveoli and more integrate alveolar structures compared with the untreated group (Fig. 4F). The histopathologic picture of the control group was shown in Fig. 4E. The results of this study show that AMF can play an important part in easing lung lesions caused by S. pneumoniae infection.
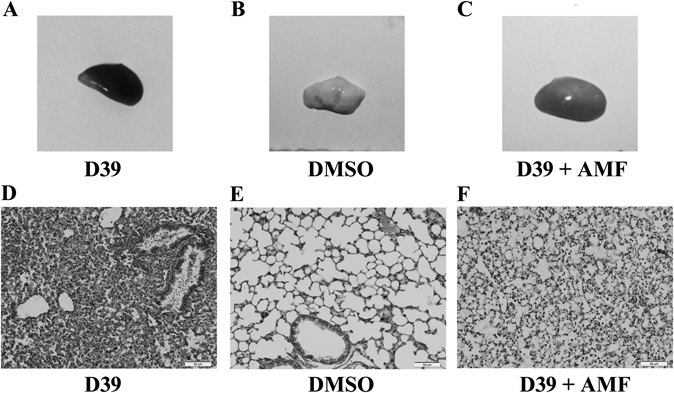
The mice were infected via the intranasal route with S. pneumoniae D39. The mice in control group were given subcutaneous injection of the same volume of solvent (DMSO). A–C, gross pathological changes. D–F, histopathology changes. The lung tissues were dyed with hematoxylin and eosin (original magnification, ×200).
PLY, a member of the CDC family, leads to lysis via two vital processes, namely, pre-pore formation (by binding to cell membrane cholesterol and inserting itself into the cytomembrane) and oligomerization into a large pore. Thus, the oligomerization mechanism plays a crucial role in cytolysis. AMF can neutralize the hemolytic effects of PLY significantly, 16 µg/mL of AMF can approximately completely inhibit hemolysis (Fig. 1B). Also AMF can disrupt the process of PLY oligomerization (Fig. 1C) and hinder the formation of cytomembrane lesions (Fig. 2). As a result of disrupting PLY oligomer formation, it is too difficult for the protein to form a large intact pore in the cytomembrane to hemolyze erythrocytes, AMF can thereby inhibit the hemolytic effects when a certain concentration of AMF existing in the reaction system. Molecular modeling revealed that AMF could bind to the cleft between domains 3 and 4 in PLY by making strong contact with Ser254, Glu277, and Arg359. And the binding of AMF to PLY can cause a conformational change of domains 3 and 4 of PLY, directly leading to a decrease in the distance between domains 3 and 4. The diminished LDH release levels in human alveolar epithelial cells (Fig. 3A) indicated the molecules’ protective effect on target tissues. Cytotoxic activity levels of PLY-variants (S254A, E277A, R359A) are much the same compared with that of WT-PLY. PLY-variants produce cytotoxic activities that can only be slightly influenced by AMF (Figs. 3B–D), suggesting that the mutated residues affect binding between PLY and AMF and thereby providing evidence for our molecular modeling results. Due to diverse experiment systems and processes, the final concentrations of PLY in different experiments are 0.033 µg/mL (hemolysis assay), 20 mg/mL (oligomerization analysis) and 0.825 µg/mL (cytotoxic testing), respectively. In spite of this, it is obvious that AMF possesses effective anti-virulence activity, resisting PLY-mediated erythrocyte rupture and lung cell damage.
Prior researches have noted that PLY is required for development of S. pneumoniae-induced pneumonia and bacterial survival in the blood, and PLY-deficient mutants exhibited a drastic decrease in the number of pneumococci in the blood compared with the wild type strain.22) The untreated mice infected with D39 had red dark lungs, and the accumulation of inflammatory cells appeared in pulmonary alveoli. Whereas the AMF treated group alleviated the inflammation, improved the histopathologic features of pneumonia. Here, AMF provided a large set of protection against PLY-mediated alveolar epithelial cell injury not only in vitro, but also could remedy the pulmonary inflammatory reaction caused by S. pneumoniae-induced pneumonia in a murine model. This assignment has demonstrated that AMF possesses pharmacological activities in reducing the virulence of PLY both in vitro and in vivo.
Consumption of antibiotics used by humans, livestock and poultry stays at a high level, not including the residue of livestock antibiotics in food, which is hard to measure. The abuse of antibiotics may result in super-bacteria which endanger all human beings. Aiming at the status quo, people have to reduce the usage of antibiotics to a reasonable level, more important, neotype agents target on other mechanisms should be developed for infections induced by the existing drug-resistant bacteria. The main aim of this research project has therefore attempted to find and verify a category of natural compounds which could relieve the pathological symptoms of bacterial infection, meanwhile, not apply a selective pressure for the development of resistance by killing microbes.
Verbascoside (VBS), a phenylpropanoid glycoside compound, has been reported to be an effective PLY antagonist and exhibits a remarkable influence on reducing the mortality of S. pneumonia infected mice by inhibiting PLY oligomer formation.12) According to the molecular simulation results and experimental validation, AMF and VBS share similar binding sites to WT-PLY. However, VBS oxidizes easily restricting its application.23) Whereas, AMF is comparatively more stable than VBS, thereby making it a better potential anti-virulence agent. In agreement with these considerations, we consider AMF as a collaborative therapeutic with other antibacterial agents to decrease the degree of organ damage and attenuate symptoms caused by S. pneumonia infection.
This research is supported by the National Nature Science Foundation of China [Grant 31130053 to X.M.D] and the China Postdoctoral Science Foundation [Project No. 2016M591486].
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.