2017 年 40 巻 11 号 p. 2001-2004
2017 年 40 巻 11 号 p. 2001-2004
In obese and diabetic patients, plasma free fatty acid (FFA) levels are often elevated and may play a causal role in insulin resistance and reactive oxygen species (ROS) production. We have previously shown that ursodeoxycholic acid (UDCA) has antioxidative activity through the phosphatidylinositol 3-kinase (PI3K)/Akt signaling-mediated glutathione production. In this study, we investigated the effects of UDCA on insulin response by analyzing intracellular ROS and the activation of the PI3K/Akt signaling pathway in HepG2 cells treated with palmitate. The level of ROS was quantified using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), and the activation of the PI3K/Akt signaling pathway was determined by Western blotting assay using appropriate antibodies. The intracellular ROS levels were increased by palmitate but were reduced by treatment with UDCA and insulin. Furthermore, insulin significantly stimulated the phosphorylation of Akt. When the cells were pre-treated with palmitate, insulin-induced Akt-phosphorylation was markedly inhibited. However, when the cells were treated with palmitate and UDCA, the effects of insulin were partially restored. UDCA may have protective effects against palmitate-induced decreases in responsiveness to insulin.
Visceral adipose tissue in obese patients is associated with hepatic steatosis and insulin resistance. Elevated plasma free fatty acid (FFA) levels, which are often associated with obesity and diabetes, may play a causal role in insulin resistance similar to other factors such as glucose and certain cytokines (e.g., tumor necrosis factor-α (TNF-α)).1,2)
Palmitic and stearic acid, which are saturated fatty acids, induce mitochondrial dysfunction and increase the production of reactive oxygen species (ROS) while oleic acid, a major non-saturated fatty acid, does not.3) Nakamura et al. reported that a high level of palmitate accelerated β-oxidation and caused an excess electron flux in the mitochondrial respiratory chain, resulting in the increased production of ROS and suppression of the insulin signaling pathway.4)
Ursodeoxycholic acid (UDCA) is a minor bile acid in humans and has been administered occasionally as a hepatoprotective drug for primary biliary cirrhosis (PBC) and chronic hepatitis. Although the detailed mechanisms of its hepatoprotective effects remain unclear, it is proposed that UDCA has antioxidative action through the induction of glutathione (GSH) synthesis and prevents apoptosis caused by mitochondrial injury.5,6) Recently, we showed that UDCA activated the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway and induced the translocation of nuclear factor-E2-related factor 2 (Nrf2) into the nucleus. These findings suggest that UDCA increases the gene expression of enzymes associated with GSH synthesis and induces the down-regulation of intracellular ROS levels.7)
Oxidative stress is reduced by insulin, a process that is dependent on the activation of PI3K and extracellular signal-regulated protein kinase in HepG2 cells.8) Therefore, both UDCA and insulin may have cytoprotective effects against oxidative stress and may reduce fatty acids-induced insulin resistance. However, to our knowledge, there are no reports that show UDCA reduces fatty acids-induced insulin resistance. Bile acids have gained attention as drug candidates to control obese and/or diabetic conditions because they affect lipid and glucose metabolism by stimulating farnesoid X receptor (FXR) and the G-protein-coupled receptor, TGR5, respectively.9) In this study, to evaluate the effects of UDCA on the action of insulin under conditions of elevated plasma fatty acids, we examined the effects on ROS levels and Akt phosphorylation in palmitate-exposed HepG2 cells.
Ursodeoxycholic acid (UDCA) was kindly supplied by Tanabe-Mitsubishi Pharmaceuticals (Osaka, Japan). Palmitic acid (c16:0) and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) were purchased from Sigma Japan (Tokyo, Japan). Insulin was obtained from Cell Science & Technology Institute, Inc. (Sendai, Japan). Fatty acid free bovine serum albumin was purchased from Nacalai Tesque (Kyoto, Japan).
Preparation of Palmitate–Albumin ComplexesPreparation of palmitate–albumin complexes was performed by a saponification technique according to Goldstein et al.10) The palmitate–albumin complex was sterilized by filtering and was subsequently aliquoted and frozen for future use.11)
Cell CultureHuman hepatoma HepG2 cells (obtained from RIKEN, Ibaraki, Japan) were cultured in Dulbecco’s modified Eagle’s medium (Sigma) supplemented with 5% (v/v) heat-inactivated fetal calf serum (BioWest, Nuaillé, France), 100 U/mL of penicillin G (Invitrogen Japan, Tokyo, Japan), 100 µg/mL of streptomycin (Invitrogen), and 0.25 µg/mL of amphotericin B (Invitrogen) in 35-mm plastic dishes in the presence of 5% CO2 at 37°C until semi-confluent. Cells were pretreated with UDCA for 30 min and treated with the indicated concentrations of palmitic acid. After the cells were incubated in serum-free medium for 3 h, the cells were treated with 1 ng/mL of insulin for 15 min. UDCA was dissolved in dimethylsulfoxide (dimethyl sulfoxide (DMSO), Nacalai Tesque) and subsequently added to the culture medium. The concentration of DMSO was adjusted to 0.1% (v/v) of the culture medium for each group.
Determination of Intracellular ROSThe ROS levels in the cells were quantified using 10 µM H2DCFDA as described previously.4) The fluorescence of DCF was measured at 485 nm/535 nm (excitation/emission) using ARVO MX multilabel reader (PerkinElmer, Inc., MA, U.S.A.).
Western Blotting of Akt and Phosphorylated AktAs previously reported,7) HepG2 cells were lysed in a buffer containing 20 mM Tris–HCl (pH 7.4), 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM sodium orthovanadate (Sigma), 0.5 mM phenylmethylsulfonylfluoride (Sigma), 0.05% Protease Inhibitor Cocktail (Sigma), and 0.1% Triton-X 100 (Sigma). The lysate was spun down at 14000×g, and the resulting supernatant fractions were used for Western blot analysis using anti-Akt (Cell Signaling Technology Japan, Tokyo, Japan) and anti-phospho-Akt (Ser-473, Cell Signaling) antibody. Horseradish-labeled goat anti-rabbit immunoglobulin G (IgG) antibody (Cell Signaling) was used as a secondary antibody. Specific immunoreactive bands were detected using Immobilon Western Chemiluminescent HRP Substrate (Merck Millipore Japan, Tokyo, Japan) and a luminescence imager (Light-Capture II, ATTO, Tokyo, Japan). Densitometric analysis was conducted using Image J for Windows by National Institutes of Health (Boston, MD, U.S.A.). The cellular protein concentration was determined using a DC protein assay kit (Bio-Rad Laboratories, Hercules, CA, U.S.A.) with bovine albumin as a standard.
Statistics and Data AnalysisData are expressed as the mean±standard deviation (S.D.) Statistical significance was determined by one-way ANOVA test followed by Holm’s multiple-comparison test using R Commander Plug-in for the EZR (Easy R) Package (RcmdrPlugin.EZR).12) p<0.05 was considered significant.
FFAs have been reported to generate ROS in various cells including adipocytes13) and pancreatic islets cells.14) As shown in Fig. 1A, the intracellular ROS levels increased with the FFA levels in HepG2 cells.

(A) HepG2 cells were incubated in the presence or absence of Pal for 24 h. The intracellular ROS level was then determined using the fluorogenic dye H2DCFDA. Fluorescence intensity was evaluated as a relative value to the amount of intracellular proteins. (B) HepG2 cells were pre-incubated with or without 100 µM UDCA and then cultivated with 0.7 mM Pal in the presence or absence of 100 µM UDCA for 24 h. After the cells were further incubated in serum-free medium for 3 h, the cells were treated with insulin (1 ng/mL) for 15 min. Column graph data are expressed as the mean±S.D. (n=3). * p<0.05, compared with the control. # p<0.05, compared with the Pal group. Statistical significance was determined by one-way ANOVA followed by Holm’s multiple-comparison test.
In Fig. 1B, the effects of UDCA and insulin on the palmitate-induced ROS production were investigated. Although insulin (1 ng/mL) and UDCA (100 µM) alone did not decrease ROS production, the combined treatment decreased the ROS level. In addition, the combined treatment of UDCA and insulin partially inhibited palmitate-induced ROS production. However, both insulin and UDCA did not show inhibitory effects against palmitate-induced ROS.
Effects of UDCA and Insulin on the Phosphorylation of Akt in HepG2 Cells Treated with PalmitateSince Akt is a mediator of insulin signaling in hepatocytes, the effects of UDCA and insulin on the phosphorylation of Akt were investigated (Fig. 2). Both insulin (1 ng/mL) and UDCA increased Akt phosphorylation. Pretreatment of cells with 0.7 mM palmitate markedly inhibited Akt phosphorylation induced by insulin or UDCA. When both UDCA (100 µM) and insulin (1 ng/mL) were added, the palmitate-induced inhibition of Akt phosphorylation was significantly suppressed.
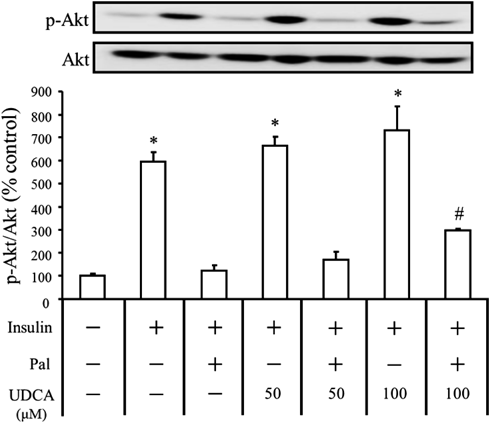
HepG2 cells were pre-incubated with or without 50 or 100 µM UDCA and then cultivated with 0.7 mM Pal in the presence or absence of UDCA for 24 h. After the cells were further incubated in serum-free medium for 3 h, the cells were treated with insulin (1 ng/mL) for 15 min. Whole cell lysates were used for Western blot analysis of phosphorylated Akt (p-Akt) and Akt. The image shown is typical of the three independent experiments. Column graph data are expressed as the mean±S.D. (n=3). * p<0.05, compared with the untreated control group. # p<0.05, compared with the Pal and insulin group. Statistical significance was determined by one-way ANOVA followed by Holm’s multiple-comparison test.
In addition to contributing to FFA-induced insulin resistance, oxidative stress may also have an important role in decreasing insulin responsiveness.15,16) Recently, it has been shown that chronic exposure to elevated concentrations of fatty acids is cytotoxic to several cell types through various mechanisms of which oxidative stress is a common cause in cell dysfunction.17,18) Increased FFA metabolism may also lead to increased ROS production and has been shown in adipocytes and pancreatic β-cells.19,20)
In this study, we showed that the combined treatment of UDCA and insulin decreased intracellular ROS production. In addition, palmitate-induced ROS production was suppressed by the treatment with UDCA and insulin in HepG2 cells. These results suggest that UDCA acts with insulin to enhance the protective effects against palmitate-induced ROS production. In regards to UDCA, we previously indicated its effectiveness against excessive iron- and doxorubicin-induced ROS.7,21) The results in this study indicate that UDCA alone is insufficient for FFA-induced ROS.
Insulin decreases oxidative stress via cellular signaling systems and is dependent on the activation of PI3K and extracellular signal-regulated protein kinase in HepG2 cells.8) Nakamura et al.4) reported that palmitate impaired insulin-induced tyrosine phosphorylation of IRS-2, serine phosphorylation of Akt, and serine phosphorylation of GSK-3α through mitochondrial β-oxidation. Furthermore, Gao et al.22) recently showed that the effects of palmitate on hepatic insulin resistance were mediated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 3-derived ROS, which is induced by the phosphorylation of c-Jun N terminal kinase (JNK) and p38 followed by the suppression of Akt phosphorylation.
In this study, we found that UDCA restored the palmitate-induced low responsiveness of Akt phosphorylation to insulin. UDCA has been shown to activate the PI3K/Akt signaling pathway and induce the translocation of Nrf2 into the nucleus. Through these activation systems, UDCA increases the gene expression of proteins associated with GSH synthesis and down-regulates the intracellular level of ROS.7) Therefore, UDCA may be involved in a protective mechanism against palmitate-induced ROS production; however, its combined stimulatory effects with insulin on PI3K/Akt signaling are weakened by palmitate.
Bile acids influence lipid and glucose metabolism through FXR and TGR59) and are gaining attention as novel drug candidates.23) Our results may support the theoretical basis for the role of bile acids in controlling diabetic conditions.
In conclusion, UDCA may have protective effects against palmitate-induced ROS production and helps to restore insulin sensitivity.
We would like to thank Mr. Keisuke Fujita for his technical assistance.
The authors declare no conflict of interest.