2017 年 40 巻 2 号 p. 187-194
2017 年 40 巻 2 号 p. 187-194
The current study evaluated the effects of Xiao Yao San (XYS) on anxiety-like behaviors and sought to determine whether the c-Jun N-terminal kinase (JNK) signaling pathway is involved. A total of 40 rats were divided into 5 groups (n=8): the control group (deionized water, per os (p.o.)), the model group (deionized water, p.o.), the SP600125 group (surgery), the per se group (surgery), and the XYS group (3.9 g/kg/d, p.o.). A 1% dimethyl sulfoxide (DMSO) citrate buffer solution (2 µL/ventricle/d) and SP600125 (10 µg/ventricle, 2 µL/ventricle/d) were separately and bilaterally injected into the rats of the two surgery groups via the ventricular system of the brain. All but the control group underwent 14 d of chronic immobilization stress (CIS; 3 h/d). On day 15, the body weights of all of the rats were measured; additionally, the rats were subjected to the elevated plus maze (EPM) and novelty suppressed feeding (NSF) tests. Finally, JNK signaling pathway indices, including phosphorylated JNK (P-JNK), JNK, phosphorylated c-Jun (P-c-Jun) and cytochrome C (Cyt-C), were examined. After modeling, the body weight and behavioral analyses of the model rats indicated that this modeling method induced anxiety-like behaviors. P-JNK, JNK, and P-c-Jun were altered in the hippocampus of the model rats. After 14 d of treatment with XYS and SP600125, rat body weight and behaviors as well as P-JNK, JNK, and P-c-Jun had changed. However, no significant difference in Cyt-C was found. XYS improves the anxiety-like behaviors induced by CIS, which might be related to the JNK signaling pathway in the hippocampus.
Xiao Yao San is a well-known traditional Chinese medicine (TCM) formula that has been most frequently used to treat anxiety and depression1) by smoothing the liver, strengthening the spleen and nourishing the blood. The combination of Xiao Yao San (XYS), acupuncture and moxibustion significantly relieves the anxiety and depression of patients following test-tube fertilization and placenta transplantation.2) A mixture of modified XYS and Kunbao pill improves the negative feelings of perimenopausal syndrome.3) In a previous study, our team revealed that XYS produces anxiolytic effects in rats exposed to 14 d of chronic immobilization stress (CIS).4)
Mitogen-activated protein kinase (MAPK) exists in the cytoplasm of most eukaryotes, and it is involved in various physiological and pathological processes. c-Jun N-terminal kinase (JNK), which is a stress-activated protein kinase, is a member of the MAPK family. Many stressors can trigger JNK/MAPK, which can damage organ cells and even cause apoptosis.5–7) The JNK signaling pathway is summarized as stress-germinal center kinase-JNK-apoptosis.
Previous research has reported that 21 d of chronic unpredictable stress (CUS) induces a rat model of depression. According to this model, phosphorylated cAMP response element binding protein (PCREB), phosphorylated JNK (P-JNK) protein expression, PCREB/camp response element binding protein (CREB) and P-JNK/JNK significantly decreased in the hippocampus. The JNK signaling pathway plays a key role after 21 d of a CUS rat model of depression.8) In addition, many similarities exist between anxiety and depression.9)
The anxiolytic effects of XYS and the role that the JNK signaling pathway plays in chronic stress raises two questions: Does the JNK signaling pathway contribute to anxiety-like behavior in rats? Do the anxiolytic-like effects of XYS involve the JNK signaling pathway? This study attempts to answer these questions by evaluating rat behaviors and testing various JNK signaling pathway indices.
A total of 40 male Sprague Dawley (SD) rats weighing 210–230 g (SCXK(Jing)2012-0001) were housed under standard laboratory conditions at 22±2°C with a relative humidity of 30–40% and a 12-h/12-h dark/light cycle with food and water freely accessible. The rats were divided into 5 groups, including the control group, model group, SP600125 group, per se group and XYS group (8 rats per group). The animal ethics committee of Beijing University of Chinese Medicine approved this study.
Drugs and ChemicalsThe extract power of XYS was prepared from the mixture of the following crude drugs: 300 g Poria (the fungus nucleus of Poria cocos [SCHW.] WOLF), 300 g Radix Paconiae Alba (the root of Paeonia lactiflora PALL.), 150 g Radix Glycyrrhizae (the root and rhizome of Glycyrrhiza uralensis FISCH.), 300 g Radix Bupleuri (the root of Bupleurum chinense DC.), 300 g Radix Angelicae Sinensis (the root of Angelica sinensis [OLIV.] DIELS), 300 g Rhizoma Atractylodis Macrocephalae (the rhizome of Atractylodes macrocephala KOIDZ.), 100 g Herba Menthae (the aboveground elements of Mentha haplocalyx BRIQ.), and 100 g Zingiberis Rhizoma Recens (the rhizome of Zingiber officinale ROSE.). These eight medicines were purchased from Beijing Tongrentang Co., Ltd. (Beijing, China) and then processed in the Chinese medicine preparation room of the China-Japan Friendship Hospital (Beijing, China). Definite authentication and extraction procedures were described in our previous articles.4,10)
XYS, dissolved in deionized water, was administered via gavage at a daily dosage of 3.9 g/kg/d in a solution of 10 mL/kg body weight. Dosage of XYS (3.9 g/kg/d) for rats was calculated as below: 6.25 (convert coefficient)×185 g crude medicines (normal human daily dosage)÷60 kg (normal human body weight)×20% extraction rate (actual dry power/actual crude medicines).
SP600125 and 1% dimethyl sulfoxide (DMSO) were purchased from Sigma Co., LLC (St. Louis, U.S.A.).
MethodologiesIntracerebroventricular InfusionAfter 7 d of adaptive feeding, an intracerebroventricular catheter was inserted into the rats of the per se and SP600125 groups. The rats were anaesthetized with pentobarbital sodium (130 mg/kg, intraperitoneally (i.p.)) to ensure that they were calm during this process. The head of the anaesthetized rats was placed on the stereotaxic apparatus (Product type: 39463001, Benchmark, Clute, TX, U.S.A.), and cannulae were implanted into the lateral ventricle based on The Rat Brain in Stereotaxic Coordinates by PAXINOS and WATSON using the following coordinates: 0.8 mm posterior to the bregma; 1.5 mm lateral to the saggital suture; and 4.5 mm ventral from the surface of the brain. Rats were allowed 7 d to recover after the operation.
Experimental Protocol (Fig. 1)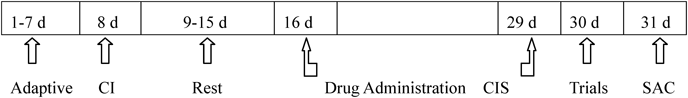
Days 1–7: All rats adapted to their surroundings; day 8, cannula implantation (CI); days 9–15: all of the rats were allowed to recover; days 16–29: XYS group rats were intragastrically administered XYS (3.9 g/kg/d), and all of the other rats were intragastrically administered deionized water (10 mL/kg/d); in addition, the SP600125 and per se rats were given SP600125 (10 µg/2 µL/ventricle/d) and a citrate buffer solution (2 µL/ventricle/d) intracerebroventrically, respectively; day 30: all of the rats underwent behavioral trials, including the EPM and NSF tests; day 31, the rats were sacrificed (SAC).
Days 1 to 7: All rats showed adaptive feeding;
Day 8: The per se and SP600125 group rats underwent the intracerebroventricular catheter procedure;
Days 9 to 15: All rats were allowed a 7-d recovery period, and one rat in the per se group died of a post-operation infection;
Days 16 to 29: All rats except those in the control group received 3 h of continuous immobilization stress each day, and one rat each in the model group, SP600125 group and XYS group died due to immobilization stress;
Day 30: The body weights of the rats were measured, and their behaviors were recorded and assessed;
Day 31: All of the rats were sacrificed to remove the hippocampus for additional testing.
Control group: Deionized water was intragastrically administered 10 mL/kg/d from days 16 to 29;
Model group: Deionized water was intragastrically administered 10 mL/kg/d from days 16 to 29;
SP600125 group: SP600125 dissolved in a 1% DMSO citrate buffer solution was injected via an intracerebroventricular catheter at a dosage of 10 µg/2 µL/ventricle/d, and deionized water was intragastrically administered 10 mL/kg/d from days 16 to 29;
Per se group: a 1% DMSO citrate buffer solution was injected via an intracerebroventricular catheter at a dosage of 2 µL/ventricle/d, and deionized water was intragastrically administered 10 mL/kg/d from days 16 to 29;
XYS group: XYS was dissolved in deionized water and intragastrically administered 3.9 g/kg/d from days 16 to 29.
Rats ModelAll of the rats except those in the control group were restrained on a bound frame 3 h per day for 14 continuous days to establish an anxiety model with liver Qi stagnation and spleen deficiency syndrome.11)
Behavioral AssessmentGeneral StatusHair color, gestures, movements, auricle colors, responses to immobilization stress, bowel movements, and other behavioral characteristics were observed daily.
Body WeightBody weight was measured before the experiments and once a week after modeling was established.
Elevated Plus Maze (EPM) TestThe EPM test consists of two open arms and closed arms resembling a crossing 50 cm off the ground. A camera was placed over the central zone of the maze to record the movement of the rats. Window shades were hung to avoid reflection. The surroundings remained quiet, and the rats were sent into the cabinet 1 h ahead for adaptation. When the test began, the rats were released into the central zone, and their heads were pointed toward one closed arm. Then, their movements were recorded for 5 min, including their total movement, closed arm entry times, retention latency, open arm entry times, retention latency, and so on. After each rat was finished, the maze was cleaned with a low-concentration alcohol and allowed to dry before the next trial began.
Novelty Suppressed Feeding (NSF) TestAll rats were deprived of food for 24 h before the test. An NSF apparatus of 50×40×40 cm was created, and a piece of food was placed at the center of the apparatus. All of the rats were placed in the test room to adapt to the new environment. One rat was placed in the apparatus at one corner until it began to bite the food; then the next rat was placed. Latencies were recorded for up to 5 min.
Biochemical Parameters EstimationP-JNK, JNK, Phosphorylated c-Jun (P-c-Jun), and Cytochrome C (Cyt-C) Protein Expression According to a Western BlotThe protein extraction reagent was cooled on ice, and the protease inhibitor mixture was added at a ratio of 1 : 99. The hippocampus tissue was weighed and added to the protein extraction reagent at a ratio of 1 : 10 (g : mL) to grind on ice. The sample was then processed in a centrifuge. Supernatants were pumped into a 1.5-mL EP tube for use. Bicinchoninic acid (BCA) was prepared to calculate the protein density. Then, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transfer to membrane, antibody addition, incubation, immunity response, coloration, computer scanning and image formation were implemented in that order.
JNK, c-Jun, and Cyt-C Gene Expression According to Quantitative Real-Time (qRT)-PCRRNA was first extracted using an RNeasy Plus Mini Kit from QIAGEN (Duesseldorf, Germany). The extracted RNA then underwent reverse transcription using a RevertAid First Strand cDNA Synthesis Kit from Thermo Fisher (Waltham, MA, U.S.A.). The RNA was then amplified using primers (Table 1) and Power SYBR Green PCR Master Mix in a PCR instrument. Last, the results were saved and tested.
| Genome | Primer | Annealing temperature (°C) |
|---|---|---|
| GAPDH | F:5′-CAAGGTCATCCATGACAACTTTG-3′ | 55 |
| R:5′-GTCCACCACCCTGTTGCTGTAG-3′ | ||
| JNK | F:5′-TCTCAGCATCCGTCGTCTTC-3′ | 55 |
| R:5′-TCTACAGCAGCCCAGAGGTC-3′ | ||
| c-Jun | F:5′-CGCACGCTCCTAAACAAACT-3′ | 55 |
| R:5′-GAACAGTCGGTCACTTCACG-3′ | ||
| Cyt-C | F:5′-CCAGGGATGAGAAAGTCCAA-3′ | 50 |
| R:5′-CAGTGAAGCCGATGAAGAAC-3′ |
All numerical data were expressed as the mean±standard deviations (S.D.) X̄±S. SPSS 17.0 was used for analysis, and p<0.05 was considered significant. A one-way ANOVA was used to analyze differences among the groups, followed by Bonferroni’s multiple comparison tests.
On the 30th day of the experiment (i.e., the 15th day of modeling), the model and per se group rats were restless and had dry hair. They tended to curl up in a corner, move slowly, and move only short distances. They were irritable and resisted fiercely to the restraint stress. Their ears became pale, and their bowel movements became loose. In contrast, the control, XYS and SP600125 rats had tidy and bright hair, moved quickly, resisted stress, and rarely broke off. The rats in these three groups showed light-red ears and normal bowel movements.
Body Weight AssessmentThe rats were randomly divided into 5 groups based on body weight before the experiments. On the 22nd day of the experiment, no significant differences were found with regard to body weight among the 5 groups (p>0.05); on the 29th day, the body weights of the model and per se groups were significantly reduced compared with those of the other groups (p<0.05; Fig. 2).
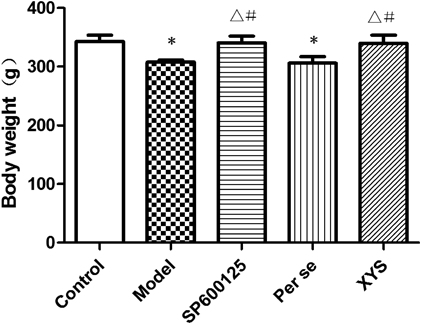
No significant differences were found among the 5 groups before the experiment or on the 22nd day of the experiment. On the 29th day, the body weights of the model and per se rats showed a significant decrease compared with those of the control group (p<0.05); no significant differences were found among the control, SP600125 and XYS groups (p>0.05). Data are expressed as the mean±S.D. (X̄±S), n=8 per group. * p<0.05 versus control, △ p<0.05 versus model, # p<0.05 versus per se.
EPM was used to evaluate the anxiety status induced by CIS in the rats of the 5 groups. The model and per se rats showed less total movement distance (a) than those in the control group (p<0.05). No significant differences were found in total movement distance among the control, SP600125 and XYS groups (p>0.05), although these rats showed more total movement distance than those in the model and per se groups (p<0.05). The open arms retention latency (b) was briefer in the model and per se groups than in the control group (p<0.05), whereas the SP600125 and XYS groups demonstrated longer latencies than the model and per se groups (p<0.05). The rats in the model and per se groups entered the central zone fewer times (c) than the rats in the control group (p<0.05 and p<0.01, respectively); however, no significant differences were observed among the control, SP600125 and XYS groups (p>0.05). The rats of per se group entered the closed arms fewer times (d) than the rats in the control and SP600125 groups (p<0.05), but no significant differences were found among the control, model, SP600125 and XYS groups (p>0.05; Fig. 3). In addition, closed arms retention latency, open arms entry times, central zone movement distance showed no differences among 5 groups.
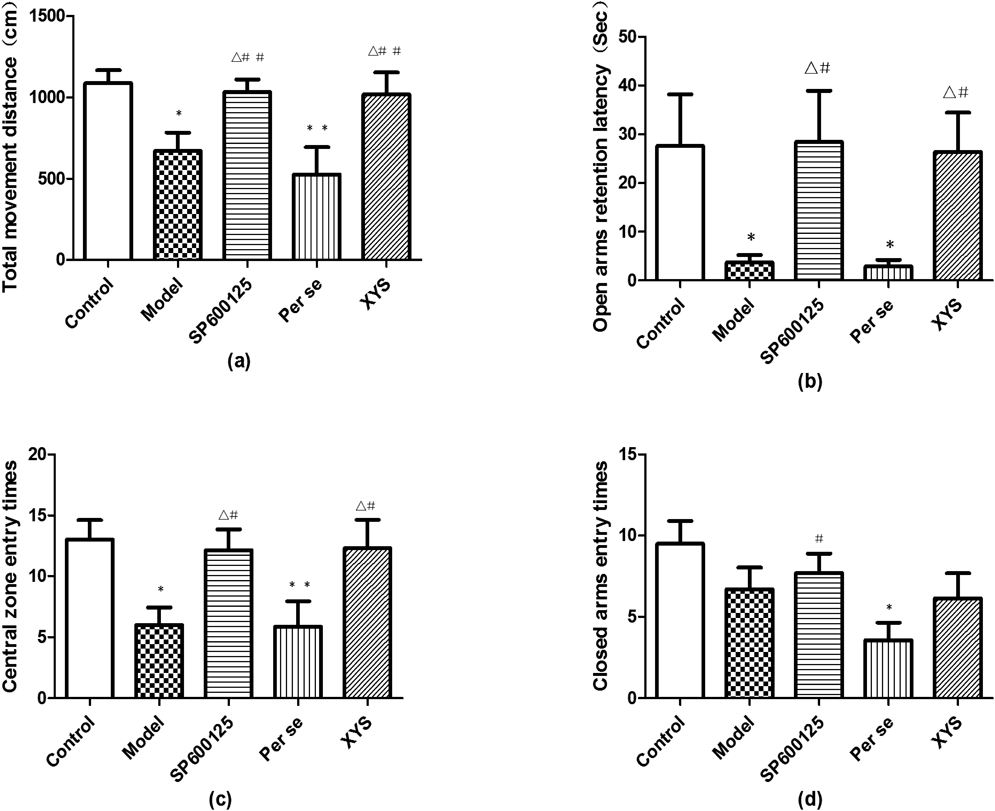
On the 30th day, the 5 groups of rats underwent behavioral observations after 2 weeks of CIS. (a) Total movement distance; (b) Open arms retention latency; (c) Central zone entry times; (d) Closed arms entry times. Data are expressed as the mean±S.D. (X̄±S), n=8 per group. * p<0.05, ** p<0.01 versus control; △ p<0.05 versus model, # p<0.05, ## p<0.01 versus per se.
On the 30th day, the NSF latencies of the model and per se groups were longer than that of the control group (p<0.05); no significant differences were found among the control, SP600125 and XYS groups (p>0.05; Fig. 4).
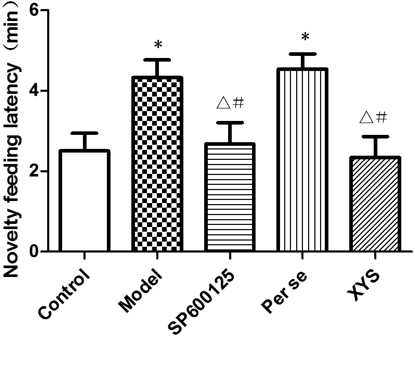
NSF latencies were recorded after 2 weeks of CIS in the 5 groups of rats. Data are expressed as the mean±S.D. (X̄±S), n=8 per group. * p<0.05 versus control; △ p<0.05 versus model, # p<0.05 versus per se.
The P-JNK, JNK and P-c-Jun protein expression levels of the model and per se groups were higher than those of the control group (p<0.05 and p<0.01, respectively); however, the expression levels of the SP600125 and XYS groups were lower than those of the model and per se groups (p<0.05 and p<0.01, respectively). No differences were found with regard to the Cyt-C protein expression among the 5 groups (Figs. 5, 6).
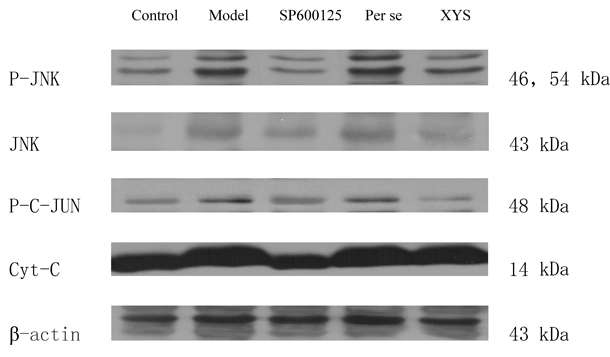
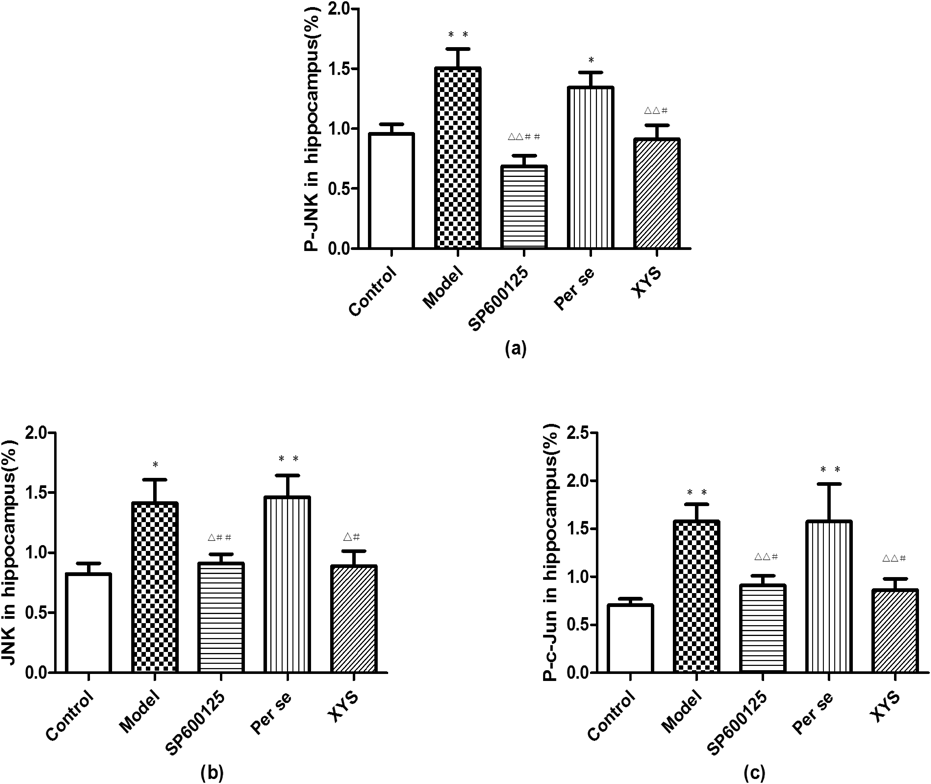
Data are expressed as the mean±S.D. (X̄±S), n=8 per group. * p<0.05 versus control, ** p<0.01 versus control; △ p<0.05 versus model, △△ p<0.01 versus model; # p<0.05 versus per se, ## p<0.01 versus per se.
The JNK and c-Jun gene expression levels in the model and per se groups increased compared with those in the control, SP600125 and XYS groups (p<0.05 and p<0.01, respectively), and no differences were among them (p>0.05). Cyt-C gene expression did not differ among the 5 groups (p>0.05; Fig. 7).
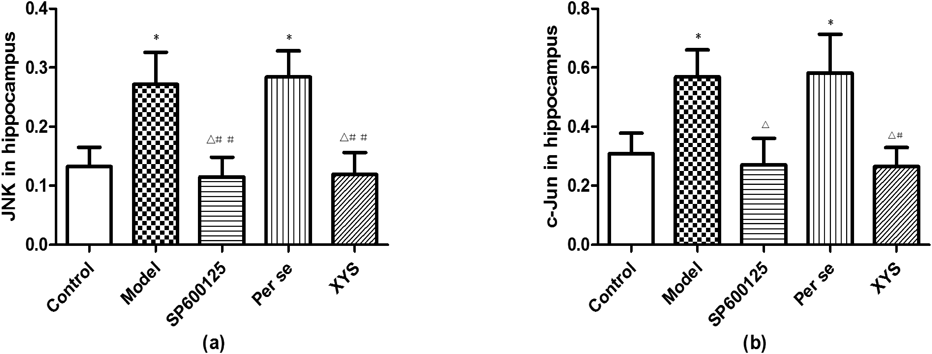
(a and b) The gene expression levels of JNK, c-Jun in the hippocampus were measured using qRT-PCR. Data are expressed as the mean±S.D. (X̄±S), n=8 per group. * p<0.05 versus control; △ p<0.05 versus model; # p<0.05 versus per se, ## p<0.01 versus per se.
Anxiety was not recorded as a malady in the classic texts of TCM. However, it might be related to the TCM concepts of pavor, palpitation and insomnia. The most common syndromes are liver Qi stagnation and liver Qi transforming into fire.12) XYS has definite clinical effects on anxiety; however, the mechanism through which this effect occurs remains unclear.
Establishing a Rat Model of AnxietyThe best method of establishing a rat model of anxiety is controversial. Currently, several established methods have been developed, including the uncertainty empty bottle,13) Vogel’s drinking conflict, the predator-exposure test,14) and others. However, these aforementioned models involve uncertainties and disturbed factors. The 21-d stress depression model in rats is well recognized. Anxiety and depression frequently co-occur, and even anxiety can contribute to depression.15) Therefore, additional discussion is needed regarding short-time stress inducing anxiety-like behavior.
After experiencing immobilization stress for 14 d, the model rats were restless and irritable; these effects are likely related to liver Qi stagnation and spleen deficiency syndrome in TCM.
According to the EPM test, the total movement distance, open arm retention latency and central zone entry times were reduced in the model group rats compared with the control group rats. These outcomes suggest that the model rats showed reduced exploratory behaviors and were in a pathological escape status.16) Interestingly, pathological escape is the core characteristic of most patients with anxiety.
The NSF test was used to evaluate anxiety based on the contrast between the fear of a novel environment and the desire to obtain food. After fasting for 24 h, all of the rats were likely extremely hungry. After they were placed into the box with food, they had to overcome their fear to acquire the food. The model rats demonstrated a longer feeding latency than the control group, which indicated that these rats were hyper-responsive to the new environment,17,18) which hindered their normal activity.
In conclusion, 14 d of CIS induced rat anxiety and established a model of anxiety. These results are consistent with those of a previous experiment.4)
Anxiety and the Hippocampus, JNK and StressAs a part of the cerebral limbic system, the hippocampus is the center of emotion and mood. A close connection was found between anxiety and the hippocampus using nuclear magnetic resonance imaging (MRI) in a clinical setting.19) In a rat experiment, the ventral hippocampal-medial prefrontal pathway, the GABAA receptor, and Galanin receptor 2 played a key role in anxiety.20–23)
The MAPK signaling pathway is involved in many pathological processes. JNK is part of this pathway and plays important roles in cell apoptosis and neurosystemic diseases. An immobilization stress of 30 d influences JNK expression in the prefrontal cortex.24) JNK phosphorylation was tested in the hippocampus of depression model rats; JNK phosphorylation accumulated in damaged neuron regions, accelerated the process of damage, and eventually led to neuronal apoptosis.25–27)
SP600125 as ContrastThe JNK signaling pathway blocker, SP600125, is a highly selective inhibitor. It inhibits c-Jun phosphorylation, decreases X-ray repair complementing defective repair in Chinese hamster cells 1 (XRCC1) downregulation, restores DNA function, reduces lactate dehydrogenase (LDH) expression, and reduces neuronal apoptosis.28–30) It also alleviates damage to the CA1 region of the hippocampus to protect neurons.31) In our experiment, SP600125 was used as a contrast to explore the possible connection between the JNK signaling pathway and stress due to chronic immobilization.
After immobilization stress for 14 d, the status of the rats in the SP600125 group had improved, and they had normal body weights as well as continued to demonstrate explorative interest in new environments. This behavior was likely because the rats were less anxious and more adaptive.
To avoid the interference of enterohepatic circulation and the blood brain barrier, SP600125 was injected into the lateral ventricle to act on the hippocampus. In addition, the per se group was established to exclude the influences of operation, DMSO, citrate buffer solution, and other factors. In comparing the SP600125 group with the control, model and per se groups, we found that the operation and solvents did not affect the body weight, behavior or cytokine levels in the JNK signaling pathway. In addition, no reports of lateral ventricle injection interference were found in previous experimental results.30,32)
In the current experiment, SP600125 inhibited JNK, P-JNK, and P-c-Jun expression levels in the hippocampus of rats, which blocked the JNK signaling pathway.
Regulating Effects of XYS on Behaviors of Anxiety Model RatsXYS is able to smooth the liver Qi and strengthen the spleen and is used to relieve anxiety in the clinical setting. In the experiment, the rats of the XYS group demonstrated an improved status, had faster growth in body weight, and showed significantly improved behaviors compared with those of the model group.
Most research has reported that the mechanism of XYS is related to lowering the excitability of the sympathetic nervous system,2) increasing insulin sensitivity, providing neuroprotection to glial cells, and activating cerebral 5-HT1A receptors.33) Moreover, the mechanism may be related to the mediation of neurosteroid synthesis,34) the modulation of the locus coeruleus-norepinephrine system,35) and the serum levels of 5-HT, brain derived neurotrophic factor (BDNF), CORT and interleukin-6.36)
Furthermore, some components of XYS are effective treatments for chronic stress; for example, curcumin reversed BDNF expression and phosphorylation of CREB,37) Radix Angelicae Sinensis blocked apoptosis in cardiomyoblast cells,38) and paeoniflorin inhibited MAPKs/nuclear factor-kappaB (NF-κB)-mediated inflammatory responses to protect the brain.39)
Our previous research showed that XYS relieved CIS by regulating enkephalin mRNA and prodynorphin mRNA gene expression in the rat hippocampus.40)
Regulating Effects of XYS on JNK Signaling PathwayIn recent years, most research on Chinese medicines and the JNK signaling pathway has almost covered all types of organism systems. JNK has attracted more attention, and the extracts of some Chinese medicines were found to regulate relative proteins and kinases in the JNK signaling pathway of the hippocampus and inhibit the apoptosis of hippocampal cells to protect neurons.41–43)
Compared with the control group, the P-JNK, JNK, and P-c-Jun protein and gene expression levels increased in the model and per se groups according to Western blot and qRT-PCR methods, and no differences were found among the XYS, SP600125 and control groups.
XYS downregulates indoleamine 2,3-dioxygenase (IDO)44) and corticotropin-releasing hormone (CRH) receptor 2 and upregulates BDNF/tropomyosin receptor kinase B (TrkB)45) in the hippocampus to suppress the JNK signaling pathway. In addition, XYS directly downregulates c-Fos and c-Jun expression levels in the hippocampus,46) decreases neuron apoptosis, and protects neurons.
Cyt-C did not significantly differ among the 5 groups, although a trend was observed in which the levels of the model and per se groups were slightly higher than those in the other groups. Although Cyt-C is a key index of the mitochondrial activity of the JNK signaling pathway, it cannot be used to show that the mechanism of immobilization stress over 14 d originates in the mitochondria of the JNK signaling pathway.
XYS blocked JNK activation and suppressed the JNK signaling pathway to reverse deterioration. The current study found that XYS improved the anxiety-like behaviors of rats, and the JNK signaling pathway and the related cytokines were most likely involved.
Because the sample sizes of the rats in this study were small and only a few indices of the JNK signaling pathway were selected, additional experiments with larger sample sizes are needed to explore more indices of the JNK signaling pathway.
XYS improved anxiety-like behaviors in rats and likely inhibited the JNK signaling pathway in the hippocampus. These findings add to our knowledge of the mechanism of the effects of XYS on anxiety. This information will be valuable in future studies.
The National Natural Science Foundation of China (No. 81473597, 81630104), the China National Funds for Distinguished Young Scientists (No. 30825046), and the Beijing Municipal Natural Science Foundation (No. 7152093) supported this work.
The authors declare no conflict of interest.