2017 年 40 巻 5 号 p. 630-637
2017 年 40 巻 5 号 p. 630-637
The aim of the present study was to examine the inhibitory roles and mechanisms of hirsutenone (HTN) in the regulation of osteoclastogenesis. Gene levels were compared to assure the effects of HTN on osteoclastogenesis in mouse splenocytes/CD4+ T cells, mouse macrophage-like cell line RAW264.7 (preosteoclast), MG63 (osteoblast), and RPMI1788 (B cell) cells. The mechanism by which HTN regulates the degradation of tumor necrosis factor receptor-associated factor 6 (TRAF6) and inhibits inhibitor of kappaB (IκB) and nuclear factor-kappaB (NF-κB) signaling was examined by Western blotting and luciferase reporter assays. Our results demonstrated that HTN effectively downregulated the expression of interferon γ (IFNγ), interleukin-22 (IL-22), IL-1β, and tartrate-resistant acid phosphatase (TRAP) in splenocyte-/CD4+-RAW264.7 co-culture system. Moreover, receptor activator of nuclear factor-κB ligand (RANKL) and CD25 expression were also significantly inhibited in MG63 and CD4+ single culture system, suggesting an additional independent effect of HTN on osteoclastogenesis. Notably, TRAF6 was markedly degraded along with a decrease in nuclear factor of activated T-cells (NFATc) and NF-κB activities in RAW264.7 cells. Finally, we concluded that HTN directly or indirectly inhibits osteoclastogenesis via the inhibition of NF-κB signaling by promoting TRAF6 degradation, and plays a crucial role in suppressing the expression of RANKL and cytokines expressed in IFNγ-producing T-helper 1 (Th1) cells. These findings suggest that HTN may be a promising therapeutic candidate for diseases resulting from bone loss.
Bone serves as an ion reservoir for the body, as well as the location of several specialized bone cells, such as osteoblasts, osteocytes, and osteoclasts, which reside within this complex tissue.1,2) Bone loss, which may induce an imbalance between bone formation and resorption, as a result of aging and after menopause, can lead to osteoporosis, which is characterized by reduced bone mass, deterioration of bone architecture, and an increased risk of fatal bone fractures.3,4) Osteoclasts (OC), the bone-resorbing cells generated from the same precursors as monocyte/macrophages, crosstalk with activated T cells to form activated osteoclasts directly by producing interleukin-7 (IL-7) and receptor activator of nuclear factor-κB ligand (RANKL), and indirectly by promoting the production of RANKL by osteoblasts and fibroblasts via the production of pro-inflammatory cytokines (i.e., IL-1, -6, -17, and interferon (IFN)-γ).5,6) Of these, interleukin-1β (IL-1β), one of the most well-known proinflammatory cytokines, has been reported as an effective stimulator of bone resorption through the upregulation of RANKL.7) In addition, IL-22 produced by activated T cells has been shown to promote osteoclastogenesis, leading to bone erosion in rheumatoid arthritis.8)
As the binding of RANKL to its receptor on the cell surface of osteoclasts and preosteoclasts increases the formation, activity, and survival of osteoclasts, the monoclonal antibody against RANKL has emerged as a promising new candidate for the treatment of osteoporosis.9,10) In addition, an increasing body of evidence has focused on activated T cell-derived IFNγ for the induction of osteoclastogenesis, although IFNγ has also been shown to have a direct anti-osteoclastogenic effect via the suppression of RANKL signaling and the subsequent degradation of tumor necrosis factor receptor-associated factor 6 (TRAF6).11,12) In light of this, we previously reported that diarylheptanoid hirsutenone (HTN), 1,7-bis-(3,4-dihydroxyphenyl)-4-heptene-3-one and one of the major diarylheptanoids, isolated from the bark of Alnus japonica. Cumulative evidences suggest that HTN has bioactive properties as a diarylheptanoid and plays a key role in inducing tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-dependent apoptosis and in the inhibition of T cell-dependent cytokines, such as IL-2, 4, 5, 10, 13, and IFNγ.13–15) We further demonstrated that HTN inhibited IFNγ-related gene expression in mouse splenocytes. Therefore, in this study, we investigated whether HTN effectively inhibits osteoclastogenesis by blocking the expression of T cell-mediated cytokines via indirectly the inactivation of T cells or directly attenuating osteoclastogenesis, thus indicating potential therapeutic advantages for bone resorption.
The bark of A. japonica was collected at Mt. Chung-gei, Seoul, Korea in November 2002. Two and half kilograms of fresh bark was incubated for 72 h at room temperature with 80% aqueous acetone. After the removal of acetone under vacuum, the aqueous solution was filtered, concentrated, applied to a Sephadex LH-20 column (25–100 µm, 8×150 cm; Pharmacia, Uppsala, Sweden), and eluted with increasing concentrations of methanol (0–100%). Column chromatography of the target fraction was repeated using MCl-Gel CHP 20P (75–150 µm, 5×80 cm; Mitsubishi Chemical Co., Tokyo, Japan). Elution was performed with proportionally increasing gradients of methanol (40 to 80%). Finally, the eluate was applied to a column filled with YMC ODS-gel (s-75 µm, 5×60 cm; YMC Co., Ltd., Kyoto, Japan), and low-pressure liquid column chromatography was performed with increasing proportions of methanol (40 to 80%). The column was connected to a peristaltic pump (Miniplus 3; Gilson, Inc., Middleton, WI, U.S.A.) with a Gilson 112 UV/VIS detector (254/280 nm), and processed using the Gilson 740 ProTech System Controller Software program. From these procedures, we obtained optimally purified HTN (0.08 g, 0.0032% (w/w)) with >93% purity by HPLC16) (Fig. 1A). The lyophilized isolate was dissolved in dimethyl sulfoxide and then further diluted to the desired concentrations from the stock solution (50 mg/mL). The structure of the compound has been characterized by spectral analyses (Fig. 1).

BALB/c splenocytes were seeded in a 12-well culture plate, and treated with varying concentrations of anti-CD3 (1 µL/mL). Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, U.S.A.), according to the manufacturer’s instructions. cDNA was synthesized from total RNA via the reverse transcription of 1 µg of total RNA using the TOP script™ reverse transcription system (Enzynomics, Daejeon, Republic of Korea) and oligo dT primers in a total volume of 20 µL. The mouse sRANKL gene (GenBank Accession No. AF019048) was amplified using conventional PCR. PCR reactions were performed on a T-Gradient Thermoblock (Biometra, Göttingen, Germany) using the 2X ReddyMix PCR Master Mix (Thermo Fisher Scientific, Waltham, MA, U.S.A.) in 20 µL reaction. The PCR products were cloned into the pGEM® T vector (Promega, Madison, WI, U.S.A.), and sequenced.
Subcloning sRANKL cDNA into the pET32a Expression VectorsRANKL cDNA was subcloned into an expression plasmid (pET32a) to express the protein product in a prokaryote system. In brief, the sRANKL cDNA was PCR-amplified using an upstream primer and a downstream primer including NotI and XhoI at the beginning and the terminal sites of the open reading frame (ORF). Amplified DNA was subcloned into NotI and XhoI-digested pET32a to generate the recombinant plasmid vector using conventional methods. The subcloned DNA in the recombinant plasmid vector was confirmed by PCR and subsequent DNA sequencing.
Expression of sRANKL in BL21 (DE3)The expression vector containing sRANKL was transformed into Escherichia coli (BL21) and plated on a Luria–Bertani (LB) agar plate containing ampicillin (100 µg/mL), and incubated at 37°C overnight. Transformants were selected from the LB plates, inoculated them in liquid LB media with ampicillin (100 µg/mL), followed by incubation at 37°C overnight in a shaking incubator (200 rpm). Next, the cells were harvested at an OD600 of 1.0, at which point the cells were resuspended in LB medium and subsequently induced by adding varying concentrations of isopropyl-f-D-thiogalactopyranoside (IPTG) for up to 24 h. After the optimal induction, the lysed cell pellets were electrophoresed on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel to detect the target protein. The proteins were visualized after staining with 0.01% Coomassie Brilliant Blue R-250 (Sigma-Aldrich, St. Louis, MO, U.S.A.). The fusion protein was isolated using the MagneHis™ Protein Purification System (Promega), which selectively purifies polyhistidine-tagged proteins.
Cytotoxicity (Lactate Dehydrogenase) Assay and Transwell Co-culture SystemThe cytotoxicity induced by HTN was quantified by measuring lactate dehydrogenase (LDH) release. LDH content was determined using a commercial nonradioactive LDH assay kit, CytoTox 96 (Promega). The increase in the amount of formazan produced in the culture supernatant directly correlates with the increase in the number of lysed cells. The formazan was quantified spectrophotometrically by measuring its absorbance at 490 nm (SpectraMax 340, Molecular Devices, Sunnyvale, CA, U.S.A.). Cytotoxicity in experimental samples was determined as the percentage of LDH released in comparison to cells treated with 1% Triton X-100. Transwell co-culture was performed by using Transwell® Permeable Supports (COSTAR, Tewksbury, MA, U.S.A.). To avoid any carry-over effect between the cultures, stimulators (HTN or lipopolysaccharide (LPS)) were completely removed after the indicated periods of treatment, followed by replacing cells with fresh culture media prior to the main study. RAW264.7 cells were used as a preosteoclastic cell model which may differentiate into osteoclast-like cells in the presence of RANKL.17)
Visualization of Tartrate-Resistant Acid Phosphatase (TRAP)RAW264.7 cells were cultured with α-MEM containing sRANKL (5 µg/mL) and varying concentrations of HTN for 5 d, with a change of medium every 2 d. The cells were fixed with 4% paraformaldehyde and stained for TRAP using the Acid Phosphatase, Leukocyte (TRAP) staining kit (Sigma-Aldrich) according to the manufacturer’s instructions. Cells that were stained red were considered to be differentiated osteoclast-like cells along with multinucleated cells (≥3 nuclei).
Splenocyte Isolation from BALB/c MiceSpleens were aseptically isolated from BALB/c mice after sacrifice, and splenocytes were prepared by mechanical dissociation in cold phosphate buffered saline (PBS) at pH 7.2. Erythrocytes were depleted using a red blood cell lysis buffer containing ammonium chloride (eBioscience, San Diego, CA, U.S.A.). Then, splenocytes were cultured in RPMI1640 complete medium with 10% fetal bovine serum (FBS) and maintained at 37°C in a humidified atmosphere containing 5% CO2. The splenocytes were cultured at 1.5×105 cells/mL in a 96-well plate or 5×106 cells/mL in a 60 mm culture dish for gene expression or Western blot assays.
Splenocyte-Derived CD4+ T CellsCD4+ T cells were isolated from BALB/c splenocytes using a CD4+ T cell isolation kit (Miltenyi Biotec, Redwood, CA, U.S.A.). All procedures were performed with a MACS® separation column according to the manufacturer’s instructions. CD4+ T cells were cultured in RPMI1640 complete medium with 10% FBS and maintained at 37°C in a humidified atmosphere containing 5% CO2. CD4+ T cells were cultured at 2×106 cells/mL in 12-well plates for mRNA expression.
Quantitative Real-Time Polymerase Chain Reaction (qPCR) AssayTotal RNA extracts from cells were prepared using the Trizol method (Invitrogen). cDNA was synthesized from RNA by reverse transcribing 1 µg total RNA using the Improm-II Reverse Transcription System (Promega) and oligo dT primers in a total volume of 20 µL. PCR amplification was performed using the primers listed in Table 1 (Bioneer, Deajeon, Republic of Korea). qPCR reactions were run on a Rotor-Gene 6000 cycler (Corbett Research, Sydney, Australia) using the SensiMix™ SYBR Hi-ROX Kit (Bioline, London, U.K.) in a 20 µL reaction volume. Each real-time-PCR master mix contained 10 µL 2X enzyme master mix, 7 µL ribonuclease (RNase) free water, 1 µL of each primer (10 pM each), and 1 µL of diluted template. The PCR was performed with an initial pre-incubation step for 15 min at 95°C, followed by 45 cycles of 95°C for 15 s, annealing at 52°C for 15 s, and extension at 72°C for 10 s. A melting curve analysis was used to confirm the formation of the expected PCR products, and products from all assays were additionally visualized using 1.2% agarose gel electrophoresis to confirm correct lengths. An inter-run calibrator was used, and a standard curve was created for each gene to obtain PCR efficiencies. The relative expression levels of each sample were calculated using Rotor-Gene 6000 Series Software version 1.7, were expressed relative to β-actin, and were corrected for between-run variability. Data for the experimental samples are expressed as percentages of the internal control gene. The primers for the target genes are listed in Table 1 (Bioneer, Daejeon, Republic of Korea).
| Gene | Primers | Sequences | Product size (bp) | Accession No. | |
|---|---|---|---|---|---|
| Mouse | TRAP | Forward | 5′-GGACGTGTTCTCTGACCGTG-3′ | 207 | BC_029644 |
| Reverse | 5′-CAGCATCACTGTGTCCAGCA-3′ | ||||
| IL-17 | Forward | 5′-GTCACCCTGGACTCTCCACC-3′ | 173 | NM_010552 | |
| Reverse | 5′-CAGCTCTCAGGCTCCCTCTT-3′ | ||||
| IL-22 | Forward | 5′-CTCCCCCAGTCAGACAGGTT-3′ | 200 | AJ_249491 | |
| Reverse | 5′-AAACAGCAGGTCCAGTTCCC-3′ | ||||
| CD25 | Forward | 5′-TAGTACCCAGTTGTCGGGCA-3′ | 211 | BC_114437 | |
| Reverse | 5′-CGATTTGTCATGGGAGTTGC-3′ | ||||
| IFNγ | Forward | 5′-TGAAAATCCTGCAGAGCCAG-3′ | 169 | NM_008337 | |
| Reverse | 5′-TGGACCTGTGGGTTGTTGAC-3′ | ||||
| IL-1β | Forward | 5′-AGCTGTGGCAGCTACCTGTG-3′ | 522 | NM_008361 | |
| Reverse | 5′-GCTCTGCTTGTGAGGTGCTG-3′ | ||||
| β-Actin | Forward | 5′-TACAGCTTCACCACCACAGC-3′ | 205 | NM_007393 | |
| Reverse | 5′-AAGGAAGGCTGGAAAAGAGC-3′ | ||||
| Human | RANKL | Forward | 5′-TGGAGAGGAAATCAGCATCG-3′ | 208 | NM_003701.3 |
| Reverse | 5′-GGGGCCATGCCTCTTAGTAG-3′ | ||||
| β-Actin | Forward | 5′-AGAGCTACGAGCTGCCTGAC-3′ | 183 | NM_001101 | |
| Reverse | 5′-AGCACTGTGTTGGCGTACAG-3′ |
For reporter gene assays on the activity of NF-κB, subconfluent RAW264.7 cells were transfected with a luciferase reporter plasmid (0.1 µg/well, 96-well plate) containing multiple copies of the NF-kB consensus sequence in the pGL4.32[luc2P/NFkB-RE/Hygro] vector (Promega) encoding the firefly luciferase reporter gene (luc2P) using FuGENE Transfection Reagent (Promega) during the first 24 h in culture. Subsequently, the cells were exposed to LPS, HTN, or a combination of LPS and HTN for four hours, and cells were harvested using Glo Lysis Buffer (Promega). Firefly luciferase activities were measured, using the Bright-Glo™ Luciferase Assay System (Promega), according to the manufacturer’s instructions, in a VICTOR™ X plate reader (PerkinElmer, Inc., Waltham, MA, U.S.A.).
Western Blot AnalysesCells were lysed in 1% RIPA buffer containing protease and phosphatase inhibitors (Roche, Mannheim, Germany), and whole-cell lysates were separated by 10% SDS polyacrylamide gel electrophoresis (PAGE). Following electrophoresis, the proteins were transferred to polyvinylidene fluoride membranes, and the membranes were blocked with 5% skim milk in a Tris-buffered saline solution containing 0.1% Tween-20. The membranes were immunoblotted with primary antibodies, including anti-nuclear factor of activated T-cells (NFATc), anti-phospho-inhibitor of kappaB (IκB), anti-TRAF6, anti-NFATc, and anti-β-actin (Cell Signaling Technology, Danvers, MA, U.S.A.), followed by incubation with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies (Cell Signaling Technology). Blots were developed using an enhanced chemiluminescent solution (Thermo Fisher Scientific).
Statistical AnalysesStatistical comparisons between groups were performed using a one-way ANOVA with Dunnet’s post-hoc test in SPSS v. 17 (IBM Corp., Armonk, NY, U.S.A.). Differences were considered significant at a p value of <0.05.
To obtain the full-length mouse sRANKL genes, cDNAs from BALB/c splenocytes were synthesized, and mouse sRANKL genes were amplified using conventional PCR and cloned based on sequence information from the National Center for Biotechnology Information (NCBI). sRANKL was estimated to be 964 base pairs and 319 amino acids in length, with a molecular mass of about 35 kDa (Figs. 2A, B). The proteins expressed in the presence of 10 µM IPTG were confirmed using a histidine (His)-Tag Purification system (Fig. 2C). The cloned RANKL cDNA was 100% identical the open reading frame (ORF) of a previously confirmed mouse RANKL gene (GenBank Accession No. AF019048).
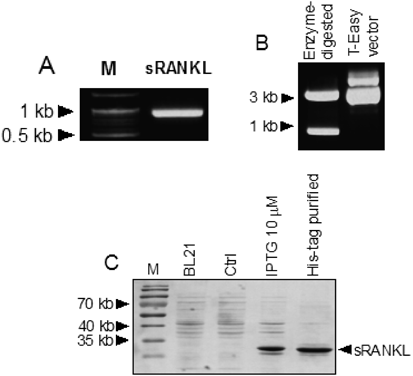
(A) Full-length sRANKL cDNA was identified in RT-PCR and (B) were successfully cloned. (C) E. coli (BL21) cells containing sRANKL were grown in the presence of IPTG (10 µM) at 30°C overnight, and cell lysates were analyzed on a 10% SDS polyacrylamide gel. Protein bands were visualized by staining with 0.01% Coomassie blue. M; marker, Ctrl; control.
The cytotoxicity induced by the HTN was quantified by measuring the release of LDH at varying ranges of concentration (10 to 0.3125 µg/mL). Incubating RAW264.7 cells with HTN did not result in cell cytotoxicity except at the highest dose (10 µg/mL) (Fig. 3A). TRAP expression was detected during osteoclastogenesis, which is regarded as a marker of osteoclast function and bone resorption.18) As shown in Fig. 3B, cells were clearly stained with TRAP (in red) and multinucleated cells were clearly identified by the presence of sRANKL. Interestingly, TRAP expression during the treatment of sRANKL was remarkably inhibited when treated with 5 µg/mL HTN (Fig. 3C).
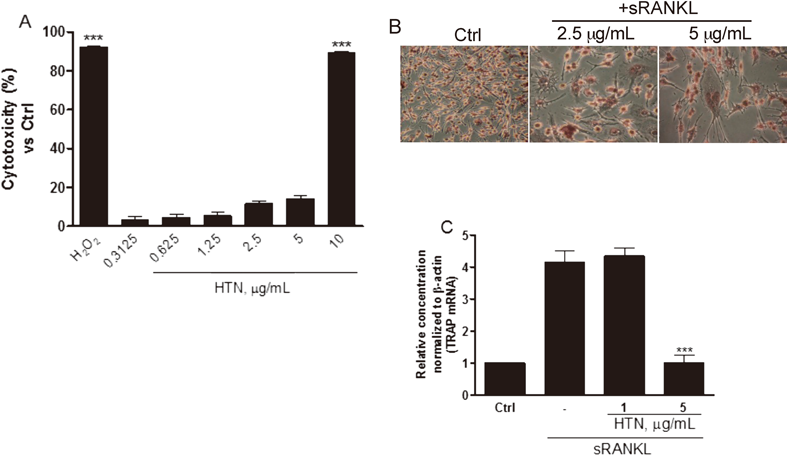
(A) Dose-dependence of cytotoxicity, measured as %LDH released into culture supernatant, was compared with cells treated with 1% Triton X-100. (B) RAW264.7 cells were exposed to the soluble sRANKL (5 µg/mL) for five days to assure multinucleated cells. (C) HTN was treated for five days during the periods of osteoclast-like differentiation with sRANKL and the level of TRAP gene expression was quantified using qPCR. Results were internally confirmed by the comparative cycle count (Ct, cycle number threshold) against β-actin as a standard. Data are expressed as mean±standard deviation (S.D.) (n=3). *** p<0.05 versus control (A) or sRANKL (C) group.
To identify and establish IFNγ+ T cells, mouse splenocytes were treated with 0.1 to 5 µg/mL LPS as a B cell stimulator, for 24 h, and IFNγ mRNA quantified using qPCR. Our results effectively demonstrated that IFNγ mRNA was substantially expressed in the presence of higher doses of LPS (5 µg/mL), which may indicate the polarization into T-helper 1 (Th1)-like cells (Fig. 4A).19) Interestingly, IFNγ, IL-22, and IL-1β mRNA transcripts were dose-dependently inhibited when treated with HTN, which are closely related with direct or indirect osteoclastogenesis through the upregulation of RANKL (Figs. 4B–D).
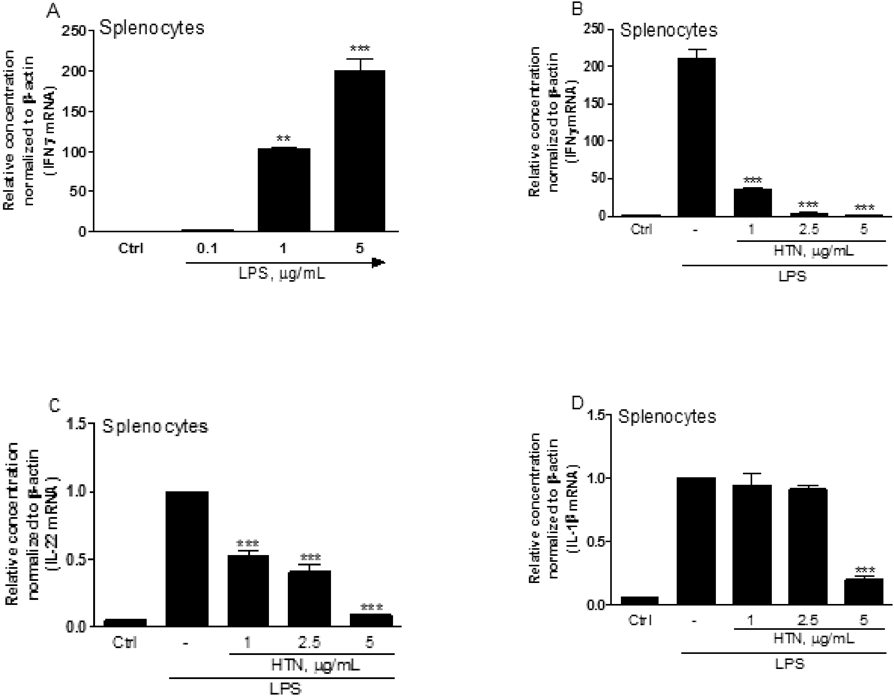
Cells were cultured for 24 h in the presence or absence of HTN (1 to 5 µg/mL) and the cells were stimulated with LPS (5 µg/mL). Isolated mRNA transcripts were analyzed by real-time reverse transcription polymerase chain reaction for IFNγ, IL-22, and IL-1β. (A) IFNγ production was identified at high level of LPS (1 and 5 µg/mL) in splenocytes. Subsequently, (B) IFNγ, (C) IL-22 and (D) IL-1β mRNAs were compared in the presence or absence of HTN. Results were internally confirmed by the comparative cycle count (Ct, cycle number threshold) against β-actin as a standard. LPS-treated group was used to calibrate for a relative comparison within each group. Results are mean±S.D. (n=3). ** p<0.01, *** p<0.001 versus control (A) or LPS group (B–C). Ctrl, control.
The crosstalk between splenocytes and RAW264.7 cells, as well as the effect of HTN, were investigated in a transwell co-culture system of HTN- and LPS-treated splenocytes and RAW264.7 cells, and levels of mRNA expression were compared in both. As shown in Figs. 5A and B, the transcripts of IFNγ and IL-22 from splenocytes were dose-dependently reduced in the presence of HTN, and TRAP mRNA expression from RAW264.7 cells was remarkably inhibited in the HTN-treated co-culture system, which indicates the inhibitive role of HTN in IFNγ+ T cells, thereby resulting in the downregulation of TRAP (Fig. 5C). Notably, IL-1β mRNA from RAW264.7 cells was concomitantly inhibited when the cells were co-cultured with HTN-treated splenocytes in a dose-dependent manner (Fig. 5D).
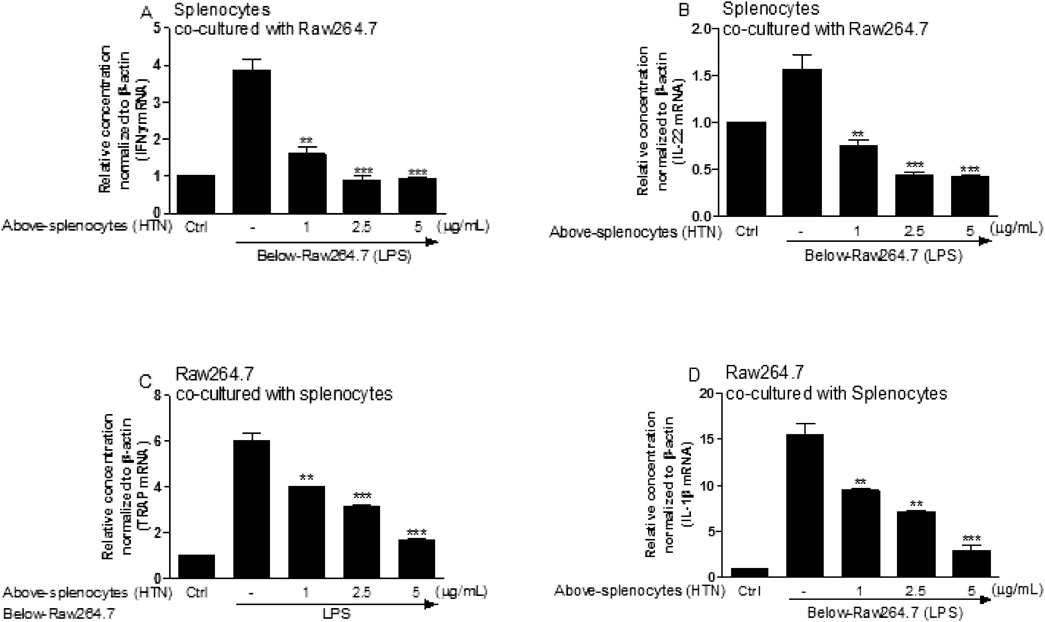
After removing HTN- and LPS-pretreated media, splenocytes were co-cultured with RAW264.7 cells (above, splenocytes-HTN and below, RAW264.7-LPS) for 24 h in a fresh media. The expression of (A) IFNγ and (B) IL-22 mRNA were compared from HTN-treated splenocytes. (C) TRAP and (D) IL-1β mRNA transcripts were analyzed in LPS-treated RAW264.7 cells. LPS-treated group was used to calibrate for a relative comparison within each group. Data are the representative results of three independent experiments. ** p<0.01, *** p<0.001 versus LPS group. Ctrl, control.
To verify that interferon-producing T cells polarized to IFNγ-producing Th1 cells via crosstalking with LPS-stimulated B cells, CD4+ cells were isolated from splenocytes and co-cultured with B cells (RPMI1788) after pretreatment of HTN in CD4+ cells and LPS in RPMI1788 cells. The results of this analysis show that IFNγ and IL-22 were downregulated, similar to the results observed in the co-culture of splenocytes and RAW264.7 cells, suggesting that HTN effectively inhibits the production of IFNγ, which can be induced by LPS-stimulated B cells (Figs. 6A, B). Moreover, RANKL gene expression from osteoblasts (MG63) was also significantly inhibited when the cells were treated with HTN (Fig. 6C). Finally, we confirmed that CD25, an early activation marker of T cells, was dose-dependently inhibited when treated with HTN (Fig. 6D).
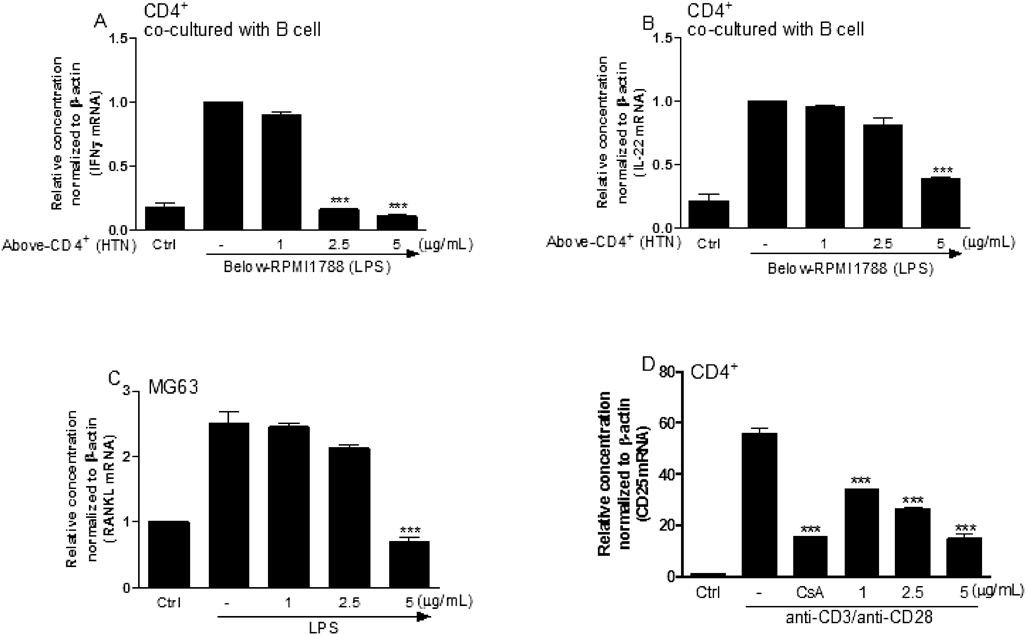
For transwell co-culture, after removing HTN- and LPS-pretreated media from CD4+ and RPMI1788 cells, CD4+ cells were co-cultured with RPMI1788 (B cell) for 24 h in a fresh media. RANKL and CD25 mRNAs were investigated in MG63 and CD4+ cells, respectively. The expression of (A) IFNγ and (B) IL-22 mRNA were compared in CD4+ cells co-cultured with LPS-treated RPMI1788 cells. (C) RANKL and CD25 mRNAs were analyzed in MG63 and CD4+ cells, respectively. For CD25 analysis, purified splenic CD4+ cells were cultured with anti-CD3 and anti-CD28 monoclonal antibodies. LPS-treated group was used to calibrate for a relative comparison within each group. Cyclosporin A (CsA) was used as a positive control. Data are the representative results of three independent experiments. *** p<0.001 versus LPS (A–C) or anti-CD3/anti-CD28 group (D). Ctrl, control.
To elucidate the intracellular inhibition pathways of HTN against osteoclastogenesis, RAW264.7 cells were treated with sRANKL and cultured for 24 h. Figure 7A indicates that HTN regulates the activation of the NFATc1 protein via a decrease in NFATc1 dephosphorylation in sRANKL-stimulated RAW264.7 cells. Upstream signaling pathways (TRAF6 and IκB) of NF-κB were also effectively inhibited when treated with HTN (Fig. 7B). Next, we examined whether HTN does in fact inactivate NF-κB using a luciferase reporter assay. RAW264.7 cell cultures were treated with the pGL4.32[luc2P/NF-κB-RE/Hygro] vector encoding the firefly luciferase reporter gene (luc2P), driven by five copies of an NF-κB enhancer element, during the first 24 h in culture in presence of HTN and/or LPS. The result of the promoter activity clearly revealed that HTN markedly inhibited LPS-mediated NF-κB promoter activation in a dose-dependent manner (Fig. 7C)
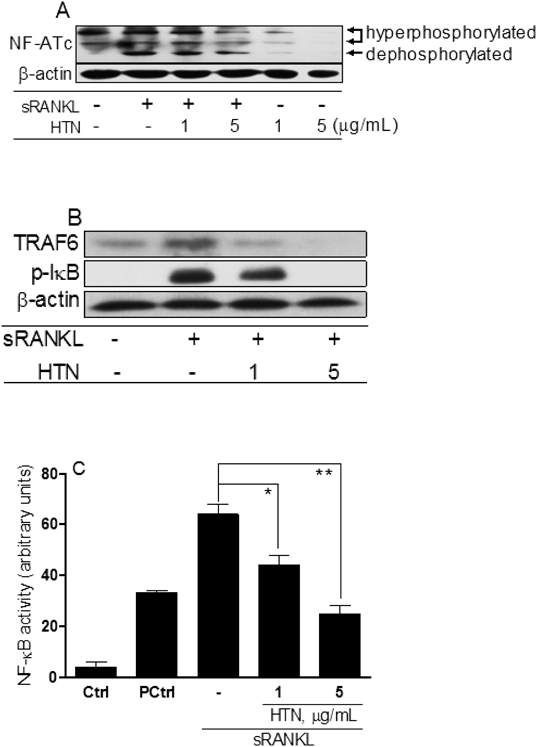
(A, B) RAW264.7 cells were treated with HTN (1, 5 µg/mL) for 2 h and stimulated with sRANKL (5 µg/mL) for 24 h. Cells were then harvested and whole cell extract were prepared for Western blot analysis of NFATc1, TRAF6, phospho-IκB, and β-actin. (C) For the measurement of luciferase activity, RAW264.7 cells were transiently transfected with pGL4.32[luc2P/NF-κB-RE/Hygro] for 24 h and treated with HTN (1 and 5 µg/mL) for 2 h before stimulation with LPS for another 24 h. The results are expressed as the mean±S.D. for three independent experiments. * p<0.05 and ** p<0.01 compared to indicated groups.
Although there have been in vitro reports that IFNγ potentially suppresses RANKL-induced osteoclastogenesis by inducing the degradation of TRAF6, multiple in vivo experiments have demonstrated that high levels of IFNγ secreted from innate immune cells and activated T cells promote osteoclastogenesis and play a crucial role as a pro-osteoclastogenic factor.12,20) In addition, high-dose LPS-stimulated B cells (1 to 10 µg/mL) can polarize to IFNγ-producing human Th1 cells, followed by a direct induction of the differentiation of human macrophages into osteoclasts through the expression of RANKL. In contrast, low doses of LPS promote the production of IL-4, which is a representative cytokine in Th2 cells and thus may inhibit the process of osteoclastogenesis.11,18) Moreover, Th1 cells preferentially produce RANKL, suggesting that Th1 cells play a direct role in bone resorption in Th1-dominant diseases.11,21)
Recently, we have reported that HTN isolated from A. japonica had a strong anti-atopic effect via the inhibition of cytosolic NFAT dephosphorylation, which results in the successful inhibition of Th1 cytokines, including IFNγ.13,14) Interestingly, our system successfully expressed high levels of IFNγ and RANKL in splenocytes in the presence of high doses of LPS (>1 µg/mL). It is also important to note that IFNγ, a Th1-dependent cytokine, and IL-22, mainly produced by IFNγ-secreting cells, were remarkably downregulated when treated with HTN in LPS-stimulated splenocytes. This may reflect the fact that HTN contributes to the process of osteoclastogenesis.
Although it has been controversially shown, primarily in vitro, that IFNγ has an inhibitory effect on osteoclastogenesis, multiple reports have demonstrated that IFNγ-producing T cells induce RANKL, and may therefore stimulate osteoclast differentiation, leading to subsequent bone damage.22,23) Moreover, the crosstalk between antigen-activated lymphocytes and monocytes produced soluble osteoclast-activating factors, such as IL-1β, which then induce an increase in RANKL in osteoblasts.24) In our co-culture system, splenocytes produced high levels of IFNγ mRNA in the absence of HTN, which may be stimulated by IL-1β mRNA increased in RAW264.7 cells. This corresponds to the prior studies that T cell-derived IFNγ activates macrophages, resulting in the production of IL-1β, and macrophage-derived IL-1β is involved in T cell sensitization, inducing IFNγ production.25–27)
Our results show that HTN inhibited the expression of IFNγ/IL-22 and IL-1β mRNA in a dose-dependent manner, suggesting an inactivation of T cells by HTN, followed by the inhibition of soluble osteoclast-activating factors (i.e., IL-1β) in the splenocyte and monocyte co-culture system. These data provide an indication that HTN can effectively reduce osteoclast differentiation in the face of high levels of IFNγ produced by T cells, thereby directly or indirectly via decreasing RANKL in osteoblasts.28) Moreover, TRAP was significantly downregulated in RAW264.7 cells. In CD4+ T cells and B cell lines, IFNγ and IL-22 transcripts were concomitantly inhibited when treated with HTN. Furthermore, HTN remarkably inhibited RANKL expression in osteoblast cells (MG63). These data strongly indicate that HTN played a key role in antiosteoclastogenesis by blocking the production of IFNγ and the IFNγ-dependent cytokine IL-22, which may lead to the downregulation of osteoclast formation via a reduction in RANKL and TRAP in both osteoblasts and preosteoclasts.29) Fundamentally, HTN significantly inhibited the expression of CD25 mRNA in CD4+ cells, indicating that HTN effectively plays a role in the inhibition of T cell receptor-triggered activation in early stages. The data demonstrated that HTN effectively inhibited the expression of IFNγ via both macrophages and B cells which are stimulated by LPS, and thus blocked sequential induction of osteoclastogenesis-related genes (i.e., TRAP, IL-1β, IL-22). Notably, HTN alone inhibited the RANKL and CD25 mRNA in osteoblastic MG63 cells and CD4+ cells, respectively, indicating that HTN may play direct and/or indirect roles in suppressing osteoclast formation in preosteoclastic cells.
Our analysis also revealed that signaling pathways (TRAF6→NF-κB→NFATc1), which are involved in the activation of downstream pathways responsible for osteoclastogenesis and thereby downregulate NFAT1c through NF-κB inhibition, were substantially downregulated by HTN in sRANKL-treated RAW264.7 cells.30) During osteoclastogenesis, RANKL binds to RANK, followed by the recruitment of TRAF6, which activates NF-κB and mitogen-activated protein kinases (MAPKs), and induces NFATc1, which is a key transcription factor in osteoclastogenesis.31,32) Although all of the experiments performed in this study took place in vitro, it is believed that HTN most likely directly or indirectly suppresses osteoclast differentiation by inhibiting the signaling cascades of RANKL-triggered osteoclastogenesis, such as TRAF6 and its downstream signaling protein, NF-κB.
In summary, we provide interesting evidences that HTN plays an important role in osteoclastogenesis, likely by downregulating the expression of IFNγ and IL-22 from Th1-polarized cells, in conjunction with an effective reduction in IL-1β upon cell–cell contact with monocytes. Given the essential role of TRAF6 in osteoclastogenesis, our study demonstrates that HTN may directly degrade the upstream signaling protein, TRAF6. In conclusion, HTN blocks the polarization of T cells to IFNγ-producing T cells, and thereby inhibits the expression of RANKL in both T cells and osteoblasts. Mechanistically, HTN directly inhibits the signal cascade of osteoclastogenesis, most likely by degrading TRAF6 proteins in preosteoclasts, indicating that HTN may be a new and promising candidate in the prevention of excessive bone resorption during bone remodeling cycles.
This research was supported by the Chung-Ang University Research Grant in 2014.
The authors declare no conflict of interest.