2018 年 41 巻 2 号 p. 190-197
2018 年 41 巻 2 号 p. 190-197
Application of food-grade Lactococcus lactis (L. lactis) as a safe delivery tool for DNA vaccines and therapeutic proteins has been well investigated. Although some studies showed that eukaryotic expression plasmids were transferred from L. lactis to enterocytes, the precise mechanism of the DNA transfer remains unknown. In this study, we generated an invasive L. lactis strain that expresses “murinized” Internalin A, an invasin of intracellular bacteria Listeria monocytogenes with two amino acid alterations for invasion into murine cells, and confirmed that this L. lactis strain delivered DNA in an invasin-dependent manner into a monolayer of epithelial cells polarized to mimic the gastrointestinal tract environment. Although invasive L. lactis inoculated orally can deliver DNA into enterocytes in the gastrointestinal tract of mice, the efficiency of DNA transfer was similar to that of non-invasive L. lactis strain, suggesting that the in vivo DNA transfer from L. lactis occurs invasin-independently. A ligated-intestinal loop assay, a method for a short-term culturing of the whole intestine filled with materials to evaluate the interaction of the materials with intestinal cells, demonstrated that both non-invasive and invasive L. lactis strains were present in the Peyer’s patches of the small intestine. On the other hand, few L. lactis was detected in the non-Peyer’s patch epithelial region. Thus, our observations lead us to speculate that DNA transfer from L. lactis occurs predominantly in the Peyer’s patches in an invasin-independent manner.
Vaccination with a plasmid containing the DNA sequence encoding an antigen has been of great interest of late as it can induce both humoral and cell-mediated immune responses.1) Despite numerous studies, no DNA vaccines have been licensed for human use to date. The administration of naked DNA is less immunogenic in large animals and humans, probably due to the low level of protein expression.2) To improve the potency of DNA vaccination, various DNA delivery platforms, including electroporation, needle-free jet injection and liposomes, have been developed.3,4) The use of bacteria as a delivery vehicle for DNA vaccines has emerged recently as a promising approach to enhance potency,5) and one of the attractive features of the use of bacteria is the potential for oral administration to induce not only systemic but also mucosal immune responses.5) Salmonella typhimurium and Listeria monocytogenes (L. monocytogenes) have been studied as experimental delivery vehicles as they are known to invade intestinal epithelial cells.6) Even though the strains employed are extensively attenuated for virulence, the possibility of reversion to virulence as well as preexisting immunity limits their clinical use, especially for infants, the elderly and immunocompromised hosts.7,8) The mechanisms involving DNA transfer from bacteria to mammalian cells and tissues remain to be clarified.
The use of food-grade Lactococcus lactis (L. lactis), a model lactic acid bacteria, to transfer DNA vaccines and therapeutic proteins to intestinal epithelial cells has been investigated.9) L. lactis is considered as an advantageous vector for oral DNA vaccines as it has been shown to have an extremely safe profile through its long history of use in the dairy industry as a starter for food fermentation.9) Guimaraes et al. showed that co-incubation of L. lactis carrying an eukaryotic expression plasmid for β-lactoglobulin (BLG) with human intestinal epithelial cell line Caco-2 cells resulted in the cellular expression of BLG.10) To improve the efficiency of L. lactis-mediated DNA delivery to intestinal cells, recombinant L. lactis strains expressing Internalin A (InlA), an invasin of intracellular bacteria L. monocytogenes, and those expressing fibronectin binding protein A (FnBPA) of Staphylococcus aureus were developed.11,12) Those invasive L. lactis showed higher invasiveness to epithelial cells in vitro in comparison to the wild-type (wt) L. lactis, and resulted in the cellular expression of genes encoded by an eukaryotic expression plasmid.11,12) The interaction of InlA with E-cadherin induces the endocytosis of InlA-expressing bacteria. However, the use of InlA-expressing strains could be limited to guinea pigs or transgenic mice expressing human E-cadherin, as InlA readily binds to human and guinea pig E-cadherin molecules but not to those of mice.13,14) Recently, it has been reported that murinized InlA (mInlA) with two amino acid alterations can bind to murine E-cadherin.15) Although oral administration of L. lactis carrying two expression plasmids, one for mInlA in L. lactis and the other for BLG in mammalian (host) cells, resulted in BLG expression in the mouse small intestine, mInlA did not increase the efficiency of DNA delivery, suggesting the lack of mInlA accessibility to its receptor E-cadherin.16) These previous studies left some questions unresolved, such as whether their observations can be generalized to include any gene of interest, which parts of the gastrointestinal tract can be reached by L. lactis for DNA delivery, and how eukaryotic expression can be achieved in the absence of molecules promoting invasion into epithelial cells in vivo.
In this study, to address the issues raised above, especially to elucidate the mechanism underlying DNA delivery by L. lactis, we generated L. lactis expressing mInlA, which has the same genetic background as strains in the reported studies, and analyzed its ability to deliver a eukaryotic expression plasmid encoding NanoLuc, as NanoLuc is a highly luminescent luciferase suitable for quantitative and sensitive measurement in vivo.17) In addition, to locate L. lactis in the tissues, we performed an ex vivo analysis using fluorescence-labeled L. lactis, and found that L. lactis accumulated in the Peyer’s patches (PPs) irrespective of mInlA expression. Our observations suggested that DNA transfer from L. lactis occurred predominantly in the PPs not in the intestinal epithelial cells.
The bacterial strains and plasmids used in this study are listed in Table 1. L. lactis strain NZ9000 (MoBiTec, Germany) was cultured in M17 media containing 0.5% glucose (GM17).18) Antibiotics were added, when necessary, at the following final concentrations: erythromycin at 5 µg/mL and chloramphenicol at 10 µg/mL.
| Strains and plasmids | Characteristics | Ref. |
|---|---|---|
| Strains | ||
| L. lactis NZ9000 (wt) | MG1363 (nisRK genes in chromosome) derivative, plasmid-free | 18) |
| L. lactis wt (empty) | L. lactis NZ9000 carrying an appropriate empty vector(s) | This study |
| L. lactis minlA+ | L. lactis NZ9000 carrying pNZ-minlA | This study |
| L. lactis nluc+ | L. lactis NZ9000 carrying pNZ8148 and pCMV-nluc | This study |
| L. lactis minlA+nluc+ | L. lactis NZ9000 carrying pNZ-minlA and pCMV-nluc | This study |
| Plasmids | ||
| pNZ8148 | E. coli-L. lactis shuttle plasmid, Cmr | 21) |
| pNZ-minlA | E. coli-L. lactis shuttle plasmid carrying the minlA gene under the control of the Nisin-inducible promoter, Cmr | This study |
| pBluescript SK II (+) | E. coli vector, Ampr | |
| pIL253 | L. lactis vector, Emr | 22) |
| pCMV253 | pIL253-derivative E. coli-L. lactis shuttle plasmid, containing pUC ori, CMV promoter/enhancer and the SV40 poly A sequence from pCMV-Script, Emr | This study |
| pCMV-nluc | E. coli-L. lactis shuttle vector carrying the NanoLuc gene under the control of eukaryotic CMV promoter, Emr | This study |
Cmr, Chloramphenicol resistant; Ampr, Ampicillin resistant; Emr, Erythromycin resistant; Kmr, Kanamycin resistant.
A 2.4-kb fragment covering the full-length inlA gene was amplified from the genomic DNA of L. monocytogenes (kindly provided by Satoshi Inoue19,20)) with primers 5′-ATT GGA TCC ATG GTG AGA AAA CGA TAT GTA TGG-3′ and 5′-TTT CTC GAG CTC CTA CTT CTA TTT ACT AGC ACG TGC-3′, and cloned between the BamHI and XhoI sites of pBluescript (pBS) SK II (+). pBS-minlA, pBS encoding murinized InlA with S192N and Y369S alterations, was generated by the site-directed mutagenesis of pBS-inlA using a commercial kit (QuikChange II XL Site-Directed Mutagenesis Kit, Agilent Technologies, U.S.A.) according to the manufacturer’s protocol with the following primers: for S192N; 5′-CTA GTC TAC AGC AAT TAA ACT TTG GTA ATC AAG TGA C-3′ and 5′-GTC ACT TGA TTA CCA AAG TTT AAT TGC TGT AGA CTA G-3′, and for Y369S; 5′-CAA AGA TTA TTT TTC TCT AAT AAC AAG GTA AGT GAC GTA AGC-3′ and 5′-GCT TAC GTC ACT TAC CTT GTT ATT AGA GAA AAA TAA TCT TTG-3′. The minlA gene in pBS-minlA was re-cloned into pNZ8148 (MoBiTec), which allows expression of the gene of interest under the control of a Nisin-inducible promoter,21) to generate pNZ-minlA.
Construction of Eukaryotic Luciferase Expression PlasmidsL. lactis plasmid pIL253 was kindly provided by Phillipe Moreillon (University of Lausanne, Switzerland).22) A fragment containing the replication origin for replication in Escherichia coli (pUC ori), cytomegalovirus (CMV) promoter, and SV40 poly A was amplified from pCMV-Script (Agilent Technologies) with primers 5′-GGC AGA TCT TTC CAC TGA GCG TCA GAC-3′ and 5′-GCA CTA GTA CGT TGG AGT CCA CGT TC-3′. The digestion of this amplicon with BglII and Spe I allowed it to be cloned between the BamHI and XbaI sites of pIL253, resulting in an E. coli-L. lactis shuttle vector pCMV253.
An eukaryotic NanoLuc expression plasmid (pCMV-nluc) was constructed as follows. The NanoLuc gene was amplified from pNL1.1 (Promega, U.S.A.) with primers 5′-AAA GCG GCC GCA GCC ACC ATG GTC TTC AC-3′ and 5′-CCC GGA TCC CCT TAC GCC AGA ATG C-3′, and cloned into pCMV253 digested with BamHI and NotI, resulting in pCMV-nluc.
Generation of Recombinant L. lactisElectrocompetent L. lactis NZ9000 was transformed with pNZ-minlA and/or pCMV-nluc and/or pCMV-nluc or with empty vector pNZ8148 and/or pCMV253 (Table 1). The transformants were selected on GM17 agar plates containing chloramphenicol and/or erythromycin at 30°C.
Confirmation of the Expression of mInlA in L. lactisOvernight cultures of L. lactis wt (empty) and L. lactis minlA+ were diluted (1 : 25) in fresh media and grown until OD600=0.3–0.4, then stimulated with 10 ng/mL of Nisin for 3 h. Bacteria were then harvested and lysed by sonication. The lysates [equivalent to 1×109 colony forming units (CFU)] were separated in 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gels, and then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, U.S.A.). Non-specific sites were blocked with TBST (20 mM Tris buffer pH 7.6, 137 mM NaCl and 0.1% Tween-20) containing 3% skim milk (Cell Signaling, U.S.A.) for 1 h. The membrane was then incubated with anti-glutathione S-transfetase (GST)-InlA serum diluted 1 : 1000 with Immuno Enhancer (Wako Pure Chemical Industries, Ltd., Japan) for 1 h at room temperature (r.t.) followed by incubation with horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin G (IgG) (Thermo Fisher Scientific, U.S.A.) diluted 1 : 1000 with Immuno Enhancer for 1 h at r.t. After each step, the membrane was washed three times with TBST. Reactive proteins were detected using a chemiluminescent reagent (Immunostar LD, Wako) and LAS3000UVmini (FUJIFILM, Japan).
Cell CultureHuman intestinal epithelial cell line Caco-2 (RCB0988, RIKEN, Japan) cells were maintained in minimum essential media (MEM, Sigma, U.S.A.) supplemented with 20% (v/v) fetal bovine serum (FBS, Hyclone, U.S.A.), 0.1 mM non-essential amino acid (Thermo Fisher Scientific) and 100 units/mL penicillin/100 µg/mL streptomycin (Gibco, U.S.A.). Murine kidney epithelial cell line TCMK-1 (CCL-139, ATC C) cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Wako) supplemented with 10% (v/v) FBS (Hyclone) and 100 units/mL penicillin/100 µg/mL streptomycin (Gibco). Both cell populations were cultured at 37°C in 5% CO2.
Polarization of Caco-2 cells were performed as follows. Transwells with 0.3 µm pores (Corning, U.S.A.) were inserted into 96-well plates and coated with 0.1 mg/mL collagen (Sigma). Caco-2 cells were then seeded at 1.25×104 cells/insert and the culture medium was changed every other day. The cells were cultured for 21 d to reach a polarized state. The transepithelial electrical resistance of the cultures was measured using Millicell ERS-1 (Millipore) to confirm a high degree of polarization.
Gentamicin Survival AssayL. lactis strains were stimulated with Nisin for InlA expression as described above, washed with phosphate-buffered saline (PBS, pH 7.0) and suspended in cell culture medium without antibiotics. The bacterial suspension was added to confluent Caco-2 or TCMK-1 cells grown in 24-well plates at a multiplicity of infection (MOI) of 103 CFU/cell. After 1 h infection, the cells were washed with PBS to remove non-adherent bacteria, and then incubated for an additional 2 h in the presence of 20 µg/mL gentamicin to kill the extracellular bacteria. The cells were lysed with sterile water containing 0.2% Triton X-100, and serial dilutions of the lysate were plated on GM17 agar plates. After incubation for 24 h at 30°C, colonies formed on the plates were counted.
DNA Transfer from L. lactis to Cultured Epithelial CellsConfluent Caco-2 or TCMK-1 cells grown in 96-well plates were co-cultured with the indicated strains of L. lactis at an MOI of 103 CFU/cell. After gentamicin treatment, the cells were maintained for an additional 24 h prior to lysing with passive lysis buffer (Promega). The lysates were mixed with luciferase assay reagent (Nano-Glo® Luciferase Assay System, Promega), and luciferase light emission was measured using the GloMax®-Multi Detection System (Promega).
DNA Transfer from L. lactis to Caco-2 MonolayersCaco-2 cells grown on Transwell® Inserts (Corning) for 1 d (unpolarized) or 21 d (fully polarized monolayers) were co-cultured with the indicated strains of L. lactis at an MOI of approximately 103 CFU/cell. After gentamicin treatment, cells were maintained for an additional 24 h prior to measurement of the luciferase activities of the cell lysates.
Oral Inoculation of MiceSix-week-old C57BL/6 female mice (Japan SLC, Inc.) were maintained under normal husbandry conditions in the Animal Facilities of Gifu University. All animal experiments were approved by the institutional Animal Care and Use Committee.
Mice (n=3–5/group) were orally inoculated with 1×109 CFU/mouse/d of L. lactis wt (empty), L. lactis nluc+, or L. lactis minlA+nluc+ for three consecutive days. Mice were euthanized at 1–3 d after the final inoculation. The small and large intestines, spleen and mesenteric lymph nodes were isolated, rinsed with PBS, cut into small pieces, and lysed with passive lysis buffer. The luciferase activities of the resultant tissue lysates were then measured.
In Vivo Persistence of L. lactisSix-week-old C57BL/6 female mice (Japan SLC, Inc.) were inoculated with 1×109 CFU of L. lactis wt (empty) per mouse. One day after the inoculation, mice were euthanized, and the small intestine were excised and mechanically homogenized with PBS containing 0.2% Triton X-100 at 100 mg of tissue/mL. Serially diluted homogenates were plated onto the selective media (Ellicker) containing chloramphenicol and incubated for 2 d before enumeration.
Ligated-Intestinal Loop AssayL. lactis was cultured, stimulated with Nisin, harvested by centrifugation, and suspended in PBS as described above. The suspension was incubated for 30 min at 30°C in the presence of 1 mM 5-cyano-2,3-ditolyl-2H-tetrazolium chloride (CTC) solution (Dojindo, Japan) for fluorescent labelling of the bacteria. After incubation, the bacteria were washed two times with PBS to remove excess CTC solution, and then re-suspended in DMEM supplemented with 10% (v/v) FBS.
Six-week-old C57BL/6 female mice (Japan SLC, Inc.) were euthanized after 4 h fasting. The small intestines were excised and cut into segments (3 cm in length) containing one PP. One end of the segment was closed by tying with suture thread. The intestinal segments were filled with CTC-labelled L. lactis (approximately 1×109 CFU/segment). The open end was tied with a suture, and the segments were incubated in DMEM supplemented with 10% (v/v) FBS for 1.5 h at 37°C. The experiments using these segments were conducted within 1 h of collection of the tissue to protect it from deterioration. After incubation, the tissues were vigorously washed with PBS to remove mucus and free bacteria, fixed with 4% paraformaldehyde overnight at 4°C, washed with PBS (pH 6.0) and then auto-fluorescence was quenched by incubation in 50 mM NH4Cl for 30 min at r.t.23) The specimens were blocked with 2% (w/v) BSA in PBS, followed by incubation with anti-GP2 monoclonal antibody (2F11C3, MBL, Japan) overnight at 4°C, and then washed with PBS and probed with the Alexa Fluor 488-labelled secondary antibody (#ab150157, abcam, U.S.A.) for 2 h at r.t.24) After immunofluorescence staining, specimens were mounted in 90% (v/v) glycerol in PBS and visualized by fluorescence microscopy (BZ-9000, KEYENCE, Japan).
Statistical AnalysesStatistical analyses were performed using GraphPad Prism 5 (Graph pad, U.S.A.). Results are expressed as mean±standard error (S.E.). Statistical significance between groups was calculated using the Student’s t-test or one-way ANOVA followed by Dunnett’s post-test, and values of p<0.05 were considered significant.
We prepared a polyclonal antiserum specific to InlA by immunization of mice with a GST-InlA fusion protein. Two major bands were detected in the lysates prepared from L. lactis minlA+, L. lactis transformed with pNZ-minlA, but not in those prepared from L. lactis wt (empty), in the immunoblotting analysis using the serum (Fig. 1A). As InlA protein contains a signal peptide sequence, the immature and mature forms were expected to be detected as products before and after cleavage of the signal peptide, respectively.11)

Bacterial lysates prepared from L. lactis wt (empty) and L. lactis minlA+ were analyzed by immunoblotting using polyclonal anti-GST-InlA mouse serum. Arrows indicate immature and mature forms of mInlA protein. Positions of molecular weight markers are indicated at left.
The ability of L. lactis minlA+ to invade epithelial cells was evaluated by gentamicin survival assay, which allows quantification of viable intracellular bacteria. In human epithelial Caco-2 cells, L. lactis minlA+ showed an approximately 100-fold higher invasion rate than that of L. lactis wt (empty) (Fig. 2A). As it has been reported that mInlA can interact with murine E-cadherin.15) Since the invasiveness of L. lactis minlA+ was demonstrated mainly in human epithelial cell line Caco-2 but not in the murine epithelial cells,16) we evaluated the ability of L. lactis minlA+ to invade murine epithelial TCMK-1 cells. L. lactis minlA+ exhibited a significantly higher invasion rate than did L. lactis wt (empty) (Fig. 2B). These results indicated that L. lactis minlA+ can invade not only human but also murine epithelial cells.

(A) Caco-2 and (B) TCMK-1 cells were co-incubated with L. lactis wt (empty) and L. lactis minlA+ for 1 h, then treated with gentamicin for 2 h. The cells were lysed and the number of bacteria internalized into the cells was measured as colony forming units (CFUs). The dashed lines represent the detection limit. Data are shown as means±S.E. (n=3; Student’s t-test, ** p<0.01, *** p<0.001).
To demonstrate the fact that mInlA can mediate DNA transfer from L. lactis to cultured mammalian cells, we prepared L. lactis nluc+ and L. lactis minlA+nluc+, which are wt and minlA+ L. lactis strains containing pCMV-nluc, a plasmid carrying a eukaryotic NanoLuc expression cassette, respectively (Table 1). No luciferase activities were detected in the L. lactis strains themselves (data not shown). Caco-2 and TCMK-1 cells were co-incubated with these strains for 1 h, and the luciferase activity of the cell lysates was measured after 24 h culturing in the presence of gentamicin. Significant luciferase activity was detected in the cell lysate co-cultured with L. lactis minlA+nluc+, whereas little luciferase activity was detected in that co-cultured with L. lactis nluc+ (Figs. 3A, B). We also analyzed the ability of L. lactis minlA+nluc+ to deliver DNA to monolayers of epithelial cells that were polarized to mimic the environment of the gastrointestinal tract. As shown in Fig. 3C, L. lactis minlA+nluc+ could deliver plasmid DNA to the polarized epithelial cells, although the efficacy of DNA delivery to the polarized epithelial cells was one-fifth that of the delivery to unpolarized cells. These observations indicated that L. lactis minlA+ delivered plasmid DNA to epithelial cells in a mInlA-dependent manner.

(A) Caco-2 and (B) TCMK-1 cells were co-incubated with L. lactis wt (empty), L. lactis nluc+ or L. lactis minlA+nluc+ for 1 h, then treated with gentamicin for 24 h. The cells were lysed and luciferase activities in the cells were measured. (C) Unpolarized and polarized Caco-2 cells cultured on Transwell inserts were co-incubated with L. lactis nluc+or L. lactis minlA+nluc+ for 1 h, then treated with gentamicin for 24 h. The cells were lysed and luciferase activities were measured. RLU: relative luminescence units. Data are shown as means±S.E. (n=5; one way ANOVA followed by Dunnett’s test, * p<0.05, *** p<0.001) .
First, the number of L. lactis in the small intestine 1 d after oral administration was determined by plating serially diluted homogenates of the tissues. There were no viable L. lactis in the tissue homogenate (data not shown), suggesting that orally administrated L. lactis were cleared from the small intestine within 1 d. Next, to evaluate the ability of L. lactis to deliver DNA in animals, mice were orally administrated with L. lactis wt (empty), L. lactis nluc+ and L. lactis minlA+nluc+ for three consecutive days, the small and large intestines were isolated, and the luciferase activities in the homogenates of those tissues were measured. The luciferase activities in the small intestine of mice treated with L. lactis nluc+ and L. lactis minlA+nluc+ were significantly higher than that in mice treated with L. lactis wt (empty), indicating that the DNA transfer to the cells in the small intestine occurred in a mInlA-independent manner (Fig. 4A). In contrast to the small intestine, the luciferase activities in the large intestine of mice treated with any of the three strains were almost at the background level (Fig. 4A). Since dendritic cells residing in the mucosal lamina propria are known to sample luminal antigens and then migrate to the mesenteric lymph node and spleen,25,26) luciferase activities in these tissues were measured. Luciferase activities in these tissues from mice inoculated with any of the three strains remained at the background level (Fig. 4B).
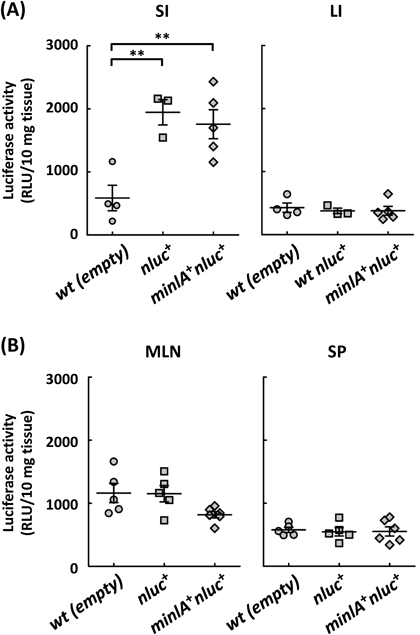
Mice were orally administered with L. lactis wt (empty), L. lactis nluc+ or L. lactis minlA+nluc+ for 3 consecutive days. At 1–3 d after the last gavage, the mice were euthanized and luciferase activities in the homogenates of (A) the small and large intestines (SI and LI), (B) mesenteric lymph node (MLN) and spleen (SP) were measured. RLU: relative luminescence units. Data are shown as means±S.E. (n=3–5; One way ANOVA followed by Dunnett’s test, ** p<0.01) .
To understand the differences in the InlA-dependency of DNA delivery between that in cell cultures and that in mice, we analyzed the localization of L. lactis in the small intestine by a ligated-intestinal loop assay, an ex vivo assay commonly used for evaluation of the interaction of intestinal cells with materials injected into the ligated-intestine. After incubation of viable fluorescent-labeled L. lactis wt (empty) and L. lactis minlA+ strains within ligated-intestinal segments containing one PP, sequential whole-mount immunostaining to visualize the PPs with antibodies against GP2, which is well known to express in the M cells of PPs, was performed (Fig. 5A). Both strains accumulated in the region containing the PPs but not in the region lacking PP (Fig. 5B).

(A) Schmatic drawing of a ligated intestinal loop. Ligated intestinal segment containing one Peyer’s patch was filled with suspension of the CTC-labelled L. lactis. (B) After incubation of CTC-labelled L. lactis wt (empty) and L. lactis minlA+ in the ligated-intestinal loops, the whole mount specimens were incubated with anti-GP2 antibody to detect the PPs (green), and analyzed by fluorescent microscopy. The red signal indicated the localization of L. lactis. Scale bars, 1 mm.
In this study, we aimed to elucidate the mechanism of DNA delivery by L. lactis and demonstrated the following: 1) L. lactis minlA+ can invade and deliver DNA not only to human Caco-2, but also to murine TCMK-1 cells in a mInlA-dependent manner, 2) L. lactis minlA+ can also deliver DNA to polarized Caco-2 monolayers that mimics the environment of the gastrointestinal tract, 3) DNA delivery to intestinal cells by L. lactis occurred in the small but not in the large intestine, and the delivery was independent of mInlA, and 4) L. lactis accumulated in PPs of the small intestine.
Previous studies quantified the BLG eukaryotic expression plasmid delivery by L. lactis using BLG protein-specific enzyme-linked immunosorbent assay (ELISA).16,27) They suggested that plasmid DNA delivery by L. lactis in mice may be a stochastic event that is dependent on environmental factors, as some mice (30–50%) did not produce any BLG protein.16) In contrast, our data indicated that DNA delivery in the small intestine by L. lactis occurred in all mice inoculated with L. lactis strains carrying a plasmid for NanoLuc expression, irrespective of mInlA expression. This discrepancy is probably due to the sensitivity of the assays used to evaluate DNA delivery. Our study used NanoLuc as a reporter for the evaluation, as it is the most highly luminescent luciferase and provides 150-fold brighter bioluminescence than firefly luciferase.17) In other words, the detection limit of our NanoLuc assay is sub pM, which is at least 1000-fold more sensitive than the ELISA used in the published reports.
Our demonstration of DNA delivery to both human and murine epithelial cells in a murinized InlA-dependent manner indicates that mInlA was fully functional in the cell cultures. Furthermore, mInlA significantly increased DNA delivery, even to the polarized epithelial cells, although the efficiency of DNA delivery to polarized cells was lower than that to unpolarized cells. This reduction in the invasion rate to polarized cells was consistent with reports describing differences in the invasion rate of L. monocytogenes to polarized and unpolarized cells, with those studies suggesting that the tight junctions between adjacent epithelial cells limited the access of InlA to E-cadherin expressed on the basolateral side of the epithelial cells.28–30) It has been reported that L. monocytogenes can enter the extruding epithelial cells at the top of the villi and mucus-secreting goblet cells, as the E-cadherin surrounding these cells is luminally accessible,14,31) leading to a working hypothesis that L. lactis may enter the enterocytes or goblet cells in a similar manner. However, we could detect few L. lactis in the epithelial cells in the non-PP regions where epithelial cells and goblet cells exist, rejecting the hypothesis. Furthermore, in spite of the use of a sensitive assay, we did not observe any increase in the efficiency of DNA delivery to the cells in the gastrointestinal tract of mice by mInlA expression, confirming the results reported previously.16) These observations suggested that mInlA expressed by L. lactis would not readily interact with E-cadherin in the intestinal environment.
We demonstrated the increase of luciferase activities only in the small intestines, which have multiple PPs, but not in the large intestines, which have no PPs. In addition, our ex vivo analysis clearly demonstrated L. lactis accumulation in the PPs but not in the non-PP epithelia. Furthermore, this accumulation was independent of mInlA. These results suggest that the PPs are the portal of L. lactis. To confirm this hypothesis, we may need to examine whether L. lactis also accumulates in the PPs immediately after the oral administration in the future study. Our observation is similar to the case of invasive E. coli expressing Yersinia pseudotuberculosis invasin. The bacteria could not invade epithelial cells in vivo due to the basolateral localization of the receptor, β-1 integrin, and accumulated in PPs.32) The PPs are one of the gut-associated lymphoid tissues that regulate mucosal immune responses against luminal bacteria and other antigens.25) The follicular-associated epithelium (FAE) that covers the PPs is the major entry site of many pathogenic and non-pathogenic microorganisms, as the immature microvilli and thin mucus layer cannot inhibit the access of microorganisms to epithelial cells.33) Recently, the mechanism of antigen sampling by PPs has been clarified.24) In PPs, abundant antigen-presenting cells, such as dendritic cells, process the antigens entering through the M cells, a subset of intestinal epithelial cells residing in the FAE and taking up intestinal microbial antigens.33) Several microbial products were known to bind to molecules specifically expressed on the apical side of M cells.34) To our knowledge, there are no reports describing the molecules associated with the binding of L. lactis to M cells. Identification of such molecules may improve our understanding of the accumulation of L. lactis in PPs. In addition, identification of the cell types that express the gene of interest delivered by L. lactis may expand our insight into the application of L. lactis as a vector for DNA vaccines, as some cell types in PPs play a key role in the induction of mucosal immune responses. It has been reported that L. lactis can deliver DNA to dendritic cells by phagocytosis in vitro.29) We did not detect any increase in luciferase activity in the spleen or mesenteric lymph node by L. lactis-mediated DNA delivery. However, these results may not exclude the involvement of dendritic cells in gene expression, as the cells expressing luciferase might be only a small fraction of the cells in those tissues.
DNA vaccines have some theoretical concerns, including a risk of DNA integration. There have been little information about the potential plasmid DNA integration in use of the bacterial vector. Therefore, it would be desirable to examine such an issue before any practical use of L. lactis vectors.
In conclusion, our study suggests that DNA transfer from L. lactis occurs predominantly in the PPs, but not in the intestinal epithelial cells, in an invasin-independent manner. To confirm this, further studies will be required to see the correlations between the amounts and localization of L. lactis orally inoculation and the type and numbers of cells expressing a gene of interest delivered as a plasmid DNA from L. lactis in mice. In addition, comparing the efficiency of DNA delivery in mice lacking M cells35) or PPs36) to that in wild type will reinforce the hypothesis.
We thank Satoshi Inoue (National Institute of Infectious Disease, Japan) and Phillipe Moreillon (University of Lausanne, Switzerland) for providing the genomic DNA of L. monocytogenes and eukaryotic expression plasmid pIL253, respectively. This study was supported by a Grant-in-Aid for Young Scientists B (JP 16K19127) from the Japan Society for the Promotion of Science to KT and by a scholarship fund from the Lactic Acid Bacteria Foundation to NI.
The authors declare no conflict of interest.