Abstract
Fungal β-glucan is a potent immunological stimulator, and that it activates both the innate immune system and adaptive immunity. Curdlan is (1→3)-β-glucan, a linear form of β-glucan with a high molecular weight; it modulates the immune response. However, its role in bone tissue is controversial, and the effects of curdlan on bone tissues are unknown. Toll-like receptors (TLRs) play critical roles in innate immunity, and various ligands for TLRs are thought to regulate the host defense mechanisms against pathogens. TLR2 is known to form heterodimers with TLR6, and the TLR2-TLR6 heterodimer (TLR2/6) recognizes diacylated lipopeptides from Gram-positive bacteria. In the present study, we prepared low molecular-weight curdlan, (1→3)-β-D-glucan, and examined its effects on bone resorption induced by TLR2/6 signaling. In co-cultures of bone marrow cells and osteoblasts, low molecular-weight curdlan suppressed the osteoclast formation induced by TLR2/6 ligand, and attenuated bone resorption in mouse calvarial organ cultures. Curdlan acted on mouse osteoblasts and suppressed the expression of receptor activator of nuclear factor-kappa B (NF-κB) ligand (RANKL), a key molecule for osteoclastogenesis. Curdlan also acted on mouse bone marrow macrophages and suppressed RANKL-dependent osteoclast differentiation from osteoclast precursor cells. The present study indicates that low molecular-weight curdlan attenuated TLR2-induced inflammatory bone resorption. Curdlan, (1→3)-β-glucan may be a natural agent with beneficial effects on bone health in humans.
Bone remodeling is regulated by bone resorption and bone formation, and osteoclasts, primary bone-resorbing cells are differentiated from monocyte-macrophages by a mechanism involving the receptor activator of nuclear factor-kappa B (NF-κB) ligand (RANKL) expressed on the cell surface of osteoblasts. Osteoclast precursors possess RANK, a receptor for RANKL, and can differentiate into mature osteoclasts via a RANK/RANKL-mediated mechanism.1)
Toll-like receptors (TLRs; TLR1-TLR13) play critical roles in innate immunity, and various ligands for TLRs are thought to regulate the host defense mechanisms against pathogens.2) TLR4 was identified as the receptor for lipopolysaccharide (LPS), which is an outer membrane component of Gram-negative bacteria. We have reported that LPS induces inflammatory bone resorption by TLR4 signaling.3) TLR2 is known to form heterodimers with TLR1 or TLR6, and the TLR2-TLR6 heterodimer (TLR2/6) recognizes diacylated lipopeptides from Gram-positive bacteria.4) We previously reported that TLR2 signaling induces osteoclast formation and inflammatory bone resorption.5)
It is known that fungal β-glucan is a potent immunological stimulator, and that it activates both the innate immune system and adaptive immunity.6) Curdlan is (1→3)-β-glucan, a linear form of β-glucan with a high molecular weight; it modulates the immune response by a receptor-dependent mechanism. Several receptors of β-glucan have been identified, including dectin-1, complement receptor 3 (CR3), and lactosylceramide (LacCer).7) Dectin-1 is a major receptor for the recognition of β-glucan, and is found on various immune cells including macrophages and T cells. Dectin-1 dependent β-glucan signaling is thought to be coordinated by TLR2 in the innate immune system.8) In addition to immune system regulation, β-glucan exhibits anti-cancer activity, anti-viral activity and reduces the blood glucose level.9) However, the role of β-glucan in bone tissues is unclear. The biological activity of β-glucan is closely related to its molecular size and the degree of polymerization of glucose. In the present study, we prepared low molecular-weight curdlan, (1→3)-β-glucan, and examined its effects on the inflammatory bone resorption induced by TLR2/6 signaling.
MATERIALS AND METHODS
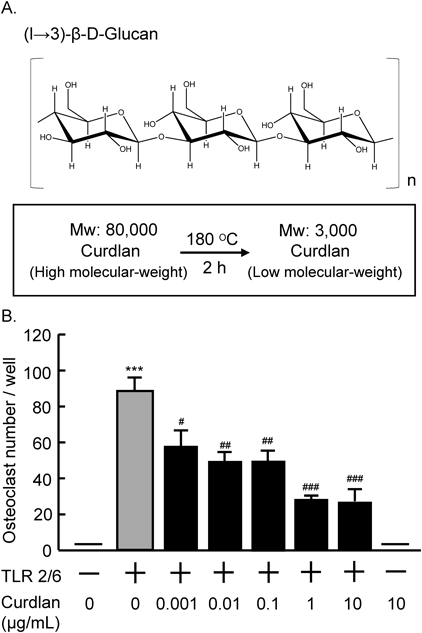
Animals and ReagentsNewborn and six-week-old ddy mice were obtained from Japan SLC, Inc. (Shizuoka, Japan). High molecular-weight curdlan, (1→3)-β-glucan (MW 80000), was obtained from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan), and heated to 180°C for 2 h in distilled water (1 mg/mL) using high pressure reactor (Highpreactor BR, Berghof Group, Germany) to prepare low molecular-weight curdlan (MW 3000) (Fig. 1A). The molecular size of the sample was determined by Gel Permeation Chromatography using HPLC (Prominence, Shimadzu Co., Ltd., Japan) with columns (TSKgel G4000PWXL, G4000PWXL and G2000PWXL; Tosoh Co. Ltd., Japan). The TLR2/6 ligand, synthetic diacylated lipopeptide (Pam2CSK4) was obtained from InvivoGen Co., Ltd., CA, U.S.A. The data were analyzed by a one-way ANOVA, followed by Tukey’s test for a post hoc analysis.
Osteoclast Formation in Co-cultures of Mouse Bone Marrow Cells and OsteoblastsPrimary osteoblastic cells were isolated from newborn murine calvariae, as reported previously.10) Bone marrow cells (3×106 cells) were isolated from 6-week-old mice, and co-cultured with primary osteoblast cells (1×104 cells) in αMEM containing 10% fetal calf serum (FCS). On day 7, the cells adhering to the well surface were stained for tartrate-resistant acid phosphatase (TRAP), a marker enzyme for osteoclasts. TRAP-positive multinucleated cells were counted as osteoclasts.10)
Osteoclast Differentiation from Bone Marrow MacrophagesTo examine the differentiation of osteoclasts from precursor cells, mouse bone marrow cells were collected and cultured with macrophage colony-stimulating factor (M-CSF) and soluble RANKL (sRANKL) to generate bone marrow macrophages. The macrophages were further cultured for 3 d in the presence of M-CSF and sRANKL.5) After the cultures, TRAP-positive multinucleated cells were counted as osteoclasts.
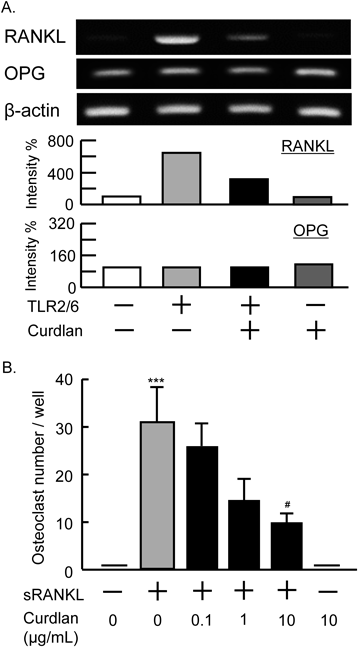
RT-PCR AnalysisTotal RNA was extracted from cultured osteoblasts, and an RT-PCR was performed to examine the mRNA expression of RANKL and osteoprotegerin (OPG) in osteoblasts. The primer sequences were as follows: mouse RANKL: 5′-AGG CTG GGC CAA GAT CTC TA-3′ (forward) and 5′-GTC TGT AGG TAC GCT TCC CG-3′ (reverse), mouse OPG: 5′-AGC AGG AGT GCA ACC GCA CC-3′ (forward) and 5′-TTC CAG CTT GCA CCA CGC CG-3′ (reverse).
Bone-Resorbing Activity in Mouse Calvarial Organ CulturesTo measure the bone-resorbing activity in the mouse calvarial organ cultures, the calvariae were collected from newborn mice, dissociated into halves, and cultured for 24 h in BGJb medium containing bovine serum albumin (BSA) (1 mg/mL). After 24 h, the calvariae were transferred into new medium with or without test compounds, and cultured for 5 d. The bone-resorbing activity was determined by measuring the calcium in the medium.10)
Statistical AnalysesThe data were analyzed by a one-way ANOVA, followed by Tukey’s test for a post hoc analysis, and expressed as the mean±standard error of the mean (S.E.M.).
RESULTS
Effects of Curdlan, (1→3)-β-Glucan, on Osteoclast FormationWe examined the effects of low molecular-weight curdlan, (1→3)-β-glucan, on osteoclast formation in co-cultures of bone marrow cells and osteoblasts. As reported previously, the TLR2/6 ligand markedly induced osteoclast formation, and TRAP-positive multinucleated osteoclasts were formed. The addition of curdlan (0.001–10 µg/mL) suppressed the osteoclast formation induced by TLR2/6 ligand in a dose-dependent manner (Fig. 1B). Curdlan (10 µg/mL) did not induce osteoclast formation in the absence of TLR2/6 ligand (Fig. 1B).
Curdlan Acts on Both Osteoblasts and Osteoclast Precursor CellsWe next examined the action mechanism of curdlan in osteoclast formation. An RT-PCR revealed that in primary osteoblast cultures, the expression of RANKL mRNA was markedly induced by TLR2/6 ligand, and that it was clearly suppressed by the addition of curdlan (Fig. 2A). Curdlan did not influence the expression of RANKL in osteoblasts in the absence of TLR2/6 ligand (Fig. 2A). The expression of OPG mRNA was not influenced by TLR2/6 ligand or curdlan (Fig. 2A). In bone marrow macrophage cultures, sRANKL induced the differentiation of macrophages into mature osteoclasts. The addition of curdlan suppressed the differentiation of osteoclasts from the precursor cells in a dose-dependent manner (Fig. 2B). Curdlan (10 µg/mL) did not induce osteoclast differentiation in the absence of sRANKL (Fig. 2B). Thus, curdlan acts on both osteoblasts and osteoclast precursors to suppress osteoclast formation.
Effects of Curdlan on Bone Resorption in Mouse Calvarial Organ CulturesMouse calvarial organ culturing is a typical ex vivo assay system used to define the effects of test compounds on bone resorption. In this assay, the TLR2/6 ligand markedly induced the bone-resorbing activity detected by the increase levels of medium calcium. Adding low molecular-weight curdlan significantly suppressed the bone-resorbing activity induced by TLR2/6 ligand (Fig. 3). Curdlan did not influence on bone-resorbing activity in the absence of TLR2/6 ligand (Fig. 3).
DISCUSSION
In the present study, we showed that low molecular-weight curdlan, (1→3)-β-D-glucan suppressed the osteoclast formation induced by TLR2/6 ligand, and attenuated TLR2-induced inflammatory bone resorption in mouse calvarial organ cultures. Curdlan acted on mouse osteoblasts and suppressed the expression of RANKL, and it also acted on bone marrow macrophages and suppressed RANKL-dependent osteoclast differentiation from osteoclast precursor cells.
Previous studies have shown that β-glucan is a potent immunological stimulator and that its biological activities are somehow similar to those of TLR2 signaling in immune cells.8) In the present study, however, curdlan did not induce osteoclast formation, but clearly suppressed TLR2-induced bone resorption. Thus, β-glucan may act as an inhibitor of inflammatory bone resorption. Since β-glucan signaling is reported to be coordinated by TLR2 signaling in the innate immune system, it is possible that β-glucan interacts with TLR2 molecule on the osteoblast surface. Dectin-1 is a major receptor for β-glucan, but other receptors, such as CR3 and LacCer also recognize β-glucan.7) Yamasaki et al.11) reported that high molecule curdlan suppressed the differentiation of macrophage RAW 264.7 into osteoclasts through the overexpression of the dectin-1 gene. Further studies are needed to define the mechanisms of molecular interaction between curdlan and these receptors in osteoblasts and osteoclasts in bone.
The biological activity of β-glucan may be related to its molecular size, the degree of polymerization of glucose, and solubility. In the present study, we prepared curdlan (MW 3000) with low molecular solubility, and showed its effects on the regulation of bone resorption. We also tested the effects of other low molecular size β-glucan on osteoclast differentiation in the co-cultures of mouse bone marrow cells and osteoblasts. Three low molecular oligosaccharides, laminaritetraose (MW 666), laminarihexaose (MW 990), and laminarioligosaccharides (MW 1153) did not exhibit the suppressive effects on osteoclast differentiation (data not shown). High solubility may be important for it to exhibit biological activity, but the molecular size more than 1153 may be essential for the suppressive effects on osteoclast differentiation and bone resorption. Further studies are needed to define the role of the molecular size of curdlan in the biological activity and the mechanism of signaling pathway in osteoblasts and osteoclast precursors in bone tissues.
Periodontal diseases are infectious diseases that develop as a result of the accumulation of bacterial plaque in the periodontal pocket. In our original mouse model of periodontitis, we reported that the loss of alveolar bone was induced by not only the TLR4 ligand LPS but also TLR2 heterodimer signaling in vitro and in vivo.7) Thus, both Gram-positive and Gram-negative bacteria might be involved in alveolar bone loss. Silva et al.12) reported that β-glucan reduced glucose levels and attenuated alveolar bone loss in diabetic rats with periodontal disease. Further in vivo studies using β-glucan and TLRs are essential for defining the roles of β-glucan in the pathogenesis of periodontitis.
Acknowledgment
This work was partly supported by Institute of Global Innovation Research in Tokyo University of Agriculture and Technology (MI and FG).
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, San Martin J, Dansey R. Bench to bedside: elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov., 11, 401–419 (2012).
- 2) Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol., 11, 373–384 (2010).
- 3) Inada M, Matsumoto C, Uematsu S, Akira S, Miyaura C. Membrane-bound prostaglandin E synthase-1-mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharide-induced bone loss associated with inflammation. J. Immunol., 177, 1879–1885 (2006).
- 4) Kang JY, Nan X, Jin MS, Youn SJ, Ryu YH, Mah S, Han SH, Lee H, Paik SG, Lee JO. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity, 31, 873–884 (2009).
- 5) Matsumoto C, Oda T, Yokoyama S, Tominari T, Hirata M, Miyaura C, Inada M. Toll-like receptor 2 heterodimers, TLR2/6 and TLR2/1 induce prostaglandin E production by osteoblasts, osteoclast formation and inflammatory periodontitis. Biochem. Biophys. Res. Commun., 428, 110–115 (2012).
- 6) Goodridge HS, Wolf AJ, Underhill DM. β-Glucan recognition by the innate immune system. Immunol. Rev., 230, 38–50 (2009).
- 7) Brown GD, Gordon S. Immune recognition: A new receptor for β-glucans. Nature, 413, 36–37 (2001).
- 8) Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med., 197, 1107–1117 (2003).
- 9) Chen J, Seviour R. Medicinal importance of fungal β-(1→3), (1→6)-glucans. Mycol. Res., 111, 635–652 (2007).
- 10) Miyaura C, Inada M, Matsumoto C, Ohshiba T, Uozumi N, Shimizu T, Ito A. An essential role of cytosolic phospholipase A2α in prostaglandin E2-mediated bone resorption associated with inflammation. J. Exp. Med., 197, 1303–1310 (2003).
- 11) Yamasaki T, Ariyoshi W, Okinaga T, Adachi Y, Hosokawa R, Mochizuki S, Sakurai K, Nishihara T. The dectin 1 agonist curdlan regulates osteoclastogenesis by inhibiting nuclear factor of activated T cells cytoplasmic 1 (NFATc1) through Syk kinase. J. Biol. Chem., 289, 19191–19203 (2014).
- 12) Silva VO, Lobato RV, Andrade EF, Macedo CG, Napimoga JTC, Napimoga MH, Messora MR, Murata RM, Pereira LJ. β-Glucans (Saccharomyces cereviseae) reduce glucose levels and attenuate alveolar bone loss in diabetic rats with periodontal disease. PLOS ONE, 10, e0134742 (2015).