2018 年 41 巻 8 号 p. 1251-1256
2018 年 41 巻 8 号 p. 1251-1256
Porcine placental extract (PPE) is used as a nonprescription drug for analeptics and in health foods and cosmetics in Japan, Korea and China. It was reported that PPE has anti-oxidative and anti-inflammatory activities; however, the mechanisms and the responsible molecules involved in these activities are still unclear. Here, we investigated how enzymatically prepared PPE affects proinflammatory factors such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α in a cultured macrophage cell line, RAW264.7, when co-stimulated with lipopolysaccharide (LPS). Enhanced production of IL-1β, IL-6 and TNF-α by LPS was significantly reduced by the addition of PPE and these effects were dose dependent. Nitric oxide (NO) production induced in cultured macrophages by LPS was also inhibited by PPE. Real-time PCR after the reverse transcription of total RNAs isolated from cells treated with PPE revealed that the mRNA expressions of IL-1β, IL-6, TNFα, and NO synthase (NOS)-2 were reduced. The necessary concentration of PPE prepared by enzymatic digestion to mediate anti-inflammatory effects compared with the reported value of that extracted by phosphate buffered saline without digestion was proportional to the amount of extracted materials from the same amount of placenta (about 10-fold). This suggests that the molecules responsible for the anti-inflammatory activity exists in the placenta and can be extracted by phosphate buffered saline, and thus might survive enzymatic digestion.
Symptoms of metabolic syndrome induced by obesity are observed in the adipose tissues.1,2) In obesity, inflammatory cell infiltration of white adipose tissue and chronic inflammation induce insulin resistance and abnormal glucose tolerance.3) Chronic inflammation is associated with many age-related pathophysiologic processes and diseases, including Alzheimer’s disease, diabetes, atherosclerosis, osteoarthritis and cancer.4) Recently, the relationship between chronic inflammation and endotoxin derived from microbiota have attracted attention.3) Placental extract (PE) was reported to have medicinal effects. For example, PE contains uracil, tyrosine, phenylalanine, and tryptophan, which have strong anti-oxidative and anti-inflammatory activities.5–8) Kawakatsu et al. demonstrated that PPE protected bone marrow-derived stem/progenitor cells against radiation injury through anti-inflammatory activity.9) Placental extracts were reported to have anti-oxidative and anti-inflammatory effects. Lee et al.10) reported that human placental extract (HPE) significantly inhibited the production of nitric oxide, tumor necrosis factor-α and cyclooxygenase-2, whereas Chakraborty et al.11) reported that HPE produced nitric oxide (NO) in mouse peritoneal macrophages. HPE decreased lipopolysaccharide (LPS)-induced microglial cell death by 24% and attenuated the LPS-induced expression of proinflammatory proteins, inducible NO synthase (iNOS) and cyclooxygenase (COX)2, in microglial cells by 34 and 28%, respectively.12)
However, it might be important to well recognize differences in the origin and the production method of each placental extract used in each experiment. Recently, various products termed “placental extract” from non-animal origins were developed, e.g., so-called marine-placental extract (fresh salmon ovary membrane extract being sold in Japan under the name of “Marine Placenta”) and rose-placental extract (cultured rose placental cells).13) In addition, some placental extracts of animal origin contain other non-placental internal organs, which can cause confusion among consumers. Furthermore, so-called “fermented porcine placental extracts” (FPPE) have been developed, where the porcine placenta is partially hydrolyzed by proteases and then fermented using yeast and black strap powder.14) In other experiments, the product was simply extracted with phosphate buffered saline (PBS) without enzymatic digestion.15) To authorize true placental extracts of animal origin to be used in foods, the Japan Health and Nutrition Food Association defined the Japan Health Food Authorization (JHFA) standard of placental extract.16) The PPE used in this study was prepared by enzymatic digestion and conforms to the JHFA standard of placental extract for food.
Several papers reported anti-inflammatory effects of PPEs prepared by different methods. Nam et al.17) reported that LPS-induced interleukin-1-beta (IL-1β), tumor necrosis factor-α (TNF-α), and IL-6 production in RAW264.7 macrophages was markedly inhibited by FPPE. Kim et al.15) reported that γ-irradiated PBS-extracted PPE (PBS-PPE) exerted more effective and potent anti-inflammatory effects compared with autoclaved PBS-PPE. We recently reported that PPE prepared by enzymatic digestion method selectively promoted the proliferation of specific progenitor cells derived from murine marrow cells,18) and modulated the expression of skin functional proteins in cultured human fibroblasts and keratinocytes.19)
In the present study, we focused on the anti-inflammatory effects of PPE prepared by enzymatic digestion method and compared the properties of its active components with those of other PPEs produced by the different methods. Specifically, we report here that the PPE prepared by enzymatic digestion method inhibited the proinflammatory factors IL-1β, IL-6, and TNF-α in LPS-stimulated RAW264.7 cells.
The extract of porcine placenta and its water-soluble portion were prepared as previously reported.5,20,21) In brief, freshly isolated placenta was washed with cold water to remove excess blood, other organs and lipid tissues. The cleaned placenta was homogenized and dissolved in distilled water, and then protease was added.5,20,21) After incubation, digested supernatants were collected and concentrated using an evaporator (EVAPOR, Okawara MFG Co., Ltd., Shizuoka, Japan) in vacuo. The concentrated extracts were further sterilized by heating at 90°C for 30 min. After sterilization, the supernatants were dried using a spray dryer (Powdering Japan Inc., Saitama, Japan) to produce a light gray powder (PPE: approximately 54 g of powder from 1 kg wet weight of placenta).
Cell CultureThe murine macrophage RAW264.7 cell line was obtained from the European Collection of Authenticated Cell Cultures (ECAC C), a Culture Collection of Public Health England (U.K.). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, U.S.A.) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, U.S.A.), 100 U/mL of penicillin, and 100 µg/mL of streptomycin (Gibco) in a humidified atmosphere of 37°C in 5% CO2 with 95% atmospheric air.
Cytotoxicity AssayRAW264.7 cells at a density of 1×106 cells/mL were cultured in a 96-well plate (100 µL) with 10% FBS-DMEM for 24 h. The cells were subsequently treated with various concentrations of porcine placental extract (0–20 mg/mL) and 50 µM of dexamethasone (DEX) dissolved in 5% FBS-DMEM was used as a positive control. After incubation for 24 h, viable cells were counted by adding 0.5 mL of medium with 20 µL of Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) solution, incubating for 1–2 h, and measuring the amount of formazan produced using a microplate reader at 450 nm (Bio-Rad Laboratories, Inc., CA, U.S.A.).
Measurement of NO ProductionRAW264.7 cells were plated at 4×105 cells/mL with 10% FBS-DMEM in 24-well plates (1 mL) and incubated for 24 h. The cells were then cultured in 5% FBS-DMEM containing various concentrations of test samples for 3 h, followed by treatment with 1 µg/mL of LPS for an additional 24 h. The culture medium (100 µL volume) was collected and mixed with an equal volume of Griess reagent containing 1% sulfanilamide, 0.1% naphthalene diamine dihydrochloride and 2% phosphoric acid and then incubated for 20 min at room temperature. The absorbance was measured at 540 nm using a microplate reader.
Measurement of TNF-α, IL-6, and IL-1β ProductionRAW264.7 cells were plated at 1×106 cells/mL with 10% FBS-DMEM in 96-well plates (100 µL) and incubated for 24 h. The cells were cultured in 5% FBS-DMEM containing various concentrations of test samples for 3 h, followed by treatment with 1 µg/mL of LPS for an additional 24 h. The concentrations of TNF-α, IL-6, and IL-1β in culture media were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Affymetrix, Inc., San Diego, CA, U.S.A.) according to the manufacturer’s instructions.
mRNA Expression Determined by Real-Time Quantitative RT-PCR (qRT-PCR)RAW264.7 cells were seeded at 4×105 cells/mL with 10% FBS-DMEM in 24-well plates (1 mL) and incubated for 24 h. The cells were then cultured in 5% FBS-DMEM containing various concentrations of test samples for 3 h, followed by treatment with 1 µg/mL of LPS for an additional 6 h. After incubation, total RNA was extracted using a NucleoSpin RNA kit (TaKaRa Bio Inc., Shiga, Japan). Reverse transcription was performed using PrimeScript RT Master Mix (TaKaRa Bio Inc.). Real-time PCR was performed using a Thermal Cycler Dice Real Time System II and SYBR Premix Ex Taq II (TaKaRa Bio Inc.). Reaction mixtures consisted of 0.2 µL each of 50 µM forward and reverse primers, 2.5 µL of template cDNA, 9.6 µL of ultra-purified water, and 12.5 µL of SYBR Premix Ex Taq II. The mRNA primer sequences for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), IL-1β, IL-6, TNF-α, and iNOS used in this study are listed in Table 1. Second-strand cDNA syntheses and PCR amplifications were carried out with the following program: 1 cycle at 95°C for 30 s, 40 cycles at 95°C for 5 s, and 40 cycles at 60°C for 30 s. The kinetics of PCR amplification were determined using data from quadruplicate samples. The expression levels were calculated using the ΔΔCt method.
| Gene name | Access No. | Sequence | Size (bp) |
|---|---|---|---|
| GAPDH | NM_008084.3 | Forward TGTGTCCGTCGTGGATCTGA | 150 |
| Reverse TTGCTGTTGAAGTCGCAGGAG | |||
| IL-1β | NM_008361.3 | Forward GCAACTGTTCCTGAACTCAACT | 89 |
| Reverse ATCTTTTGGGGTCCGTCAACT | |||
| IL-6 | J03783 | Forward AGTTGCCTTCTTGGGACTGA | 191 |
| Reverse CAGAATTGCCATTGCACAAC | |||
| TNFα | NM_013693.2 | Forward GTGGAACTGGCAGAAGAGGC | 122 |
| Reverse AGACAGAAGAGCGTGGTGGC | |||
| iNOS | NM_010927.3 | Forward TCCATGACTCCCAGCACA | 108 |
| Reverse CCATCTCCTGCATTTCTTCC |
PPE was dissolved in distilled water at a concentration of 100 mg/mL and 500 µL of the sample was applied to a Superdex Peptide 10/300GL column (GE Health Care) equilibrated with a 5-fold dilution of PBS. Each fraction was collected at 2 mL/tube using an AKTA pure 25 system (GE Health Care), pooled through repeated runs, and lyophilized.
Measurement of Molecular Weight DistributionThe lyophilized PPE fractions were dissolved in distilled water at a concentration of 20 mg/mL or 4 mg/mL. Then, 20 µL of samples were analyzed by HPLC (Agilent1200) using a Superdex Peptide 10/300GL size-exclusion column (GE Health Care) eluted with 20 mM phosphate buffer (pH 7.2) containing 0.25 M NaCl. The flow rate was 0.5 mL/min and the monitoring wavelength was 214 nm. The molecular weights of peaks were calculated using a calibration curve with glycine, triglycine, hexaglycine, insulin chain B, and cytochrome c.
Statistical AnalysisAll data are presented as the mean±standard deviation (S.D.). Statistical analyses were performed with GraphPad InStat Version 3.10 software (Graph Pad, La Jolla, CA, U.S.A.). Statistical significance was determined by Dunnett’s test after one-way ANOVA for comparisons with non-treated control cultures or non-stimulated LPS cultures. p<0.05 was considered significant.
The HPLC analysis of PPE dissolved in H2O is shown in Fig. 1. The peaks were mostly assigned to amino acids, oligopeptides, mucopolysaccharides, and nucleic acids.

PPE dissolved in H2O was analyzed by a reverse-phase ODS column (InertSustain ODS: 5 µm, 3.0×250 mm) using a linear gradient of aqueous acetonitrile (0→100%). Samples (5 µL of 1 mg/mL) were loaded and monitored by dual ultraviolet absorbance at 254 and 280 nm for 60 min. The elution rate was 0.2 mL/min.
The proliferation of RAW264.7 cells was evaluated by CCK-8 assay. As shown in Fig. 2, RAW264.7 cell viability was not significantly altered by incubation with up to 20 mg/mL PPE. Dexamethasone, the positive control, also did not affect the viability of RAW264.7 cells.

RAW264.7 cells were cultured with or without various concentrations of PPE (0–20 mg/mL) or DEX (50 µM of dexamethasone as a positive control). Cell viability was measured after 24 h of cultivation. Data are expressed as the means±S.D. of quadruplicate cultures.
RAW264.7 cells were treated with various concentrations of PPE (0–20 mg/mL, or 50 µM of dexamethasone as a positive control) for 3 h, followed by treatment with 1 µg/mL of LPS for an additional 24 h. NO production was measured with Griess reagent. LPS treatment significantly increased NO production. However, co-incubation with PPE (5, 10, 20 mg/mL) reduced LPS-stimulated NO production dose-dependently (Fig. 3).
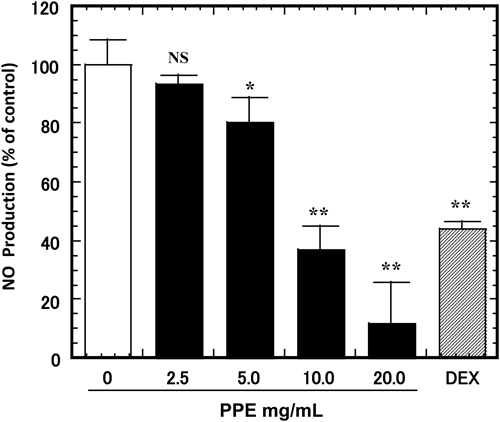
RAW264.7 cells were cultured with or without various concentrations of PPE (0–20 mg/mL) or DEX (50 µM of dexamethasone as a positive control) for 3 h. After the pre-culture, the culture plate was stimulated with LPS (1 µg/mL) for 24 h. NO production was measured with Griess reagent. Data are expressed as the means±S.D. of quadruplicate cultures. * p<0.05 ** p<0.01, PPE vs. control culture.
To determine the effects of PPE on the production of proinflammatory cytokines (IL-1β, IL-6, and TNF-α), RAW264.7 cells were pretreated with various concentrations of PPE (0–20 mg/mL or 50 µM of dexamethasone as a control) and stimulated with 1 µg/mL of LPS for 24 h. Levels of IL-1β, IL-6, and TNF-α in the culture media were significantly decreased by PPE in a dose-dependent manner (Fig. 4).
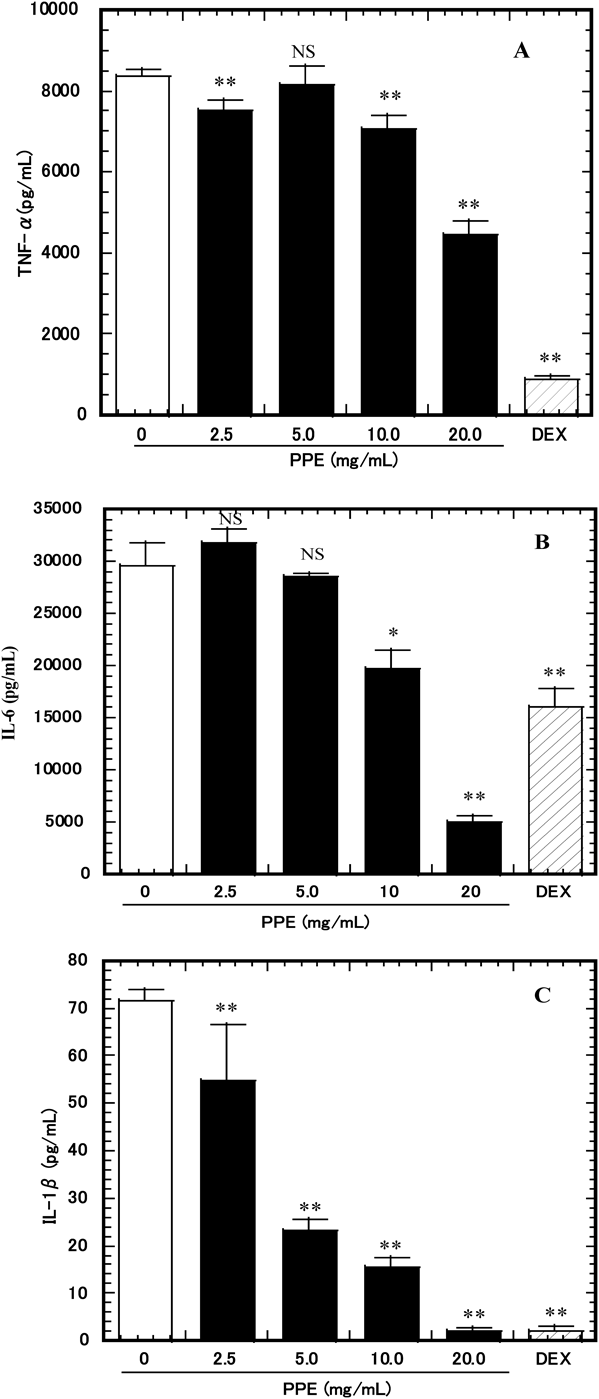
RAW264.7 cells were pretreated with various concentrations of PPE (0–20 mg/mL) or DEX (50 µM of dexamethasone as a positive control) and stimulated with 1 µg/mL of LPS for 24 h. Quantitation of TNF-α (A), IL-6 (B) and IL-1β (C) in the culture media was performed by ELISA. Data are expressed as the means±S.D. of quadruplicate cultures. * p<0.05 ** p<0.01, PPE vs. control culture.
To investigate the effect of PPE anti-inflammatory activity at the transcriptional revel, we examined iNOS, TNF-α, IL-6, and IL-1β mRNA expressions in LPS-stimulated RAW264.7 cells. As shown in Fig. 5, LPS (1 µg/mL) stimulation increased the mRNA expressions of iNOS, TNF-α, IL-6, and IL-1β compared with non-LPS-stimulated controls. At a concentration of 5–20 mg/mL, PPE significantly decreased the mRNA expressions of iNOS (p<0.01) and IL-6 (p<0.01) (Figs. 5A, C). At a concentration of 20 mg/mL, PPE significantly decreased the mRNA expression of TNF-α (p<0.05) (Fig. 5B). At a concentration of 10–20 mg/mL, PPE significantly decreased the mRNA expression of IL-1β (p<0.01) (Fig. 5D).

RAW264.7 cells were pretreated with various concentrations of PPE (0–20 mg/mL) or DEX (50 µM of dexamethasone as a positive control) for 3 h before stimulation with LPS (1 µg/mL) for 6 h. The mRNA levels of iNOS (A), TNF-α (B), IL-6 (C), and IL-1β (D) were measured by qRT-PCR with GAPDH as an internal control. Data are expressed as the means±S.D. of quadruplicate cultures. * p<0.05 ** p<0.01, PPE vs. control culture.
The lyophilized PPE fractions were dissolved in 5% FBS-DMEM at a concentration of 10 mg/mL. The effects of PPE fractions on LPS-induced NO production were examined as described in the Materials and methods. RAW264.7 cells were treated with PPE fractions for 3 h, followed by treatment with 1 µg/mL of LPS for an additional 24 h. NO production was measured with Griess reagent. The result was expressed as values relative to NO production in the absence of the PPE fraction, which was set as 100% (Fig. 6). As shown in the figure, fractions 4 and 10 reduced LPS-stimulated NO production (Fig. 6). The molecular weight of the main peak in fraction 4 was estimated to be 47 kDa and fraction 10 consisted of two peaks of 28 Da and 53 Da, respectively (Fig. 7). Fractions 6, 7 and 8 increased LPS-stimulated NO production compared with the control (Fig. 6).
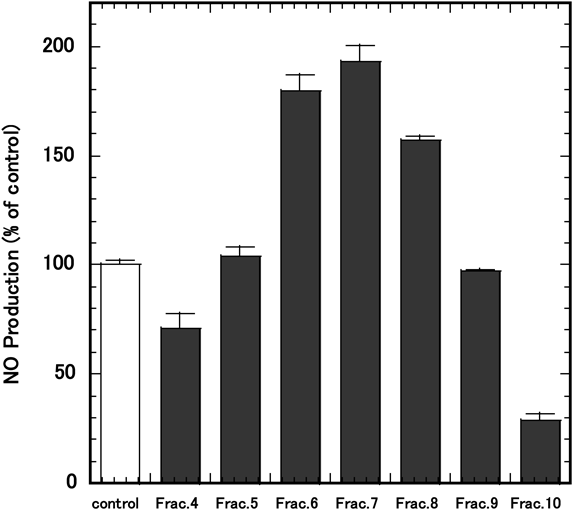
RAW264.7 cells were treated with PPE fractions (10 mg/mL) for 3 h, followed by treatment with 1 µg/mL of LPS for an additional 24 h. NO production was measured with Griess reagent. Data are expressed as the means±S.D. of triplicate cultures.

The lyophilized PPE fractions were dissolved in distilled water at a concentration of 20 or 4 mg/mL. Then, 20 µL of samples were analyzed by HPLC (Agilent1200) using a Superdex Peptide 10/300GL column. The elution used 20 mM phosphate buffer (pH 7.2) containing 0.25 M NaCl. The elution rate was 0.5 mL/min and was monitored at 214 nm.
Here, we showed that PPE markedly suppressed the production of IL-1β, IL-6, TNF-α and iNOS proteins in LPS-stimulated RAW264.7 cells. The mRNA expressions of IL-1β, IL-6, TNF-α and iNOS induced by LPS were also significantly inhibited by PPE, suggesting PPE inhibits inflammatory reactions induced by LPS at or before the expression of these genes. Our result was in agreement with the report of Lee et al.10) who used HPE prepared by enzymolysis treatment with pepsin and chemical hydrolysis with hydrochloric acid, although our PPE only underwent enzymatic digestion. When we compared the water-soluble PPE used in this study with equine placental extract (EPE, Snow placenta gold powder NH, Snowden, Tokyo, Japan), the same anti-inflammatory effect was observed. This suggests that the suppression of LPS-induced inflammation by PE is not affected by species differences, at least between human, porcine and equine extracts. Furthermore, our result was also in agreement with the study of Nam et al.17) who used FPPE, manufactured by fermenting PPE. Gly-Leu and Leu-Gly dipeptides, the proposed active components of FPEE, are considered candidate anti-fatigue and anti-inflammation agents. We cannot refer to the active constituents of the water-soluble portion that inhibited cytokine production; however, we used fractionation by gel filtration to investigate the active component of PPE. As a result, the constituent of PPE that suppressed NO production induced by LPS stimulation was estimated to have a molecular weight of approximately 47 kDa (fraction 4) and/or approximately 28–53 Da (fraction 10), which was smaller than glycine (75 Da). The retarded peaks (fraction 10) may be derived from hydrophobic constituents of larger molecular weights because of their possible hydrophobic interaction with the column. Interestingly, NO production was enhanced by adding fractions 6, 7, and 8 from PPE. However, we cannot explain why these fractions stimulated NO production at present.
Kim et al.15) recently reported that crude PBS extract prepared directly from porcine placenta inhibited the production of TNF-α, IL-1β as well as NO in activated macrophages as in our PPE prepared by enzymatic digestion. The necessary concentrations of the water-soluble portion of enzymatically digested PPE were approximately 10 times higher than that obtained by PBS crude extracts without digestion in the study by Kim et al.15) Based on our experiences, PBS extraction from homogenized porcine placenta was not efficient and much of the tissue remained unextracted (about only 0.43 g dry weight of powder was extracted from 100 g wet weight of homogenized placenta). In contrast, enzymatic digestion solubilized most of the placenta (5.4 g from 100 g wet weight of placenta=about 6.5 g of dry weight). Therefore, the water-soluble portion of the enzymatically digested PPE might be about 12.6-fold of the PBS-extracted PPE, which might explain the difference in the amount of PBS-PPE and PPE required to inhibit LPS-induced proinflammatory cytokines. This also suggests that the molecules in PPEs that mediate the anti-inflammatory activity can survive disruptive enzymatic digestion. Furthermore, because other PPEs prepared with “enzymatic digestion and chemical hydrolysis with hydrochloric acid”10) and “enzymatic digestion and bacterial fermentation”17) also demonstrated anti-inflammatory activities, the responsible molecules might survive these destructive methods as well. Taken together, these results indicate that the anti-inflammatory components in the placenta can be extracted simply with PBS, as well as survive destructive enzymatic digestion, chemical hydrolysis, and fermentation, therefore suggesting they are chemically stable, water-soluble, small molecules. However, because Kim et al.15) reported that the anti-inflammatory activity in PBS-PPE was inactivated by autoclaving, the active components might interact with a larger molecule that is present in PBS-PPE at high temperatures. The elucidation of the molecular nature of these anti-inflammatory molecules should be determined in the future. In summary, placental extract might be a promising ingredient of anti-inflammatory health foods.
We thank Dr. Yasuhiko Komatsu at Snowden Co., Ltd. for the critical reading and discussion of the manuscript.
M.T., Y.D., H.I., and J.K. are employees of Snowden Co., Ltd.
The online version of this article contains supplementary materials.