2019 Volume 42 Issue 11 Pages 1830-1838
2019 Volume 42 Issue 11 Pages 1830-1838
Colorectal cancer (CRC) is one of the most common malignant tumors and the third leading cause of cancer-related deaths in the world. It was reported that sophocarpine could attenuate the progression of CRC in mice. However, the mechanisms by which sophocarpine regulate the proliferation and migration in CRC remain unclear. Thus, this study aimed to investigate anti-tumor mechanisms of sophocarpine in CRC cells. CCK-8 assay, wound healing assay and transwell migration were used to detect cell proliferation and migration, respectively. In addition, Western blotting and enzyme-linked immunosorbent assay (ELISA) were used to further detect protein expressions and cytokines in vitro. The results revealed that sophocarpine significantly inhibited proliferation in HCT116 and SW620 cells, respectively. Meanwhile, sophocarpine inhibited CRC cells migration via downregulation of the levels of N-cadherin, matrix metalloproteinase (MMP)-9, phosphorylated extracellular signal-regulated kinase (p-ERK), p-mitogen-activated protein kinase kinase (MEK), vascular endothelial growth factor (VEGF)-A, VEGF-C and VEGF-D. Moreover, overexpression of MEK reversed the anti-migration effects of sophocarpine on CRC cells via upregulation of VEGF-A/C/D. Our findings indicated that sophocarpine could inhibit CRC cells migration via downregulation of MEK/ERK/VEGF pathway. Thus, sophocarpine may act as a potential agent for the treatment of CRC.
Colorectal cancer (CRC) is one of the most common malignant tumors and the third leading cause of cancer-related deaths in the world.1,2) The primary causes of death in patients with CRC are due to the abnormal growth, migration, and metastasis of the tumor.3) Clinically, about 65% of patients with CRC survive for an average of 5 years.4) However, if made a definite diagnose in early stage, the 5-year overall survival could improve to 90%. But, if patients were diagnosed in late stages with CRC metastasis, the overall survival rate could be declined to merely 12%.5,6) Metastasis and migration are mediated by multiple factors, including vascular endothelial growth factor (VEGF), cytokines and so on, and VEGF signaling pathway regulates migration in multiple cell types.7) Previous study has shown that VEGF signaling triggers migration via regulating cell signaling processes in endothelial cells.8) VEGF was demonstrated to trigger tumor migration via regulation of downstream pathway the mitogen-activated protein kinase kinase/extracellular signal-regulated protein kinases (MEK/ERK).9,10) The MEK/ERK cell signaling pathway popularly play an important role in various human cancers, which involved in cellular physiological functions such as proliferation, survival, metabolism and cell migration.11) Therefore, it is imperative to illuminate the roles of migration-related molecules and the possible mechanisms they participate in CRC.
Sophocarpine is a compound derived from the foxtail-like sophora herb, which is a tetracyclic quinolizidine alkaloid.12) Previous studies have shown that sophocarpine possessed anti-cancer activities on different tumor types such as head and neck cancer, hepatocellular carcinoma and prostate cancer.12–15) However, the anti-migration activities of sophocarpine on CRC and its underlying mechanisms remain unclear.
Hence, we aimed to investigate the effects of sophocarpine on proliferation and migration of CRC cells in vitro. Moreover, the mechanisms underlying the anti-migration effect of sophocarpine on CRC cells were explored.
HCT116 (cat. no. CCL-247™) and SW620 (cat. no. CCL-227™) human colon cancer cell lines were obtained from American Type Culture Collection (ATC C, Rockville, MD, U.S.A.) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) medium (cat. no. 10569044, Thermo Fisher Scientific, Waltham, MA, U.S.A.) containing 10% fetal bovine serum (FBS, cat. no. F8192, Sigma-Aldrich, St Louis, MO, U.S.A.), penicillin and streptomycin (cat. no. P0781, 100 U/mL, Sigma-Aldrich) in a humidified incubator with 5% CO2 at 37°C.
CCK-8 Assay of Cell ViabilityHCT116 or SW620 cells were plated at 5 × 103 cells/well onto 96-well plate overnight at 37°C. After that, the cells were incubated with different concentrations of sophocarpine (0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4 or 1.6 mM) for 24, 48 or 72 h, respectively. At indicated time point, CCK-8 (10 µL, cat. no. C0037, Beyotime Biotechnology, Beijing, China) reagent was added to each well for another 1 h. Then, a microplate reader (Bio-Rad Laboratories, Benicia, CA, U.S.A.) was used to measure the absorbance at 450 nm. Sophocarpine was provided from Abcam (cat. no. ab143179, Cambridge, MA, U.S.A.).
Migration AssayCell migration assay was conducted using transwell chambers with an 8-µm-pore-sized polycarbonate filter membrane (cat. no. 354483, Corning Costar, Cambridge, MA, U.S.A.). HCT116 and SW620 cells resuspended in serum-free medium were seeded onto the upper chamber with serum-free culture medium. The lower chambers were filled with medium containing 10% FBS. After 24 h of incubation at 37°C, the invaded cells on the underside were fixed with 4% formaldehyde, and stained with 0.05% crystal violet for 2 h. The migrated cells were counted under microscope and quantified by ImageJ software (NIH, Bethesda, MD, U.S.A.).
Wound Healing AssayWound healing assay was carried out to determine migration of CRC cells. In brief, HCT116 and SW620 cells (4 × 105 cells per well) were seeded into 6-well plate overnight. When cells were grown to 80% confluences in 6-wells plates, a ‘scratch’ was made in the cell monolayer to create a wound. Then cells were washed with warmed PBS and treated with sophocarpine (0 or 0.4 mM) for 0 h, 24, 48 and 72 h at 37°C. Images were observed at 0 h, 24, 48 and 72 h by fluorescence microscope (Leica M165 FC, Buffalo Grove, IL, U.S.A.). The initial wound width (Wi0 h) and the final wound width (Wf) at 24, 48, 72 h after scratching were observed and photographed. Migration distance (Md24 h) = Wi0 h−Wf24 h, wound healing rate (100%) = Md24 h, sophocarpine/Md24 h, control × 100%.
Western Blot AnalysisThe cells were lysed in RIPA lysis buffer (cat. no. P0013B, Beyotime) at room temperature for 10 min. BCA protein assay kit (cat. no. P0011, Beyotime) was used to quantification the proteins concentration. Then, proteins were separated by the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After that, proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (cat. no. T2234, Thermo Fisher Scientific) at room temperature for 2 h. Later on, the PVDF membranes were blocked with 5% skimmed milk powder in TBST at room temperature for 1 h. Next, the PVDF membranes were incubated with primary antibodies at 4°C overnight: MEK (cat. no. ab178876, Abcam) (1 : 1000), p-MEK (cat. no. ab96379, Abcam) (1 : 1000), ERK (cat. no. ab54230, Abcam) (1 : 1000), p-ERK (cat. no. ab50011, Abcam) (1 : 1000), E-cadherin (cat. no. ab18203, Abcam) (1 : 1000), MMP-9 (cat. no. ab38898, Abcam) (1 : 1000), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (cat. no. ab8245, Abcam) (1 : 1000), p-VEGFR2 (cat. no. ab5473, Abcam) (1 : 1000), VEGFR2 (cat. no. ab2379, Abcam) (1 : 1000). After washing in TBST twice, the membranes were incubated with secondary antibody (goat anti-rabbit, cat. no. ab7090, Abcam) (1 : 5000) at room temperature for 1 h. Finally, the bands were visualized using the enhanced chemiluminescence system reagent (ECL, cat. no. WP20005, Thermo Fisher Scientific).
Measurement of Cytokines by Enzyme-Linked Immunosorbent Assay (ELISA)HCT116 or SW620 cells were seeded into a 24-well plate (2 × 104 cells/well) and cultured in mediu for 24 h. Then, the cells were treated with sophocarpine (0, 0.4 mM) for another 48 h. Samples of the supernatant were collected from each well to measure VEGF-A (cat. no. H044), VEGF-C (cat. no. H046), VEGF-D (cat. no. H047) levels by ELISA. ELISA kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Construction of Recombinant LentivirusThe lenti-vector and MEK plasmids were purchased by Genepharm (Shanghai, China). The lenti-vector and MEK plasmids were transfected into 293T cells and incubated at 32°C for 48 h. Then, the supernatant was collected, which was containing the retroviral particles.
Exogenous MEK OverexpressionHCT116 or SW620 cells (4 × 105 cells per well) were seed into cell plates (60 mm) at 37°C overnight. Then, HCT116 or SW620 cells (with 50–60% of confluence) were transfected with lentivirus MEK supernatants for 24 h, respectively. After that, cells were treated with puromycin (2.5 µg/mL, cat. no. A1113802, Thermo Fisher Scientific) for another 3 d. Western blotting assay were applied to determine expression of MEK in the stable cells.
Quantitative (q) Real-Time PCRTotal RNA was extracted from HCT116 or SW620 cells by using TRIzol reagent (cat. no. 15596018, Thermo Fisher Scientific). Reverse transcription was performed using the cDNA Reverse Transcription Kit (cat. no. 4368814, Thermo Fisher Scientific). After that, PowerUp™ SYBR™ Green Master Mix (cat. no. A25742, Thermo Fisher Scientific) was used with QuantStudio™ 7 Flex Real-Time PCR System (Thermo Fisher Scientific) for qRT-PCR analysis according to the manufacturer’s instructions. QRT-PCR reactions were performed as follows: 95°C for 10 min followed by 35 cycles of 94°C for 45 s, 66°C for 30 s, 72°C for 45 s. The relative gene level was normalized to GAPDH using 2−ΔΔCt method.
Statistical AnalysisAll results were expressed as the mean ± standard deviation (S.D.). Statistical analyses were performed using SPSS version 22.0 software (SPSS Inc., Chicago, IL, U.S.A.). The comparison between two groups was assessed by Student’s t-test. The one-way ANOVA followed by Dunnett’s test was used to evaluate statistical differences among multiple. p < 0.05 was considered to indicate a statistically significant difference (* p < 0.05, ** p < 0.01). All assays were performed independently at least 3 experiments
First, CCK-8 assay was used to evaluate the effects of sophocarpine on the viability of HCT116 and SW620 cells. As indicated in Figs. 1A–1C, sophocarpine suppressed the proliferation of CRC cells in a concentration-dependent manner. Since the number of cells was decreased approximately 50% following treatment with 0.4 mM sophocarpine for 48 h, 0.4 mM sophocarpine was utilized in the following experiments. These results suggested that sophocarpine could suppress the proliferation of CRC cells.

The viability of CRC cells were investigated with CCK-8 assays. (A, B, C) HCT116 and SW620 cells were treated with sophocarpine (0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4 or 1.6 mM) for 24, 48 and 72 h, respectively. (D) HCT116 cells were treated with sophocarpine (0 or 0.4 mM) for 24 h. Then, cell migration was detected with transwell assay. (E) SW620 cells were treated with sophocarpine (0 or 0.4 mM) for 24 h respectively. Cell migration was detected with transwell assay. ** p < 0.01 vs. 0 mM group.
The transwell migration and wound healing assays were used to determine the effects of sophocarpine on the migration of CRC cells. The results of transwell assay indicated that sophocarpine notably decreased the migration ability of CRC cells, compared with the control group (Figs. 1D, 1E). Meanwhile, wound healing assay further demonstrated that sophocarpine significantly decreased the migration ability of CRC cells at the time points of 48 and 72 h, compared with the control group (Figs. 2A, 2B). These data indicated that sophocarpine could inhibit the migration of CRC cells.

(A) HCT116 were treated with sophocarpine (0 or 0.4 mM) for 24, 48 and 72 h, respectively. Cell migration was detected with wound healing assay. (B) SW620 cells were treated with sophocarpine (0 or 0.4 mM) for 24, 48 and 72 h, respectively. Cell migration was detected with wound healing assay. ** p < 0.01 vs. 0 mM group.
VEGF family plays an important role in tumor growth and migration, such as inhibiting apoptosis, promoting migration.16,17) Therefore, ELISA assay was used to determine the levels of VEGF-A, VEGF-C and VEGF-D in CRC cells supernatant. As indicated in Figs. 3A–3C, the levels of VEGF-A, VEGF-C and VEGF-D in CRC cells supernatant were significantly downregulated in sophocarpine treated group, compared with control group. These results suggested that sophocarpine could inhibit the level of VEGF family cytokines in CRC cells supernatant.

HCT116 and SW620 cells were treated with sophocarpine (0 or 0.4 mM) for 48 h. (A) The level of VEGF-A in the cell culture supernatant was measured with ELISA. (B) The level of VEGF-C in the cell culture supernatant was measured with ELISA. (C) The level of VEGF-D in the cell culture supernatant was measured with ELISA. ** p < 0.01 vs. 0 mM group.
We next explored the mechanism by which sophocarpine inhibited the migration of CRC cells. The levels of p-MEK, p-ERK, E-cadherin, MMP9 and p-VEGFR2 were detected by Western blotting. The results showed the expressions of p-MEK, p-ERK, E-cadherin, MMP9 and p-VEGFR2 in CRC cells were markedly decreased in sophocarpine treated group, compared with the control group (Figs. 4A–4C). In addition, sophocarpine notably inhibited the levels of p-MEK and p-ERK at early time point (Supplementary Figs. 1A–1C). Taken together, these data suggested that sophocarpine could exhibit anti-migration effect on CRC cells via inhibition of MEK/ERK/VEGF pathway.
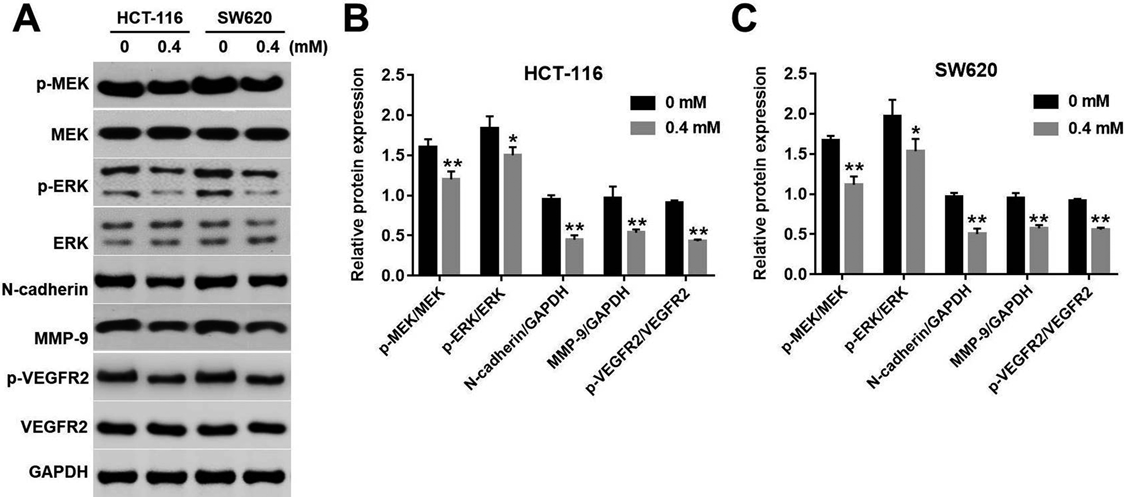
HCT116 and SW620 cells were treated with sophocarpine (0 or 0.4 mM) for 48 h. (A) Expression levels of p-MEK, p-ERK, N-cadherin, MMP9 and p-VEGFR2 in HCT116 and SW620 cells were detected with Western blotting. GAPDH was used as an internal control. (B, C) The relative expressions of p-MEK, p-ERK and p-VEGFR2 in CRC cells were quantified via normalizing to MEK, ERK and VEGFR2, respectively. The relative expressions of N-cadherin and MMP9 in CRC cells were quantified via normalizing to GAPDH. ** p < 0.01 vs. 0 mM group.
To further investigate whether the MEK/ERK/VEGF pathway was regulated by sophocarpine in CRC cells, exogenous MEK overexpression was applied. As shown in Figs. 5A–5D, the expressions of p-MEK, p-ERK, N-cadherin and MMP9 were markedly decreased in sophocarpine treated group, while the levels of MEK, p-ERK, N-cadherin and MMP9 were significantly increased in lenti-MEK transfected CRC cells. Meanwhile, the result of qRT-PCR indicated sophocarpine inhibited N-cadherin and MMP9 gene expressions in CRC cells, which were revered by MEK OE (Figs. 5E, 5F). In addition, sophocarpine-induced VEGF-A, VEGF-C and VEGF-D cytokines decrease were markedly reversed following transfection with lenti-MEK in CRC cells (Figs. 6A–6C). These results suggested overexpressing of MEK reversed the inhibitory effect of sophocarpine on MEK/ERK/VEGF pathway in CRC cells.

Lenti-MEK was transfected into HCT116 and SW620 cells with or without sophocarpine for 72 h, respectively. (A) Protein expression of p-MEK, p-ERK, N-cadherin and MMP9 in HCT116 cells were detected with Western blotting. GAPDH was used as an internal control. (B) The relative expression levels of p-MEK and p-ERK in HCT116 cells were quantified via normalizing to MEK and ERK, respectively; the relative expression levels of N-cadherin and MMP9 in HCT116 cells were quantified via normalizing to GAPDH. (C) Protein expression of p-MEK, p-ERK, N-cadherin and MMP9 in SW620 cells were detected with Western blotting. GAPDH was used as an internal control. (D) The relative expressions of p-MEK and p-ERK in SW620 cells were quantified via normalizing to MEK and ERK, respectively; the relative expressions of N-cadherin and MMP9 in SW620 cells were quantified via normalizing to GAPDH. (E, F) Gene expressions of MEK, N-cadherin and MMP9 in CRC cells were detected with qRT-PCR. ** p < 0.01 vs. control group, ## p < 0.01 vs. 0.4 mM + NC group.

Lenti-MEK was transfected into HCT116 and SW620 cells with or without sophocarpine for 72 h, respectively. (A) The level of VEGF-A in cell culture supernatant was measured with ELISA. (B) The level of VEGF-C in cell culture supernatant was measured with ELISA. (C) The level of VEGF-D in cell culture supernatant was measured with ELISA. ** p < 0.01 vs. control group, ## p < 0.01 vs. 0.4 mM + NC group.
Next, the result of wound healing assay showed that the migration cells were markedly decreased in sophocarpine treated group at 24 h time point, while the migration rate was significantly increased following transfection with lenti-MEK in CRC cells (Figs. 7A, 7B). Similarly, the data of transwell migration assay indicated that sophocarpin significantly inhibited the migration ability of CRC cells, which was reversed by following transfection with lenti-MEK (Figs. 8A, 8B). All these results confirmed that sophocarpine inhibited the migration of CRC via downregulation of MEK/ERK/VEGF pathway.

(A) Lenti-MEK was transfected into HCT116 cells with or without sophocarpine for 24, 48, 72 h. Cell migration was detected with wound healing assay. (B) Lenti-MEK was transfected into SW620 cells with or without sophocarpine for 24, 48, 72 h. Cell migration was detected with wound healing assay. ** p < 0.01 vs. control group, ## p < 0.01 vs. 0.4 mM + NC group.

(A) Lenti-MEK was transfected into HCT116 cells with or without sophocarpine for 24 h. Cell migration was detected with transwell migration assay. (B) Lenti-MEK was transfected into SW620 cells with or without sophocarpine for 24 h. Cell migration was detected with transwell migration assay. ** p < 0.01 vs. control group, ## p < 0.01 vs. 0.4 mM + NC group.
In patients with colon cancer, metastases are among the primary causes of death.18) Therefore, suppressing migratory and metastasizing abilities of cancer cells is especially important in patients with CRC.18) Our results indicated that sophocarpine could inhibit the migration of HCT116 and SW620 cells via suppressing MEK/ERK/VEGF pathway. This finding demonstrated that MEK may plays an important role in the migration of CRC.
We found that sophocarpine suppressed the proliferation of CRC cells for the first time. Similarly, Liu et al. revealed that sophocarpine inhibited head and neck tumor proliferation.14) Moreover, our results also revealed that sophocarpine suppressed CRC cells migration via downregulation of N-cadherin and MMP9 levels. E-Cadherin and MMP9 expression has been related to the progression of CRC and expression of VEGF.1,19,20) An analogue of cucumin suppressed the cell migration through downregulating N-cadherin in the CRC cells.21) These results were consistent with our finding.
However, the molecular mechanisms underlying the inhibitory effect of sophocarpine on the migration of CRC remain unclear. Meadows et al. found that VEGF is an important marker for cell migration.22) In addition, MEK/ERK signaling pathway also involved in cell growth and differentiation, which play a central role at different levels during invasion, migration and metastasis.23) Importantly, migration and proliferation of tumor cells are indispensable processes during tumor development.24) Lee et al. revealed that genipin inhibited CRC cells invasion and migration via suppressing the expression of VEGF.25) Meanwhile, Zhang et al. indicated that norcantharidin inhibited tumor migration via inhibiting MEK/ERK/VEGF signaling pathways.26) In this study, the novel finding is that sophocarpine inhibited CRC cells migration by suppressing MEK/ERK/VEGF pathways. Our results were consistent with these findings.
Another intriguing finding in this study is that overexpression MEK increased the expression of VEGF in CRC cells. Meanwhile, overexpressing MEK reversed sophocarpine-induced migration inhibition in CRC cells. In contrast, Gupta et al. indicated that knockdown of MMP9 significantly reduced VEGF expression in PC3 cells.19) In addition, downregulation of ERK1/2 inhibited VEGF secretion on human myeloma cells.27) All these reports confirm our findings that overexpression of MEK could increase the expression of VEGF in CRC cells, and MEK may play a pro-migratory role in CRC cells. The results presented in this study also suggested that MEK plays an important role in regulation of VEGF secretion in CRC cells. Sophocarpine may act as a MEK inhibitor.
In conclusion, this study demonstrated that sophocarpine significantly inhibited the proliferation and migration in CRC cells. Therefore, these findings suggested sophocarpine may serve as a potent agent for the treatment of patients with CRC.
This work was supported by the National Natural Science Foundation of China (No. 81460704).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.