2019 年 42 巻 2 号 p. 194-200
2019 年 42 巻 2 号 p. 194-200
Seventeen 13,28-epoxy triterpenoid saponins obtained from Ardisia gigantifolia STAPF. were evaluated their anti-proliferative activities on MCF-7 cells. The structure–activity relationship analysis indicated that CH3 group at C-30, four saccharide units with L-rhamnose at R6 in the sugar units are crucial for the cytotoxic activity on MCF-7. Compounds 1, 2, 6, 7, 12, and 14 were selected to identify the anti-proliferative activity on the other three breast cancer cell lines (T47D, MDA-MB-231 and SK-BR-3). Compounds 2, 6, and 7 with good activity on MCF-7 also showed activity on T47D, MDA-MB-231, and SK-BR-3. Compounds 12 and 14 without cytotoxic activity on MCF-7 almost showed no activities on the other three cell lines. For the triple-negative breast cancer MDA-MB-231, Saponins 7 and 14 showed selective cytotoxic activity, 7 showed much more activity than 14, suggesting the six saccharide units in sugar units and CH3 on C-30 were the key moieties for the anti-proliferative activities. Further molecular mechanism of saponin 7 was studied on inhibiting cell proliferation of MDA-MB-231 cells. Saponin 7 could enhance apoptosis, arrest cell cycles, decrease mitochondrial membrane potentials (MMPs), and considered the involvement of reactive oxygen species (ROS) may explain this conundrum.
The rhizome of Ardisia gigantifolia STAPF. is mainly used as Chinese folk medicine in south of China for the treatment of rheumatism, pain of muscles and bones and traumatic injury.1–3) The 13,28-epoxy triterpenoid saponins are characteristic constituents of A. gigantifolia STAPF. This type of triterpenoid saponins showed cytotoxicity against a variety of human cancer cell lines through cell cycle arresting, apoptosis inducing and microtubule disassembling, which demonstrate the potential anti-cancer properties of the saponins.4–8) We have isolated thirteen triterpenoid saponins (1, 6–17) from the rhizomes of A. gigantifolia STAPF. and obtained four new triterpenoid saponins (2–5) by biotransformation of compound 1.9–13) Some of them have prominent cytotoxicity against breast cancer cell lines.14) In order to identify the active moieties for development of novel drugs against breast cancers, we reported the structure–activity relationship (SAR) of compounds 1–17 and MCF-7. According to verify the result of SAR, some saponins were selected to test their anti-proliferative activities against the other three types of breast cancer cell lines (T47D, MDA-MB-231, and SK-BR-3). Compound 7 showed the best anti-proliferative activities against MDA-MB-231, we further demonstrated the anti-tumor effects and investigated the molecular mechanism of 7.
The rhizome of A. gigantifolia was collected from Guangdong, China in 2007 and was identified by Prof. Ping Liu of Traditional Chinese Medicine Pharmacy, Chinese PLA General Hospital. The voucher specimen (collection No. 029) is deposited in Traditional Chinese Medicine Pharmacy, Chinese PLA General Hospital, Beijing, China.
Chemicals and ReagentsCompounds 1, 6–17 (the purities were >95% by HPLC analysis) were isolated from A. gigantifolia STAPF., 2–5 were obtained by biotransformation of 1, which have been reported previously.9–13) The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and N-acetyl-L-cysteine (NAC) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). MitoTracker Red CMXRos, Annexin V-fluorescein isothiocyanate (FITC) kit and JC-1 Mitochondrial Membrane Potential (MMP) Detection Kit were purchased from Beyotime (Jiangsu, China).
Cell CultureThe MCF-7, T47D, MDA-MB-231 and SK-BR-3 cells were purchased from Cell Culture Collection of Chinese Academy of Medical Sciences (Beijing, China). MDA-MB-231 cells were grown in L-15 medium (Gibco) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. MCF-7, T47D and SK-BR-3 cells were grown in RPMI 1640 medium containing 10% FBS at 37°C in 5% CO2 conditions.
Cell Viability AssayThe cell proliferation was measured by MTT assay. Briefly, MCF-7, T47D and SK-BR-3 cells were seeded into 96-well plates (2 × 104 cells/well) and cultured in RPMI 1640 medium, the MDA-MB-231 cells cultured in L-15 medium. After 24 h, different concentrations of 7 were added and cultured at 37°C for 24, 48 and 72 h, respectively. MTT solution was added to each well and incubated for 4 h. The supernatant was aspired, the formazan crystals was dissolved by dimethyl sulfoxide (DMSO), and cellular viability was measured by an enzyme-linked immunosorbent assay (ELISA) plate reader (Perkin-Elmer, Inc., 1420-012, China) at 570 nm. NAC was added to the MDA-MB-231 cells 1 h before 7-treatment, and then the cells were cultured with NAC and 7 for 24 h before the cell viability measurement.
Measurement of Cell ApoptosisCell apoptosis was tested using Annexin V-FITC and propidium iodide (PI) double stain. After treatment by 7 (0, 0.5, 1.0, and 1.5 µM) for 24 h, MDA-MB-231 cells were collected and washed twice with pre-chilled phosphate buffered saline (PBS). Cells were stained by Annexin V-FITC stock and PI for 15 min at room temperature in dark. Samples were analyzed using flow cytometry (FACS Calibur; Becton Dickinson, San Jose, CA, U.S.A.).
Measurement of Mitochondria ActivityMDA-MB-231 cells were incubated with with 7 (0, 0.5, 1.0, and 1.5 µM) for 24 h, and then cells were incubated with 100 nM MitoTracker Red CMXRos for 30 min at 37°C. The specimens were observed by fluorescence microscopy (Olympus BX60).
Measurement of Mitochondrial Membrane PotentialMDA-MB-231 cells (1.5 × 105 cells/well) were seeded in 6-well plates for 24 h and treated with 7 (0, 0.5, 1.0, 1.5 µM) for 24 h at 37°C. Then, the cells were incubated with JC-1dye (10 µg/mL in PBS) at 37°C for 20 min. After washed by PBS, the mitochondrial membrane potential of 7-treated cells was determined by flow cytometer. The emission wavelengths of JC-1monomers and JC-l aggregates were ca. 530 and ca. 590 nm, respectively.
Analysis of Cell Cycle by PI StainingMDA-MB-231 cells (1 × 105/well) were seeded in 6-well plates, treated by various concentrations of 7 (0, 0.5, 1.0, 1.5 µM) for 24 h, washed with PBS, and fixed gently in 70% ethanol at 4°C for 1h. The cells were washed with PBS twice and stained by 0.05 mg/mL PI and 0.01 mg/mL RNase A for 30 min in the dark. The cell cycle of the stained cells was analyzed by FACS Calibur flow cytometer immediately (BD Biosciences, U.S.A.).
Statistical AnalysisAll data were presented as mean ± standard deviation (S.D.) from three independent experiments. Data were analyzed by ANOVA. Statistical comparisons were evaluated using Student’s t-test. Differences were considered to be statistically significant when p-values less than 0.05.
To study SAR, the structures of triterpenoid saponins and anti-proliferative activities on MCF-7 were compared (Fig. 1 and Table 1). Saponin 2 is similar with 3, 2 showed better activity than 3, suggested that CH2OH at C-30 can significantly decrease the activity. Saponins 1 and 14, 7 and 8, 6 and 11 have same sapogenins respectively, but in the glucosyl moiety, 14, 7, and 11 have one more β-D-glucopyranose than 1, 8, and 6. The activities of saponins 1 > 14, 7 > 8, 6 > 11, suggested that the glucosyl moiety are preferable to be five saccharide units when CHO and CH2OAc at C-30, while six saccharide units are better than five saccharide units when CH3 at C-30. Saponins 7, 11, and 14 with the same C-3 glycosyl moieties showed different activities (7 > 11 > 14) indicating that the substituents at C-30 in the sapogenin part contribute differently to the anti-proliferative activities (CH3 > CH2OAc > CHO). Compound 5 (CH3 at C-30) also showed better activity than 4 (CHO at C-30). This may be ascribed to the electron-donating ability of these substituent groups (CH3 > CH2OAc > CHO). Comparing with the activity of 8 and 9, 1 and 15, the 6-OAc-β-D-glucopyranosyl at C-2 of arabinose could decrease the anti-proliferative activities on MCF-7. Compound 2 showed best inhibitory activity to MCF-7 cells (IC50 0.73 µM), better than that of 5-fluorouracil (5-FU) (IC50 5.10 µM), 2 was similar with 17, the only difference is the L-rhamnose at R6, which indicated that the L-rhamnose lead to a significant increase of cytotoxicity activities. But the activities of saponins (8 > 10) suggesting that the cytotoxicity of saponins depend on combination of properties at both the saccharide and the moieties at C-30.

| Compounds | IC50 (µM)a) | Compounds | IC50 (µM)a) |
|---|---|---|---|
| 1 | 3.85 ± 0.15 | 10 | 3.69 ± 0.31 |
| 2 | 0.73 ± 0.09 | 11 | 5.17 ± 0.18 |
| 3 | 5.78 ± 0.33 | 12 | >100 |
| 4 | 5.83 ± 0.45 | 13 | 5.24 ± 0.29 |
| 5 | 1.14 ± 0.42 | 14 | >100 |
| 6 | 0.96 ± 0.11 | 15 | 8.32 ± 0.32 |
| 7 | 0.84 ± 0.12 | 16 | 4.78 ± 0.57 |
| 8 | 1.88 ± 0.28 | 17 | 15.32 ± 0.75 |
| 9 | 5.72 ± 0.49 | 5-FUb) | 5.10 ± 0.22 |
a) Values are means ± S.D., n = 3, IC50 in µM. b) Positive control.
According to the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2), the breast tumors can be classified into four subtypes as luminal A (ER+ or PR+ and HER-2−), luminal B (ER+ or PR+ and HER-2−), HER-2+ (ER− and PR− and HER-2+), and triple-negative (TN) (ER− and PR− and HER-2−).15) In order to verify the rules of compounds on breast cancer cell lines, compounds with no activity or good activity on MCF-7 (1, 2, 6, 7, 12, 14) were selected to test the cytotoxity on the other three breast cancer cell lines. The T47D (luminal A subtype, ER+, PR+, HER2−), SK-BR-3 (HER-2+ subtype, ER−, PR−, HER2+), and MDA-MB-231 (triple-negative subtype, ER−, PR−, HER2−) were selected for this study.16,17) According to Table 2, saponins 1, 2, 6, 7, which have good activity on MCF-7 (luminal A subtype, ER+, PR+, HER2−)16) also showed certain activity on T47D, MDA-MB-231 and SK-BR-3. Compound 12 which have no activity on MCF-7 also showed no cytotoxicity against T47D, MDA-MB-231 and SK-BR-3 cells. The structure of 12 is similar with 1 and 6, but 1 and 6 showed much more better cytotoxity than 12 indicating CH2OH at C-30 can dramatically decrease the activity.
| Compounds | IC50 (µM)a) | |||
|---|---|---|---|---|
| MCF-7 | T47D | MDA-MB-231 | SK-BR-3 | |
| 1 | 3.85 ± 0.21 | 0.88 ± 0.09 | 3.27 ± 0.13 | 13.19 ± 0.51 |
| 2 | 0.73 ± 0.06 | 6.93 ± 0.23 | 18.10 ± 0.32 | 23.44 ± 0.45 |
| 6 | 0.97 ± 0.10 | 10.17 ± 0.12 | 1.87 ± 0.14 | 0.95 ± 0.11 |
| 7 | 0.84 ± 0.07 | 15.86 ± 0.35 | 0.76 ± 0.08 | 3.91 ± 0.19 |
| 12 | >100 | >100 | >100 | >100 |
| 14 | >100 | >100 | 3.06 ± 0.24 | >100 |
a) Values are means ± S.D., n = 3, IC50 in µM.
In Table 2, compound 1 showed selectively cytotoxicity against T47D cells, which is most sensitive to PR receptor.17) The structure of 1 is similar with that of 12 and 14, but 1 showed much more activity, indicating the CHO at C-30 and four saccharide units may have effects on PR receptor. Compound 2 showed more activities against ER+ cells (MCF-7 and T47D) than ER− cells (MDA-MB-231 and SK-BR-3), indicating 2 have selective effects on ER receptor and the mechanism needs to be further studied.
Compound 14 had no activity on MCF-7, T47D and SK-BR-3, but showed middle activity on MDA-MB-231cells, which means that 14 may inhibit proliferation of MDA-MB-231 cells selectively. Saponins 7 also showed selective cytotoxic activity on MDA-MB-231, but 7 showed much more activity than 14, suggesting the six saccharide units in sugar units and CH3 on C-30 were the key moieties for the anti-proliferative activities. Among the selected saponins, 7 showed the best anti-proliferative activities on MDA-MB-231, the mechanism needed to be further researched.
Compound 7 Inhibited Cell Proliferation and Growth on MDA-MB-231The effects of 7 on MDA-MB-231 were investigated. The cytotoxicity of 7 was examined at the indicated concentrations for 24, 48, and 72 h, respectively. Saponin 7 could dramaticly inhibit the viability of MDA-MB-231 cells in a dose- and time-dependent manner (Fig. 2A). The IC50 value of 7 was 0.73 µM approximately.
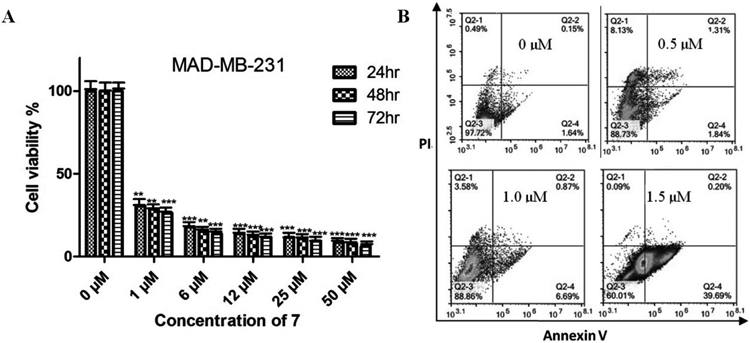
(A) The viability of MDA-MB-231 cells were assessed by MTT assays at 24, 48, and 72 h after treatment with different concentrations of 7 (0, 1, 6, 12, 25, and 50 µM); Data were presented as mean ± S.D. of three independent experiments. ** p < 0.01 and *** p < 0.001. (B) Flow cytometry detection of apoptosis with annexin V/PI in MDA-MB-231 cells treated by different concentrations of 7 (0, 0.5, 1.0, and 1.5 µM).
To determine the relationship between cell viability decrease and cell apoptosis, MDA-MB-231 cells were incubated with 7 for 24 h and then the apoptotic effects were investigated by annexin-V/PI double-staining using flow cytometry. As shown in Fig. 2B, after treatment with 7 of 0, 0.5, 1.0, and 1.5 µM for 24 h, the percentage of early apoptotic cells was 1.64, 1.84, 6.69, and 39.69%, respectively. These results demonstrated that 7 reduce the cells viability through increasing the number of early apoptotic cells in a dose-dependent manner.
Compound 7 Affected Mitochondrial Functions in MDA-MB-231 CellsMitochondria injury is one of the most causes of cell apoptosis, after treated by 7 for 24 h, the mitochondria activity of MDA-MB-231 cells was tested using MitoTracker Red CMXRos dye. In the control group, there are numbers of bright red fluorescence and dot-like structures in the cytoplasm of cells (Fig. 3A). After treated by 7, the red fluorescence in cytoplasm decreased significantly in a dose-dependent manner (Fig. 3A). This result suggested that 7 can significantly decrease the MitoTracker Red CMXRos uptake capability. After stained with Rh123, the level of MMP was tested using a flow cytometer. In the 7-treat cells, the MMP was remarkably attenuated compared to the control cells in a concentration-dependent manner (Fig. 3B). These results suggested that 7 may severely impair the mitochondrial function and induce mitochondria-related ROS accumulation and apoptosis in MDA-MB-231 cells.

(A) MDA-MB-231 cells were incubated with 7 (0, 0.5, 1.0, 1.5 µM) for 24 h and incubated with 100 nM MitoTracker Red CMXRos for 30 min. The specimens were observed by fluorescence microscopy (100×). (B) MDA-MB-231 cells were incubated with 7 (0, 0.5, 1.0, and 1.5 µM) for 24 h The mitochondrial depolarization patterns of the MDA-MB-231 cells were measured by flow cytometry.
To investigate the molecular mechanisms of 7 on cell growth inhibition, cell cycle distributions of MDA-MB-231 were evaluated by flow cytometry (Fig. 4A). Treatment of 7 increased the number of cells in G2/M phase and decreased that in G1 phase concomitantly compared to control, indicating that the DNA synthesis of MDA-MB-231 cells was significantly inhibited by 7 (Fig. 4B). These results suggested that 7 can induce G2/M-phase cell cycle arrest and proliferate of MDA-MB-231 cells in dose-dependent manner.

(A) Cells were treated with indicated concentrations of 7 for 24 h and then were analyzed by flow cytometry. (B) The phases of cell cycle were presented as mean ± S.D. of three independent experiments. * p < 0.05, ** p < 0.01 compared to the control group.
In order to test the effects of antioxidant NAC on the viability of 7-treated cells, cells were pre-treated with NAC (4 mM) prior to treatment with 7 (1.0 µM) for 24h. As shown in Fig. 5, NAC alone almost had no effects on the cell viability. After treatment of 7, cell viability dramatically decreased to 40.3% and in the 7 + NAC group, cell viability increased to 89.3%. These result suggested that NAC could counteract the negative effects of 7 on the viability of MDA-MB-231 cells significantly (Fig. 5).

Cells were treated with or without NAC (4 mM) prior to treatment with 7 (1.0 µM) for 24 h.
Triterpenoid saponins are heterogeneous group of bioactive metabolites found in many plants, which have a wide range of structural diversity and versatile biological effects.1–3) Both the aglycones and sugar moieties are essential for their biological activities.18,19) In the SAR study, some triterpenoid saponins have same aglycone but different sugar moieties, displayed different anti-proliferative activity.20–22) As part of our ongoing study for antitumor compounds from plant. We have obtained a series of triterpenoid saponins form Ardisia gigantifolia STAPF. and researched the SAR against cancer cells.23) With more saponins obtained and some of them showing significant anti-proliferative activity against breast cancers. We further studied the SAR between the triterpenoid saponins and MCF-7. We studied the roles of CH3, CH2OAc, CH2OH, and CHO at C-30, the glucosyl moiety at C-3, although further SAR studies should be carry out to achieve general conclusions, it seems that the incorporation of CH3 group at C-30, four saccharide units with L-rhamnose at R6 in the sugar units are crucial for the cytotoxic activity on MCF-7.
Some compounds were selected to test the cytotoxity on three kinds of breast cancer subtypes. However, different breast cancer cell lines have different sensitivity to compounds, which may be related to the expression of receptors of breast cancer subtypes. Molecular docking was studied to understand the interactions of saponins and cancer cells. It is reported that triterpenoids with more hydroxyls showed higher binding affinities with ERα receptor of breast cancer cells.24) Saponins molecules can also dock with proteins associated with cancers such as PAK1 Kinase, ABL1 Kinase, vascular endothelial growth factor receptor 2 (VEGFR2), tumor necrosis factor-alpha (TNF-α) and sarco-endoplasmic reticulum calcium transport ATPase (SERCA) pump.25–29) The membrane cell permeabilization of saponins is correlated with the kinds of aglycon.30) But it has been proposed that L-rhamnose units in the sugar chain facilitate the cellular uptake of saponins,31) indicating the process of saponins crossing cell membrane and binding with different receptors is complicated and the mechanisms need to be researched further.
Saponin 7 showed significant and selective activity on triple-negative breast cancer MDA-MB-231. It can be concluded that the CH3 group substituted at C-30 and the six saccharide units at C-3 were critical for the anti-proliferative activity of 7. According to these findings, we investigated the anti-proliferative mechanism of 7 against MDA-MB-231. Apoptosis is a biological cell development process that is crucial for the normal development of organisms during embryogenesis, tissue homeostasis, and immune system regulation.32) Dysregulated apoptosis is an important cause of many kinds of human diseases including cancer.33) Apoptosis induction has been reported to be a major mechanism of anticancer drugs. In this study, 7 could decrease the cell viability of MDA-MB-231 cells in a dose- and time-dependent manner. According to the results of flow cytometry, 7 could increase early apoptosis of MDA-MB-231 cells in a dose-dependent manner.
Many anticancer agents induce apoptosis by arresting the cell cycle distribution at G0/G1, S or G2/M phase and34,35) or directly induce apoptosis of cancer cell.36) To verify the anticancer mechanism, we study the effects of 7 on cell cycle distribution of MDA-MB-231 cells. After treated with 7 for 24 h, the cell percentage in G2/M phase was increased and that in G1 phase decreased concomitantly compared to control.
Mitochondria are critical in the apoptotic pathways, and mitochondrial depolarization is a major event in mitochondrial dysfunction. If the membrane integrity is destroyed in mitochondria, the MMPs will be depolarized and mitochondria-mediated apoptosis will happen.37–40) MitoTracker Red CMXRos dye is usually used to examine the integrity of membrane. In our present study, 7 can significantly decrease the MitoTracker Red CMXRos uptake capability and MMPs, demonstrating that mitochondrial function is severely impaired by 7 in MDA-MB-231 cells.
Reactive oxygen species (ROS) are generated by oxygen partial reduction and contain unpaired electrons.41) Overproduction of ROS is associated with further instability of lipids, proteins and DNA.42,43) The levels of oxidative stress in cancer cells are higher than that in normal cells, so, ROS accumulation is more harmful to cancer cells. Therefore, ROS generation is a major mechanism of antitumor drugs.44,45) Anti-oxidants have the ability to donate an electron to unstable free radicals without compromising their own stability42) and, hence, may prevent the damage caused by ROS-seeking or donating electrons. Therefore, we used an antioxidant (NAC) in an attempt to prevent reduction of oxygen. Strikingly, we found that NAC treatment prevented 7-induced reductions in cell viability in MDA-MB-231 cells compared to 7-treatment alone, suggesting 7 induce oxidative stress pathway, resulting in cell viability reducing.46)
In this study, SAR of compounds 1–17 and MCF-7 was studied. Some saponins were selected to verify their anti-proliferative activities against T47D, MDA-MB-231 and SK-BR-3. Saponin 7 showed significant cytotoxity on MDA-MB-231 through apoptosis enhancement, cell-cycle arresting, MMPs decrease. The inhibitor of ROS (NAC) could protect 7-treated MDA-MB-231 cells from apoptosis, considering the involvement of ROS as a possible explanation to this conundrum. In conclusion, although to make clear the detail mechanisms of 7 against breast cancer cells, much more research needs to be done. These studies may assist the rational design of targeted triple-negative breast cancer therapeutics.
This study was supported by Grants from the National Natural Science Foundation of China (No. 31370006).
The authors declare no conflict of interest.