2019 年 42 巻 6 号 p. 937-943
2019 年 42 巻 6 号 p. 937-943
Hydroxyoctadecadienoic acids (HODEs) are generated by oxidation of linoleic acid in vivo and thought to mediate various pathophysiological responses. In this study, we examined the effects of HODEs on EL4 mouse lymphoma cell growth and found that 9-(E,Z)-HODE inhibited EL4 cell growth in a dose-dependent manner, whereas no such growth inhibition was observed with other isomers (9-(E,E)-, 13-(Z,E)-, or 13-(E,E)-HODE), suggesting that the growth-inhibitory effect of HODEs was stereospecific. Analysis by flow cytometry (FACS) with annexin V and propidium iodide (PI) staining showed that 9-(E,Z)-HODE induced apoptosis with G2/M phase arrest. We next examined the growth inhibition profile of 9-(E,Z)-HODE against a panel of 39 human cancer cell lines (JFCR39). The fingerprint of growth inhibition by 9-(E,Z)-HODE exhibited a high degree of similarity to that by MLN4924, an inhibitor of NEDD8-activating enzyme. The intracellular NEDD8 (ubiquitin-like protein) expression in EL4 cells was decreased by the treatment with 9-(E,Z)-HODE as assessed by immunoblotting and flow cytometry. In conclusion, 9-(E,Z)-HODE specifically induced G2/M phase arrest and apoptosis, and the decrease of NEDD8 expression might be involved in this effect.
Reactive oxygen species (ROS) generation is increased in association with oxidative stress, and ROS levels in tissues and blood have been used as markers of various diseases. ROS chemically modify cellular constituents including lipids, proteins, nucleic acids and carbohydrates, and change their physical properties. A variety of important cellular processes are modulated either by ROS-modified molecules or directly by ROS themselves.1) Polyunsaturated fatty acids are one of the best targets for ROS among biological substances. For example, oxidation products of linoleic acid (hydroxyoctadecadienoic acids (HODEs) and hydroperoxyoctadecadienoic acids (HPODEs)) are pathophysiologically important fatty acid derivatives.2,3) HPODEs are produced by oxidation of linoleic acid in vivo and converted into HODEs. HODEs of biological origin usually include four isomers (9-(E,Z)-HODE, 9-(E,E)-HODE, 13-(E,E)-HODE, and 13-(Z,E)-HODE).4)
Several previous studies have indicated the important roles of HODEs in cellular responses—e.g., in the activation of peroxisome proliferator-activated receptor gamma (PPARγ),5) or as a ligand for G2A of G-protein coupled receptor (GPCR).6) It was also reported that the blood levels of HODEs were significantly correlated with diabetic parameters,7) and that increased levels of HODEs were suggested to contribute to atherosclerosis progression.8) These results strongly suggested that HODEs play crucial roles in various physiological and pathological processes. In this study, we examined the effect of four isomers of HODEs on the proliferation of EL4 lymphoma cells and found that one isomer specifically inhibited their growth.
HODEs were extracted from barley powder by homogenizing the powder in CHCl3 : CH3OH (2 : 1). The extract was dried and dissolved in methanol. The content of HODEs was quantified by HPLC (TSK-ODS-100 V column; dissolution solution, 0.01 M HCl : CH3CN : CH3OH = 48 : 44 : 8; flow rate, 1.0 mL/min; UV at 235 nm). HODEs were fractionated into four isomers, 9-(E,Z)-HODE, 9-(E,E)-HODE, 13-(E,E)-HODE, and 13-(Z,E)-HODE, and each isomer was dissolved in methanol (10 mg/mL).
Cell Culture and Cell Growth InhibitionEL4 mouse lymphoma cells (ATCC, TIB-39) were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 4.5 g/L glucose, 0.05 mM 2-mercaptoethanol and penicillin/streptomycin at 37°C under 5% CO2. Cells were treated with 9-HODE, 13-HODE or methanol for 24 h in a 48-well or 24-well culture plate. Fatty acids were dissolved in methanol and were added to final concentrations of 10, 50, or 100 µM. Cells were counted with a hemocytometer after trypan blue staining. The cell viability was measured using a cell counting kit-8 (WST-8; Dojindo, Kumamoto, Japan), and the absorbance at 450 nm was monitored and expressed as a sample/control ratio.
Annexin V and Propidium Iodide (PI) StainingEL4 cells (2.0 × 105) were treated with 9-HODE (10, 50, or 100 µM) for 24 h in a 48-well culture plate. Cells were stained with an Annexin V-fluorescein isothiocyanate (FITC) and PI Apoptosis Detection Kit (Nacalai Tesque, Kyoto, Japan) and examined immediately with a flow cytometer (FACSAria™; BD Biosciences, San Jose, CA, U.S.A.).9–11) Quadrant analysis was used to quantify viable cells (PI-negative/annexin V-negative), early apoptotic cells (annexin V-positive/PI-negative), and late apoptotic cells (annexin V-positive/PI-positive).
Cell Cycle AnalysisEL4 cells (1 × 106 cells) were treated with 9-(E,Z)-HODE (10, 50, or 100 µM) for 24 h in a 24-well culture plate and fixed with 70% ethanol. Cells were then stained with PI and their fluorescence was analyzed by FACS.
COMPARE AnalysisThe cell growth inhibition profile of 9-(E,Z)-HODE was assessed by measuring changes in total cellular proteins in each cell line of a panel of 39 human cancer cell lines (termed “JFCR39”) after 48 h of treatment with the compound as described previously.12,13) Based on the set of 50% growth inhibition (GI50) values for each compound, the fingerprint is presented in graphical format to show the relative sensitivity. The Pearson correlation coefficient (r) was calculated using the COMPARE computer algorithm as described previously.14,15) This analysis was supported by the Molecular Profiling Committee, Grant-in-Aid for Scientific Research on the Innovative Area “Platform of Advanced Animal Model Support” from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Detection of NEDD8EL4 cells (1 × 106 cells) were treated with 9-(E,Z)-HODE (10–100 µM) or MLN4924 (Focus Biomolecules, Plymouth Meeting, PA, U.S.A.) (10 µM) for 24 h in a 24-well culture plate. For immunoblotting analysis, cells were lysed with the sample buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and subjected to immunoblotting as described previously.16) Rabbit anti-NEDD8 monoclonal antibody (Y297), which recognizes both NEDD8 bound to cullin proteins (cullins-NEDD8) and free NEDD8, and mouse anti-β-actin antibody (AC-15) were purchased from Abcam (Cambridge, U.K.) and Sigma-Aldrich (St. Louis, MO, U.S.A.), respectively. Horseradish peroxidase (HRP)-conjugated goat antibodies to rabbit immunoglobulin G (IgG) or to mouse IgG were purchased from Kirkegaard & Perry Laboratories (Burlingame, CA, U.S.A.). The bands were visualized by chemiluminescence (Chemi-Lumi One Ultra, Nacalai Tesque) and detected by ImageQuant LAS 500 (GE Healthcare, Piscataway, NJ, U.S.A).
For FACS analysis, 9-(E,Z)-HODE-treated cells were fixed with 70% ethanol and stained with rabbit anti-NEDD8 monoclonal antibody (Y297, 1 µg/mL). An AlexaFlour®647-labeled F(ab′)2 fragment donkey anti-rabbit IgG (711-606-152; Jackson ImmunoResearch, West Grove, PA, U.S.A.) was used as the secondary antibody (1/2000 dilution).
We first conducted a cell growth assay with EL4 mouse lymphoma cells in the presence of 9-(E,E)-, 9-(E,Z)-, 13-(E,E)-, or 13-(Z,E)-HODE (Fig. 1a). After performing a culture with each compound for 24 h, we measured cell viability by the water-soluble formazan (WST-8) method and found that only 9-(E,Z)-HODE showed marked, dose-dependent inhibition of cell growth. The cell viability after the treatment with 9-(E,Z)-HODE was decreased to 53.6% at 50 µM and to 27.3% at 100 µM, as compared with the control (Fig. 1b). Although 13-(Z,E)-HODE (100 µM) showed a slight decrease, the difference was not statistically significant.

(a) Structures of isomers of HODEs. (b) EL4 mouse lymphoma cells (1 × 106 cells) were cultured for 24 h in the presence of HODEs (10, 50, or 100 µM) or vehicle only. The cell viability was measured using a cell counting kit-8 (WST-8), and the absorbance at 450 nm was measured and expressed as a percentage of that in the control. Experiments were performed in triplicate, and data are presented as the mean ± standard error of the mean (S.E.M.). Statistical data analysis was conducted using the Student’s t-test. * p < 0.05 vs. control.
Annexin V and PI staining analysis using FACS was performed to quantify viable cells (PI-negative/annexin V-negative), early apoptotic cells (PI-negative/annexin V-positive), and late apoptotic cells (PI-positive/annexin V-positive) after 9-(E,Z)-HODE treatment of EL4 cells. The results showed that 9-(E,Z)-HODE increased the number of early and late apoptotic cells in a concentration-dependent manner (Fig. 2a). The cell viability was reduced to 47.6% by 9-(E,Z)-HODE (100 µM) (compared to 86.8% in the control, p < 0.01). The treatment with 9-(E,Z)-HODE (100 µM) markedly increased late apoptotic cells, which accounted for 34.5% of total cells (compared to 4.2% in the control, p < 0.05) (Fig. 2b). The results of cell cycle analysis showed that 9-(E,Z)-HODE increased the number of apoptotic cells and arrest at G2/M phase (Fig. 3, Table 1). The histogram for 9-(E,Z)-HODE (50, 100 µM) showed a notable G2/M peak, which indicated a group of cells arrested at G2/M phase caused by the 9-(E,Z)-HODE treatment.
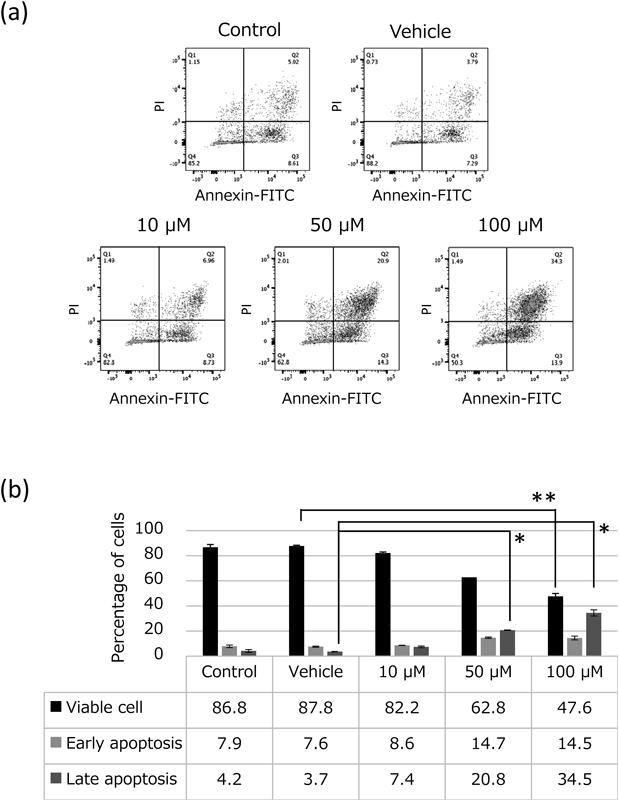
EL4 cells (2.0 × 105 cells) were treated with 9-(E,Z)-HODE (10, 50, or 100 µM) for 24 h. Flow cytometry quadrant analysis with annexin V and PI staining was performed to measure viable cells (PI-negative/annexin V-negative), early apoptotic cells (PI-negative/annexin V-positive), and late apoptotic cells (PI-positive/annexin V-positive). (a) Fluorescence profiles after 9-(E,Z)-HODE treatment were analyzed. X-Axis, annexin V; Y-axis, PI. (b) The percentages of viable cells, early apoptotic cells and late apoptotic cells were expressed as histograms. Experiments were performed in triplicate, and data are presented as the mean ± S.E.M. Statistical data analysis was conducted using the Student’s t-test. * p < 0.05, ** p < 0.01 vs. vehicle.

EL4 cells (1 × 106 cells) were treated with 9-(E,Z)-HODE (10, 50, or 100 µM) for 24 h. PI-stained cells were examined using a flow cytometer. Arrows indicate the cells at G2/M phase.
| Samples | Sub-G1 | G0/G1 | S | G2/M |
|---|---|---|---|---|
| Control | 3.5 ± 1.2 | 90.6 ± 15.1 | 2.8 ± 8.2 | 2.6 ± 6.8 |
| Vehicle | 2.9 ± 1.3 | 90.2 ± 1.4 | 3.8 ± 1.6 | 2.3 ± 3.5 |
| 9-(E,Z)-HODE 10 µM | 8.2 ± 1.9* | 68.5 ± 1.7 | 13.6 ± 1.8 | 7.3 ± 5.0 |
| 9-(E,Z)-HODE 50 µM | 15.6 ± 0.8** | 45.5 ± 1.2** | 21.0 ± 0.6** | 13.5 ± 0.3* |
| 9-(E,Z)-HODE 100 µM | 13.5 ± 0.7* | 48.3 ± 0.8** | 14.0 ± 0.9* | 20.7 ± 0.6* |
* p < 0.05, ** p < 0.01 vs. control.
To improve our mechanistic understanding of the effects of HODEs on the proliferation of cancer cells, including the molecular target of 9-(E,Z)-HODE, we applied 9-(E,Z)-HODE to the JFCR39 panel. The cell growth inhibitory activity was assessed by measuring changes in total cellular proteins in each cell line of the JFCR39 panel after cell culture for 48 h. The GI50 mean graph was obtained by computer processing (Supplementary Fig. S1). The fingerprint is presented in graphical format to show the relative sensitivity. The Pearson correlation coefficient (r) was calculated by coupling with a drug–activity database as described previously.12–15) The 9-(E,Z)-HODE-specific fingerprint generated based on the growth inhibition profile exhibited a high degree of similarity to MLN4924, an inhibitor of NEDD8 activating enzyme16) (r = 0.749).
9-(E,Z)-HODE Decreased the Amount of Intracellular NEDD8When the intracellular NEDD8 amounts in EL4 cell lysates were analyzed by immunoblotting using a specific antibody (Y297), we detected almost exclusively the cullins-NEDD8 band (ca. 75 kDa). The amount of the 75 kDa band was significantly decreased in the 9-(E,Z)-HODE (100 µM)-treated group (Fig. 4a). We next performed FACS analysis using the same antibody (Y297). The 9-(E,Z)-HODE (100 µM)-treated group gave median fluorescence intensity (MFI) of 1100, whereas the control group gave 1562, showing a statistically significant decrease in the 9-(E,Z)-HODE-treated group (p < 0.05) (Fig. 4b). We also used MLN4924 as a positive control, and found that MLN4924 decreased intracellular NEDD8 more effectively than 9-(E,Z)-HODE.

EL4 cells (1 × 106 cells) were treated with 9-(E,Z)-HODE or MLN4924 for 24 h. (a) The cullins-NEDD8 (75 kDa) was detected by immunoblotting with anti-NEDD monoclonal antibody (Y297, 1/1000 dilution). The membranes were also treated with anti-β-actin antibody (1/1000 dilution) as a control. (b) Cells were fixed with ethanol and stained with anti-NEDD8 antibody (Y297)/AlexaFlour®647-conjugated antibody. Fluorescently labeled cells were applied to a flow cytometer (upper panels). The amounts of intracellular NEDD8 were calculated and expressed as histograms (lower panels). Experiments were performed in triplicate, and data are presented as the mean ± S.E.M. Statistical data analysis was conducted using the Student’s t-test. * p < 0.05 vs. control.
In this study, we examined the effects of oxidation products of linoleic acid (9-(E,Z)-HODE, 9-(E,E)-HODE, 13-(Z,E)-HODE and 13-(E,E)-HODE) on the growth of EL4 mouse lymphoma cells. In the WST-8 assay, only 9-(E,Z)-HODE showed a cell growth-inhibitory effect on EL4 cells (Fig. 1), and apoptosis was induced by the compound in a concentration-dependent manner, as assessed by FACS (Fig. 2). Cell cycle analysis revealed that 9-(E,Z)-HODE induced G2/M phase arrest associated with apoptosis (Fig. 3).
In the process of oxidation of linoleic acid, HODEs are produced via HPODEs, yielding four isomers with different positions and configurations of the hydroxyl group. The HODEs prepared from biological origins are usually obtained as a mixture of these isomers.4) Therefore, we examined each isomer for its biological activity. The results clearly indicated that one isomer with a 9-(E,Z)-configuration specifically showed a cell growth-inhibitory effect on murine lymphoma cells. This finding suggests specific interactions of this isomer with certain cellular molecules. It is also possible that other isomers show distinct effects in other cells. It is thus very important to select an effective stereoisomer so that the target cells can develop a strong immunomodulatory effect and an anticancer effect.
We examined the effects of 9-(E,Z)-HODE on the growth inhibition against various tumor cell lines by subjecting them to the JFCR39 panel (Fig. S1). The fingerprint for 9-(E,Z)-HODE was found to be the most similar to that for MLN4924 based on the database search. MLN4924 has recently been shown to be an inhibitor of NEDD8-activating enzyme (NAE),17) and to have a significant therapeutic effect against myeloid leukemia, multiple myeloma, lymphoma, metastatic melanoma, and advanced solid tumors.18–24) We performed an experiment to measure the amount of intracellular NEDD8 with immunoblotting and FACS analyses using a specific monoclonal antibody (Y297). The amount of the cullin-NEDD8 was significantly decreased in the 9-(E,Z)-HODE (100 µM)-treated cells (Fig. 4a). Moreover, the 9-(E,Z)-HODE (100 µM)-treated cells showed an approximately 30% lower MIF (p < 0.05) than the control group in FACS analysis (Fig. 4b). NAE has been shown to regulate the turnover of the proteasome through control of the cullin-RING subtype of ubiquitin ligases.17) 9-(E,Z)-HODE may have activity similar to MLN4924 and cause the impairment of this system, leading to the apoptotic death of EL4 lymphoma cells observed in this study.
Previous studies have indicated that the oxidation products of linoleic acid, including HODEs, exhibit a variety of crucial functions in physiological and pathological cellular processes such as inflammation, immune reactions, and carcinogenesis; e.g., activating PPARγ,5) acting as ligands for G2A, a proton sensing receptor,6) and acting as agonists for TRPV1, a receptor involved in hyperalgesia and allodynia.25) Because G2A is also known to transduce a signal to regulate the cell cycle, one possibility is that the growth inhibition of EL4 cells by 9-(E,Z)-HODE involves the function of G2A. We previously confirmed the expression of G2A mRNA in EL4 cells by RT-PCR analysis (Tsuiji et al., unpublished observation). In addition to HODEs, arachidonic acid metabolites such as 11-hydroxyeicosatetraenoic acid (HETE) were reported to serve as ligands for G2A.6) Therefore, it will be interesting to examine the effects of various eicosanoids on the growth and apoptosis of EL4 cells. HODEs have also been identified as potential biomarkers for diabetes, atherosclerosis and infection.7,8,26) Moreover, conjugation of these compounds with β-glucan derived from fungi has been shown to yield agents with strong immunostimulating and anti-tumor effects (Yasuhara et al., unpublished observation). In a future study, the mechanism underlying the cell growth-inhibitory effect, including identification of target molecules for 9-(E,Z)-HODE, should be elucidated. In addition, because the results of this study revealed the stereospecific activity of HODEs, future studies investigating the activities of individual stereoisomers are merited.
We would like to thank all our colleagues, especially Ms. Chisato Kurisaka, Mr. Taiki Yamaguchi, Ms. Sakiko Okabe, Ms. Harumi Shibusawa, Dr. Katsuhiko Takahashi, and Dr. Nobuaki Higashi (Hoshi University School of Pharmacy and Pharmaceutical Sciences). This study was supported in part by JSPS KAKENHI Grant No. 18K07334, the Ministry of Education, Culture, Sports, Science and Technology (MEXT)-supported program for the Strategic Research Foundation at Private Universities, 2014–2018 (Grant No. S1411019), Grant-in-Aid for Scientific Research on Innovative Areas “Platform of Advanced Animal Model Support” (JSPS KAKENHI Grant JP 16H06276), and the RCSI and Hoshi University International Research Summer School Scholarship Program.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.