2020 年 43 巻 3 号 p. 503-508
2020 年 43 巻 3 号 p. 503-508
Obesity elevates the risk of cardiovascular disease and has been strongly associated with increases in the incidence of many metabolic diseases. Therefore, prevention of obesity leads to the prevention of metabolic diseases. In light of this, substances that exert anti-obesity effects are crucial for the prevention of obesity. Indirubin, a 3,2′ bisindole isomer of indigo, is the active component of the traditional Chinese medicine used for the treatment of chronic myelocytic leukemia. In particular, indirubin-3′-oxime (1) was shown to inhibit the differentiation of adipocytes. In this study, we investigated the inhibitory effects of nine indirubin-3′-oxime derivatives against lipid accumulation during differentiation in 3T3-L1 cells. Among the compounds tested, 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) at 5 µM exhibited significantly stronger inhibitory activity than indirubin-3′-oxime (1). Furthermore, 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) markedly suppressed the expression of CCAAT/enhancer-binding protein α, peroxisome proliferator activator γ2, and adipocyte protein 2, both of which are key adipogenic regulators at the intermediate stage of adipocyte differentiation. Our results demonstrate that 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) significantly down-regulated lipid accumulation during differentiation of 3T3-L1 cells, suggesting their potential as novel therapeutic drugs against the development of obesity.
Obesity is currently one of the greatest social problems in Japan. It increases the risk of cardiovascular disease and has been strongly associated with increases in the incidence of metabolic diseases, such as hypertension, type 2 diabetes, insulin resistance, dyslipidemia, and atherosclerosis.1–6) Therefore, improving and preventing obesity leads to the prevention of metabolic diseases. Over the last few decades, considerable attention has been focused on the improvement of obesity, therapeutic treatments, and the molecular mechanism of adipocyte differentiation.7–9) 3T3-L1 cells have been extensively used in anti-obesity studies.10–12) Therefore, these cells are a useful model in screening novel agents for the prevention of obesity and clarifying the molecular mechanism of adipocyte differentiation. We previously reported the inhibitory effects of triterpenoids and chalcones isolated from Brazilian propolis and leaves of Murta (Myreugenia euosma), respectively, against lipid accumulation during differentiation of 3T3-L1 cells.13–15)
Indirubin, a 3,2′ bisindole isomer extracted from the indigo plant, has been identified as the active component of Danggui Longui Wan, a traditional Chinese medicine used for the treatment of chronic myelocytic leukemia.16) Indirubin derivatives have also been reported to inhibit protein kinases, such as cyclin-dependent kinases and glycogen synthase kinase-3.17–20) Our previous studies have also reported that some of these derivatives exhibit beneficial pharmacological effects, such as anti-cancer and anti-inflammatory activities.21–24) Interestingly, indirubin-3′-oxime has been demonstrated to inhibit the differentiation of adipocytes.25) However, it remains unclear whether indirubin-3′-oxime derivatives exert their inhibitory effects against lipid accumulation during differentiation of 3T3-L1 cells. In the present study, we investigated the inhibitory effect of nine indirubin-3′-oxime derivatives on lipid accumulation during differentiation of 3T3-L1 cells.
The structures of the indirubin-3′-oxime derivatives are shown in Fig. 1. These compounds were prepared as previously reported.23,26)

Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F12 medium were purchased from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan). Calf serum was obtained from Thermo Fisher Scientific (Waltham, MA, U.S.A.). Fetal bovine serum and Oil Red O were obtained from Cosmo Bio. Co., Ltd. (Tokyo, Japan). Isobutyl-3-methylxanthine, dexamethasone, insulin, adenine, transferrin, glutamine, and dimethyl sulfoxide (DMSO) were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Triiodothyronine was obtained from Sigma-Aldrich Co. (St. Louis, MO, U.S.A.). Penicillin–streptomycin–glutamine and penicillin–streptomycin–neomycin were purchased from Life Technologies (Burlington, ON, Canada). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Cell Counting Kit (MTT) was obtained from Nacalai Tesque Inc. (Kyoto, Japan).
Cell CultureThe 3T3-L1 cells (Human Science Research Resources Bank, Osaka, Japan) were cultured in DMEM supplemented with 10% calf serum and 1% penicillin–streptomycin–glutamine in an incubator at 37°C with 5% carbon dioxide. Subsequently, 3T3-L1 cells were seeded in 24-well plates and 6-cm dishes. The cells were grown to confluence to induce adipose differentiation. The cells were induced with differentiation medium (DMEM and Ham’s F12 supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin–neomycin, 1.6 µM insulin, 0.0005% transferrin, 180 µM adenine, 20 pM triiodothyronine) containing 500 µM isobutyl-3-methylxanthine, and 0.25 µM dexamethasone with indirubin-3′-oxime derivatives (defined as 0 d). After 2 and 4 d, the cell culture medium containing indirubin-3′-oxime derivatives was replaced with fresh differentiation medium. The test samples were dissolved in DMSO. The final concentration of DMSO in the differentiation medium was 0.1%.
Cell Viability AssayCell viability was measured using the MTT assay. The 3T3-L1 cells (1 × 104 cells/well) were seeded in a 96-well plate. After 2 d of incubation, the cells were treated with indirubin-3′-oxime derivatives at 5 µM. After 48 h, 10 µL MTT solution (5.0 mg/mL) was added to each well, and the plate was incubated for an additional 4 h at 37°C. Subsequently, 100 µL MTT solubilization solution was added. Cell viability was calculated by determining the absorbance at 570 nm using a microplate reader.
Oil Red O StainingThe 3T3-L1 cells (5 × 104 cells/well) were seeded in a 24-well plate. At day 7, the cultured cells were washed with phosphate-buffered saline and fixed with 10% formalin for 24 h. The fixed cells were then rinsed once with H2O, and stained with Oil Red O solution (0.5% Oil Red O in 60% isopropanol, and filtered with a 1.0-µm filter) for 15 min. The stained cells were washed thrice with H2O prior to visualization and documentation. The Oil Red O was eluted using a solubilization solution (99% isopropanol), and quantified by measuring the absorbance at 540 nm using a microplate reader. The value of DMSO-treated cells was defined as 100%.
Gene ExpressionThe 3T3-L1 cells (2 × 105 cells/well) were seeded in 6-cm plates. At day 7, total RNA was isolated from 3T3-L1 cells using RNAiso Plus (TaKaRa Co., Ltd., Shiga, Japan). RNA (1.0 µg) was used for cDNA synthesis with oligo primers (Sigma-Aldrich Co.), deoxynucleoside triphosphate mixture (Nippon gene Co., Ltd., Tokyo, Japan), and reverse transcriptase (Toyobo Co., Ltd., Osaka, Japan). Aliquots of cDNA were amplified using a Stratagene Mx3000 quantitative RT-PCR (Agilent Technologies, Santa Clara, CA, U.S.A.) by GoTap qPCR Master Mix (Promega, Madison, WI, U.S.A.) according to the protocol provided by the manufacturer. The primer sequences (Sigma-Aldrich Co.) used in this study are shown in Table 1. The relative mRNA expression was normalized against the expression of 36b4.
| Gene | Primers | (5′–3′ Sequence) |
|---|---|---|
| Pparγ2 | Forward | GCTGTTATGGGTGAAACTCTG |
| Reverse | ATAATAAGGTGGAGATGCAGG | |
| C/ebpα | Forward | TGGACAAGAACAGCAACGAG |
| Reverse | TCACTGGTCAACTCCAGCAC | |
| aP2 | Forward | ATGAAATCACCGCAGACGACAGGA |
| Reverse | TGTGGTCGACTTTCCATCCCACTT | |
| Atgl | Forward | GGAGACCAAGTGGAACATCTCA |
| Reverse | AATAATGTTGGCACCTGCTTCA | |
| Hsl | Forward | TCCTGGAACTAAGTGGACGCAAG |
| Reverse | CAGACACACTCCTGCGCATAGAC | |
| Pparα | Forward | ATGCCAGTACTGCCCTTTTC |
| Reverse | GGCCTTGACCTTGTTCATGT | |
| Ucp2 | Forward | GGCTGGTGGTGGTCGGAGAT |
| Reverse | CCGAAGGCAGAAGTGAAGTG | |
| 36b4 | Forward | AAGCGCGTCCTGGCATTGTCT |
| Reverse | CCGCAGGGGCAGCAGTGGT |
The data are presented as the mean ± standard deviation. All data analyses were conducted using ANOVA. The statistical significance of differences between DMSO and test samples was determined using Tukey’s test. p-Values <0.05 denoted statistical significance.
Indirubin-3′-oxime (1) has been shown to inhibit the differentiation of adipocytes.25) Firstly, we confirmed whether indirubin-3′-oxime (1) acts as an inhibitor of adipocyte differentiation in 3T3-L1 preadipocytes for 7 d, using Oil Red O staining. The results demonstrated that treatment with indirubin-3′-oxime (1) significantly inhibited lipid accumulation during differentiation in a dose-dependent manner (Fig. 2). Secondly, the effect of the nine indirubin-3-oxime derivatives on cell viability was evaluated using the MTT assay. None of the indirubin-3′-oxime derivatives were cytotoxic at concentrations up to 5 µM (Fig. 3). Subsequent experiments were performed with indirubin-3-oxime derivatives at concentrations up to 5 µM. Oil Red O staining was conducted after induction of differentiation for 7 d to investigate the effects of the indirubin-3-oxime derivatives (1–9) on lipid accumulation. After induction, complete differentiation was observed in DMSO-treated control cells, whereas treatment with 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) significantly inhibited lipid accumulation during differentiation (Fig. 4). The presence of methoxy group in 5-position is important for decreasing lipid accumulation. Moving the methoxy group from the 5-position to the other sites was detrimental (2 vs. 3–5). In addition, incorporation of the bromine in the 5-position (6) especially in the 6-posion (7) increased activity. The quantitative analysis of Oil Red O staining also revealed that treatment with 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) drastically attenuated lipid accumulation compared with DMSO and indirubin-3′-oxime (1) (Fig. 5). It is well established that berberine chloride can act as an inhibitor of lipid accumulation during the differentiation of 3T3-L1 cells. Hence, berberine chloride at 5 µM was used as a positive control.27) 5-Methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) exhibited a similar inhibitory activity on lipid accumulation to that observed for berberine chloride. As shown in Fig. 6, 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) effectively decreased the level of lipid accumulation in 3T3-L1 cells in a dose-dependent manner. Our results show that 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) had significantly stronger inhibitory activity than indirubin-3′-oxime (1).

The cells were treated with compound 1 on days 0, 2, and 4. The levels of intracellular lipids were assayed using Oil Red O staining 7 d after the induction of differentiation. Results are the mean ± standard deviation (S.D.) of three independent experiments (* p < 0.05 vs. control, Tukey’s test). The value obtained using DMSO-treated cells was 100%.
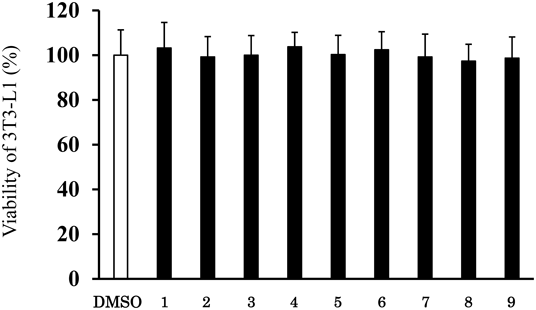
The cells were treated with each of the nine compounds. Cytotoxicity was assessed on day 2 using MTT assay. Results are means ± S.D. of three independent experiments (* p < 0.05 vs. control, Tukey’s test). The value obtained with DMSO-treated cells was 100%.

Berberine chloride (ber.) (5 µM) was used as a positive control. The cells were treated with each of the nine compounds on days 0, 2, and 4. The levels of intracellular lipids were assayed using Oil Red O staining 7 d after the induction of differentiation. Results are the mean ± S.D. of three independent experiments (* p < 0.05 vs. control, Tukey’s test). The value obtained with DMSO-treated cells was 100%.

(Color figure can be accessed in the online version.)

The cells were treated with compound 2 and 7 on days 0, 2, and 4. The levels of intracellular lipids were assayed using Oil Red O staining 7 d after the induction of differentiation. Results are the mean ± S.D. of three independent experiments (* p < 0.05 vs. control, Tukey’s test). The value obtained using DMSO-treated cells was 100%.
CAA T/enhancer-binding protein (C/EBP) α and peroxisome proliferator activator receptor (PPAR) γ2 play roles as master regulators of adipogenesis and induce the expression of lipogenic proteins, such as adipocyte protein 2 (aP2).28–30) We examined the expression levels of major adipocyte-related genes (Pparγ2, C/ebpα, and aP2) to investigate the inhibitory mechanism of 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) during differentiation. As shown in Fig. 7A, differentiated cells clearly displayed increased expression levels of these genes compared with undifferentiated cells. In contrast, 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) down-regulated the expression levels of these genes compared with DMSO. Moreover, adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) has been shown to be involved in the lipolysis of triacylglycerol in adipocytes.31) Furthermore, PPARα and uncoupling protein 2 (UCP2) are known to play a role in lipid metabolism.32,33) We also measured the expression levels of genes related to lipolysis and lipid metabolism. The results show that 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) inhibited the expression levels of the lipolysis and lipid metabolism-related genes (Figs. 7B, C). These findings suggest that 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) exert an inhibitory effect during the differentiation of 3T3-L1 cells. Therefore, 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) may represent lead compounds in the development of agents for the prevention of obesity.
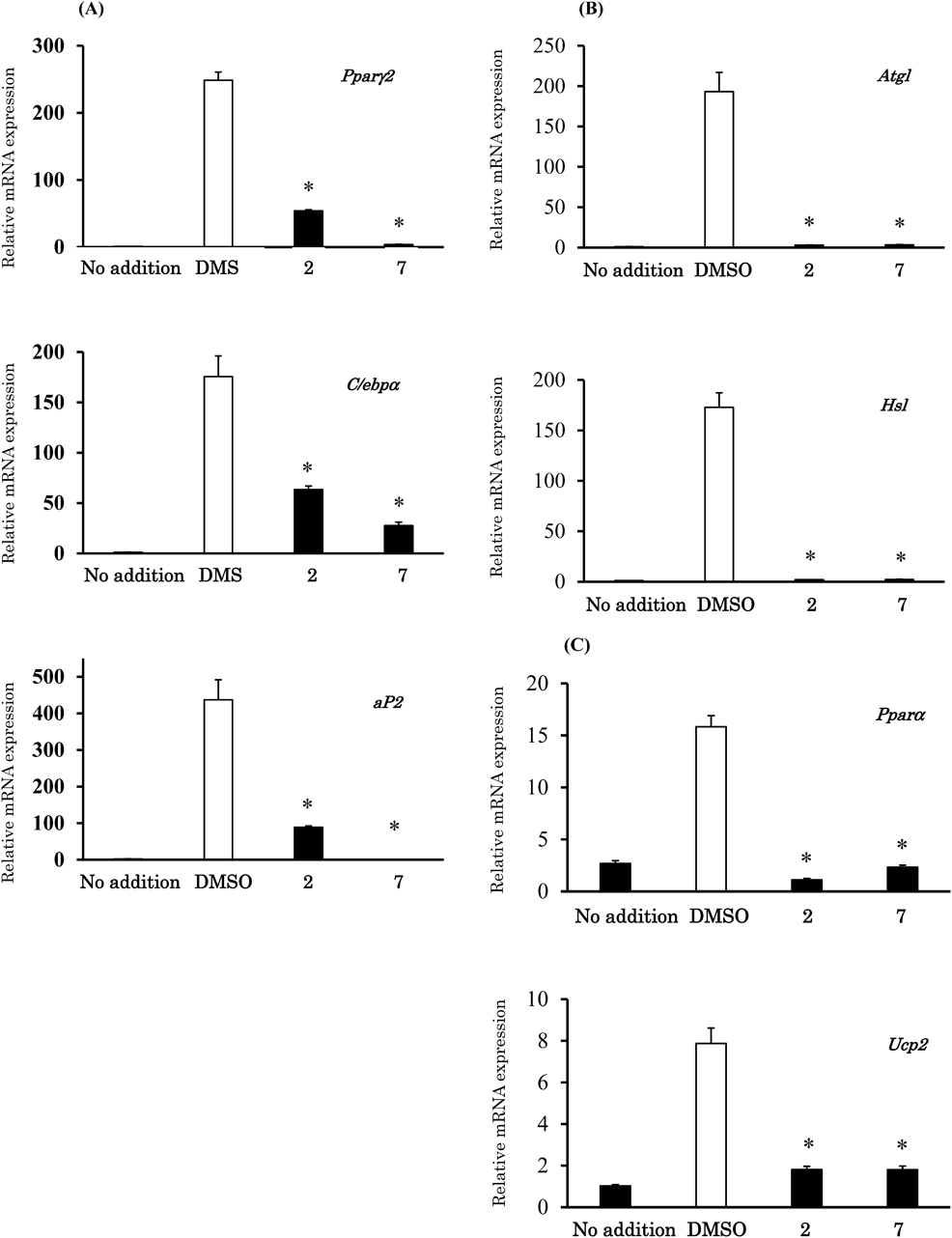
The cells were treated with compound 2 and 7 (5 µM) or DMSO as a control. The expression of the Pparγ2, C/ebpα, aP2, Atgl, Hsl, Pparα, and Ucp2 genes was normalized to that of 36b4 (loading control). Results are the mean ± S.D. of three independent experiments (* p < 0.05 vs. control, Tukey’s test).
The inhibitory effect of indirubin-3′-oxime (1) on adipocyte differentiation has been reported.25) The molecular mechanisms of adipocyte differentiation have been extensively examined using cultured pre-adipocytes, such as 3T3-L1 cells. Thus, these cells are a suitable screening model to assess the anti-obesity effects of agents and natural products. In the present study, we examined the inhibitory effects of indirubin-3′-oxime derivatives against adipocyte differentiation using 3T3-L1 cells. We selected indirubin-3′-oximes with different substituent types (i.e., methoxy group as an electron-donating group and bromine as an electron-withdrawing group) to obtain information regarding the substituents and electron density of the aromatic ring of the indirubin skeleton. Among the nine indirubin-3′-oxime derivatives selected, 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) demonstrated stronger inhibitory activity than indirubin-3′-oxime (1). The data shown in Fig. 4 indicate the need of a methoxy group in the 5-position and a bromine in the 6-position. The bromine substitution in the 6-position is seems to be important for selectivity of indirubin analogs toward critical physiological kinases (GSK-3 and CDKs).19) Interestingly, insertion of these substituents in the 5′-position instead of the 5-position was detrimental for lipid accumulation (Fig. 4). Also, indirubin-3′-oxime derivatives were not cytotoxic at concentrations up to 5 µM. Thus, the results of the cell viability analysis suggest that the inhibitory effects of 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) on lipid accumulation during cell differentiation are attributable to their anti-lipogenic properties. Moreover, berberine chloride was used as a positive control.27) The results show that 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) exhibit a similar inhibitory effect on lipid accumulation to that reported for berberine chloride.
Adipogenesis is accompanied by the expression of various adipocyte-related genes. C/EBPα and PPARγ2 are the master regulators of adipogenesis, and are activated during the intermediate stages of adipocyte differentiation.28–30) aP2 is expressed in differentiated adipocytes and plays a role in fatty acid metabolism. We confirmed that 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) suppressed the expression levels of the adipocyte-related genes Pparγ2, C/ebpα, and aP2. These results demonstrate that 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) inhibit the differentiation of 3T3-L1 preadipocytes by suppressing the expression of Pparγ2 and C/ebpα. In addition, 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) inhibited the expression of the lipolysis and the lipid metabolism-related genes Atgl, Hsl, Pparα, and Ucp2, which was not significantly different from the expression levels in undifferentiated cells. Although the precise structure–activity relationships and inhibition mechanisms have not yet been clarified, these results provide new directions for designing 5- and/or 6-substituted indirubins19,20,23) that could potentially be applied to the prevention of obesity. Further studies are warranted to elucidate the direct association between the inhibition of lipid accumulation and the structure of indirubin-3′-oximes.
In the present study, we found that indirubin-3′-oxime derivatives exhibit an inhibitory effect against lipid accumulation during differentiation of 3T3-L1 cells. The results showed that 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) exhibited significantly stronger inhibitory activity than indirubin-3′-oxime (1). In addition, 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) suppressed adipocyte differentiation, and was involved in the down-regulation of key adipogenic regulators (i.e., Pparγ2, C/ebpα). The present study is the first to investigate the anti-obesity effect of indirubin-3′-oxime derivatives. These results suggest that 5-methoxyindirubin-3′-oxime (2) and 6-bromoindirubin-3′-oxime (7) may represent lead compounds in the development of agents for the prevention of obesity.
Development and Establishment of the Center of Excellence on Anti-Doping Education Research, and Studies toward Post-Olympic/Paralympic Games at Nihon University. The authors thank Dr. Koichi Metori from the Institute of Pharmaceutical Sciences, Nihon University, for performing the mass measurements.
The authors declare no conflict of interest.