2020 年 43 巻 8 号 p. 1147-1153
2020 年 43 巻 8 号 p. 1147-1153
Gene and nucleic medicines have recently gained attention as novel drugs with the advancement of molecular biology and genetics; however, they have low bioavailability and low target delivery due to their low stability and poor membrane permeability. Therefore, the development of an effective drug delivery system (DDS) is necessary for the practical use of gene and nucleic acid medicines; however, despite considerable research, both safety and efficiency remain poor. Furthermore, the healthcare needs are not met by traditional DDS. Therefore, we developed an effective multi-functional DDS, which is constructed using materials that are safe for human consumption. This DDS involves several ternary complexes as novel gene delivery carriers constructed by coating the cationic complex of the gene and nucleic acid medicines as well as the biodegradable cationic polymer with a biocompatible anionic polymer. Early implementation of the ternary complex in clinical studies is expected due to their efficacy and safety. Furthermore, these complexes may be prepared using large-scale manufacturing. In addition, personalized DDS may be prepared according to the patient’s disease stage, which is useful for advanced therapy.
Gene and nucleic acid medicines are promising next-generation drugs that can control diseases at the genetic level. These medicines include inhibitors of transcription factor (decoy) and mRNA translation (antisense), which are agents of RNA interference (small interfering RNA (siRNA) and micro RNA (miRNA)), as well as nucleic acids that bind proteins and other molecular ligands (aptamers). However, a major challenge in their implementation is their rapid degradation by nucleases in the body.
Chemical modification of gene and nucleic acid medicines helps to improve their stability, cellular permeability, circulation half-life, and tissue bioavailability of nucleic acid medicines, which are required for clinical application. Compared with unmodified gene and nucleic acids, those that are modified have higher membrane compatibility and permeability due to its lipophilic characteristic.1,2) Gene and nucleic acid medicines are able to harness natural carriers in circulation for improved pharmacokinetics by lipophilic modulation. Lipophilic siRNA, especially cholesterol siRNA conjugates, strongly bind with blood components and greatly prolong circulation time.3) However, stable gene and nucleic acid medicines must have strong off-target effects and little specific uptake. Thus, the clinical application of gene and nucleic acid medicines is highly dependent on the development of effective and reliable drug delivery systems (DDS).
Viral and non-viral vectors have been used as carriers for safe and effective gene delivery systems.4) Due to the intracellular gene delivery pathway, viral vectors have high gene transfection efficiency5); however, their use is limited due to side effects. Therefore, non-viral vectors that have flexibility in chemical design and low immunogenic response as well as high safety and stability are gaining attention. Furthermore, it is easy to produce and chemically convert non-viral vectors on a mass scale.6) Most importantly, non-viral vectors are not restricted by the molecular size of the gene and nucleic acid. Based on these advantages, a large number of non-viral delivery systems, such as liposomes,7–9) dendrimers,10–12) peptides,13–15) and polymers,16–18) have been developed and applied to gene therapy.
There are many studies regarding cationic particles and PEGylated particles for gene delivery.19,20) Although cationic particles are able to encapsulate gene and nucleic acid medicines stably, they are taken up by non-specific cells and have high cytotoxicity in vitro and high hematological toxicity in vivo.19) Among this type of DDS, lipid-based delivery systems are the most studied with several formulations in late-stage clinical trials. As of August 2019, 8 nucleic acid medicines have been approved by drug regulatory agencies from various countries. Onpattro (Alnylam) was recently approved by the U.S. Food and Drug Administration (FDA) for clinical use as the first siRNA therapy using lipid-based delivery systems. However, polymer-based delivery systems have yet to be approved. PEGylated particles are also able to encapsulate gene and nucleic acid medicine stably with little cytotoxicity and hematotoxicity; however, challenges include low cellular uptake due to their particle size as well as their complicated structure. Thus, in order to be approved for clinical use, the DDS must be safe and biocompatible.
We successfully developed several ternary complexes as novel gene delivery carriers, which were constructed by coating the cationic complex of the gene and nucleic acid medicine well as the biodegradable cationic polymer with a biocompatible anionic polymer (Fig. 1). This ternary complex consisted of biodegradable materials found in foods and medical products that are already in clinical use, and we were able to deliver gene and nucleic acid medicines to specific organs without toxicity. Here, I will review our research regarding the development of several gene delivery systems.

(Color figure can be accessed in the online version.)
Toxicity of cationic particles in gene and nucleic acid medicines arise due to their strong cationic surface. One method for overcoming the challenges of cationic particles is the construction of a ternary complex coated by anionic compounds, which cover the surface of the cationic particles. However, non-cationic particles are generally not well taken up by cells since they electrostatically repulse the cell membrane. We discovered a new type self-assembling nanoparticles for gene delivery, which consists of plasmid DNA (pDNA), polyethyleneimine (PEI), and γ-polyglutamic acid (γ-PGA). Surprisingly, γ-PGA-coated pDNA–PEI (pDNA–PEI–γ-PGA complexes) had high cellular uptake and gene expression without cytotoxicity and blood agglutination.21)
First, we investigated the ternary complexes of the pDNA-PEI complex coated with various anionic compounds, such as polyadenylic acid (polyA), polyinosinic–polycytidylic acid (polyIC), α-polyaspartic acid (α-PAA), α-polyglutamic acid (α-PGA), and γ-PGA. Although the pDNA–PEI complex had a cationic surface charge and had high gene expression, it had high cytotoxicity and agglutination with blood components. Complex size was unaffected when the anionic compounds changed the cationic charge of the pDNA–PEI complex to anionic. The ternary complexes had lower cytotoxicity and had no agglutination activities, and most did not show cellular uptake and transgene efficiency. However, the pDNA–PEI–γ-PGA complexes had high cellular uptake and gene expression without cytotoxicity and agglutination (Fig. 2).

Reprinted with permission, Biomaterials, published by Elsevier, 2009.21)
We examined the uptake mechanism and intracellular localization of the complex in vitro. Although the addition of γ-PGA as well as hypothermic conditions significantly inhibited the cellular uptake of pDNA–PEI–γ-PGA complexes, there was no inhibition with the addition of L-glutamic acid. These results show that pDNA–PEI–γ-PGA complexes are taken up by the γ-PGA-specific receptor-mediated energy-dependent process. Most of the pDNA–PEI–γ-PGA complexes were located in the cytoplasm without dissociation, and a few complexes were also observed in the nuclei. Thus, we discovered a γ-PGA-coated vector that may be effective and safe for gene delivery.
Furthermore, with continued screening, we found several anionic compounds, such as chondroitin sulfate,22,23) fetuin,24) folic acid,25) phosphatidylserine,26) and polycytidylic acid (polyC),27) that can be covered with cationic particles with high gene transfection without toxicity.
Next, we investigated the in vivo transgene efficiency of the pDNA–PEI–γ-PGA complex. The pDNA–PEI–γ-PGA complex had high gene expression in the spleen after intravenous administration (Fig. 3a). The spleen, which is the largest secondary immune organ in the body, is responsible for initiating immune reactions to blood-borne antigens and for filtering foreign substances as well as old and damaged erythrocytes from the blood.29) Splenic dysfunction results in an increased risk of infection. Spleen-targeting nanoparticles are a promising gene therapy approach for splenic disease and vaccination. In particular, vaccinations are now gaining attention as a method to treat several cancers and autoimmune diseases as well as to prevent various infectious diseases.
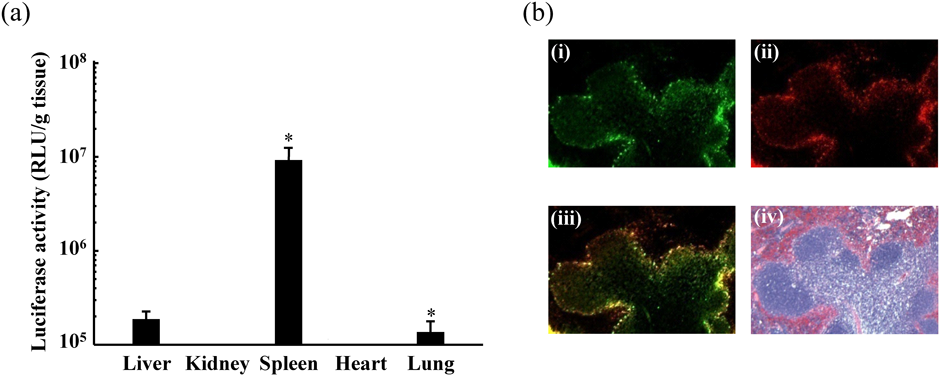
The localization of FITC–PEI (i), gene expression of ptdTomato (ii), merged picture (iii), and HE-stained sections (iv) are shown (100× magnification). Biol. Pharm. Bull., Vol. 36, No. 11, pp. 1800–1806, Copyright 2013 The Pharmaceutical Society of Japan.28) (Color figure can be accessed in the online version.)
We examined the detailed localization of the pDNA–PEI–γ-PGA complex in the spleen. The pDNA–PEI–γ-PGA complex was constructed with ptdTomato-N1 and fluorescein isothiocyanate (FITC)–PEI and was intravenously administered to mice. The spleen was dissected 24 h after administration. The pDNA–PEI–γ-PGA complexes were found to accumulate and show gene expression in the marginal zone, which is a region at the interface between the red and white pulp of the spleen (Fig. 3b). The marginal zone of the spleen is abundant in antigen-presenting cells (APCs), such as dendritic cells and macrophages.30) DNA vaccines have several advantages, such as the ease of setting up large-scale productions resulting in low-cost vaccines for worldwide use, which may benefit developing areas of the world.31,32) A new approach to enhance the efficacy of DNA vaccines is to develop a vector that enables the efficient delivery of DNA vaccines to APCs.33,34) The pDNA–PEI–γ-PGA complex improved the transgene efficiency of DNA vaccines on APCs in the marginal zone of the spleen with higher immune responses. These results showed that the pDNA–PEI–γ-PGA complex is a strong candidate as a DNA vaccine vector. We therefore applied the γ-PGA-coated complex to a melanoma DNA vaccine.
Melanoma is a malignant tumor that develops from pigment-containing cells, known as melanocytes, and most frequently arises from the skin. Although melanoma has high risk for metastasis and is also highly resistant to chemotherapy, it is one of the most immunogenic cancer, and some antigens against melanoma have been found.35,36) Therefore, a DNA vaccine against melanoma will not only help prevent metastasis and relapse but may also suppress tumor growth. We selected pUb-M, which expresses melanoma-related antigen (gp100 and tyrosinase-related protein 2 (TRP2)), as a potential DNA vaccine candidate against melanoma and prepared a ternary complex (pUb-M–PEI–γ-PGA complex) composed of pUb-M, PEI, and γ-PGA. The pUb-M–PEI–γ-PGA complex was intravenously administered to intradermal transplant mice to evaluate immune response against a mouse melanoma cell line (B16-F10). Naked pUb-M and control pDNA–PEI–γ-PGA complexes did not suppress the growth of B16-F10 cells in the intradermal transplant mice. However, the pUb-M–PEI–γ-PGA complex significantly suppressed tumor growth (Fig. 4a). Furthermore, the pUb-M–PEI–γ-PGA complex also significantly inhibited lung metastasis of B16-F10 cells (Figs. 4b, c). These results show that this novel splenic delivery system may have application to the development of DNA vaccines.

Biol. Pharm. Bull, Vol. 36, No. 11, pp. 1800–1806, Copyright 2013 The Pharmaceutical Society of Japan.28) (Color figure can be accessed in the online version.)
There were 219 million cases and approximately 435000 deaths globally due to malaria in 2017.39) Various strategies have been developed to prevent malarial infections, such as diagnosis, treatment, and vector control.40–42) However, these strategies are limited since malaria-causing parasites are resistant to most antimalarial drugs, and there is insecticide resistance in anopheline mosquitoes that transmit malaria.43) Among other strategies, vaccination may be able to control and eradicate malaria.44–47) However, despite years of substantial effort, the development of a malarial vaccine continues to be a challenge. Furthermore, the only approved malarial vaccine as of 2015 is RTS, S, which is also known by its trade name, Mosquirix. Due to the low effectiveness of RTS, S, WHO does not recommend its routine use in babies between 6 and 12 weeks of age.48)
Among the various approaches, DNA vaccination as well as its application to antimalarial drug research was developed in the last 20 years. DNA vaccination has several advantages, such as the ability to induce both humoral and cellular immune responses, the flexibility to create vectors that incorporate antigens, the presence of immunostimulatory sequences, and its formulation that elicit optimal protective immunity. However, a major challenge of this approach is the generation of a gene delivery system with the appropriate adjuvant effect for the optimal delivery of the vaccines to target cells and the induction of the desired immune phenotype. Nanoparticles would ensure that the immunogen is processed by the immune system in a similar manner to pathogens and thereby eliciting a similar response.
We therefore applied γ-PGA-coated complex to malarial DNA vaccines.37,38) We used Plasmodium yoelii merozoite surface protein-1 (PyMSP-1)-encoding plasmid DNA (pVR1020-MSP-1) as the malarial DNA vaccine and prepared the ternary complex (pVR1020-MSP-1–PEI–γ-PGA complex) composed of pVR1020-MSP-1, PEI, and γ-PGA. The particle size and ζ-potential of the ternary complex were approximately 100 nm and -15 mV, respectively.
To examine whether immunization with the ternary complex had any effects on the course of blood-stage malarial infection, mice were immunized three times in three week intervals with either 5% glucose (control), naked pVR1020-MSP-1, or ternary complex and were intraperitoneally challenged with lethal P. yoelii 17XLparasitized red blood cells (1 × 105). Mice immunized with the ternary complex had lower parasitaemia than those immunized with 5% glucose or naked pVR1020-MSP-1. Furthermore, all mice that were immunized with 5% glucose and naked pVR1020-MSP-1 died within 10 d after infection, whereas all mice that were immunized with the ternary complex survived for more than 30 d.
The pVR1020-MSP-1–PEI–γ-PGA complex showed significant immunogenicity and elicited protective levels of antigen specific immunoglobulin G (IgG) and its subclass antibody. Furthermore, in addition to observing interferon-γ (INF-γ)-producing cells in the spleen, there were an increased proportion of CD4+ and CD8+ T cells as well as increased INF-γ and interleukin (IL)-12 levels in the serum and cultured splenocyte supernatant. These results suggest that the ternary complex enhanced cellular and humoral immunity and had protective effects against a lethal strain of the parasite in a mouse model.
We also applied the γ-PGA-coated complex as a vaccine against schistosomiasis.49) From this study, we showed significant anti-fecundity effect of nanoparticle-coated SjGST DNA vaccine against murine Schistosoma japonicum infection.
Glycyrrhizin (GL) is the main sweet-tasting compound extracted from the Glycyrrhiza glabra (licorice) root. In Japan, GL has been used as a drug under the name of Stronger Neo-Minophagen C, which is an injectable solution, for the treatment of chronic active hepatitis.52–56) The cell membrane of in vitro rat hepatocytes have specific binding sites for GL.57,58) After intravenous administration, GL is rapidly removed from the blood circulation by uptake into the liver.59) These results imply that GL may be a novel ligand for hepatocyte-specific gene delivery. We hypothesized that GL coats cationic particles, including pDNA, electrostatically by its anionic charge and that the GL-coated complexes are taken up by cells via the GL-mediated pathway. Therefore, we developed a ternary complex of pDNA, PEI, and anionic GL by electrostatic interaction.
The ternary complex was stable and had high gene expression in the human hepatoma cell line HepG2. It also had high gene expression, specifically in the liver, after intravenous administration. Furthermore, the ternary complex that included the pDNA-encoding insulin was able to decrease blood glucose concentrations after intravenous administration in mice. These results suggest that the ternary complex coated by GL may be a promising liver-targeting gene vector.
N-Lauroylsarcosine (LS) is a low-toxic biodegradable detergent and is metabolized by humans into sarcosine and corresponding fatty acids.60) In a preliminary study, we found that LS-containing particles showed high transgene efficiency in the lung after intravenous administration. Therefore, we hypothesized that LS, which has an anionic charge, is able to coat cationic particles, such as pDNA, and that the LS-coated complexes have selective delivery to the lung after intravenous administration.
We then prepared a hybrid vector for pulmonary gene delivery: a lipopolyplexes composed of PEI, N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethlylammonium (DOTMA), and LS. LS-coated lipopolyplexes had markedly high transgene efficiency in the lung after intravenous administration with no cytotoxicity and hematotoxicity. We also found that LS molecules contributed to the transgene efficiency of our lipopolyplexes in the lung at 76.7% of the contribution index. There are expectations that lung-targeting nanoparticles will be a promising method in gene and nucleic acid medicines for various inherited and acquired lung diseases, such as cystic fibrosis, emphysema, asthma, and certain types of cancer.
RNA interference (RNAi) is a biological process that controls gene expression and translation by sequence-specific gene silencing, and thereby inhibiting the translation of its mRNA transcripts into proteins. Exogenous siRNA may be delivered to exert RNAi. SiRNA are 21–23 base-pair duplex oligonucleotides in which antisense chains are complementary to the target mRNA and the sense chains act as bystanders. SiRNA operates through the native RNAi machinery to assemble the RNA-induced silencing complex (RISC). In RISC, siRNA initiates sequence-specific cleavage of both sense and antisense chains. This selective degradation of mRNA results in decreased expression of proteins involved in disease pathogenesis.
Gene silencing by siRNA has potential applications in the treatment of refractory diseases, such as cancers, viral infections, autoimmune diseases, and genetic disorders.62–64) However, the therapeutic use of siRNA requires a drug-delivery system since unmodified naked siRNA is immediately degraded by nucleases. Furthermore, it has poor plasma membrane penetration and induces interferon responses after systemic injection.
Therefore, we applied the γ-PGA-coated complex to siRNA and used dendrigraft poly-L-lysine (DGL) as the cationic compound of the γ-PGA-coated complex. DGL consists entirely of lysine, including its central core, and is completely biodegradable, water-soluble, thermally stable, and non-immunogenic. However, the binding ability of siRNA with cationic compounds is weaker than that of pDNA since the molecular weight of siRNA is lower than pDNA. We were able to successfully construct a ternary complex that includes siRNA; however, it prepared in a different manner to that of pDNA. The siRNA–DGL–γ-PGA complexes were approximately 100 nm in diameter with negative surface charges. Strong silencing effects of the siRNA–DGL–γ-PGA complexes were observed in a mouse colon carcinoma cell line, Colon26, and was shown to express luciferase (Colon26/Luc cells) without cytotoxicity or hematological toxicity.61) Thus, a safe and effective siRNA delivery system was constructed using biodegradable DGL and γ-PGA.
Recently, we constructed another novel ternary complex for siRNA, which showed high cellular uptake and was able to deliver siRNA into cancer cells stably in both in vitro and in vivo conditions.
We successfully developed several ternary complexes as novel gene delivery carriers for clinical use. We anticipate our ternary complexes to be implemented soon in clinical trials due to their efficacy and safety. We have been able to submit a clinical application for the ternary complexes with the support of Program for Creating STart-ups from Advanced Research and Technology (START) of Japan Science and Technology Agency (JST). START is a program designed for creating start-ups using human resource units (“Project Promoters”) with commercialization, business development and private commercialization expertise as well as public funds for R&D.
These complexes are also expected for personalized DDS according to the patient’s disease stage, which is useful for advanced therapy. The complexes can quantitatively deliver gene and nucleic acids to target site according to combination and ratio of components. The components will be mixed by pharmacists to prepare the adequate complex for individualized patient on bedsides.
We believe that our ternary complexes will be applied to clinical practice in the near future.
I would like to thank all members of my past and present laboratories at the Department of Hospital Pharmacy, Nagasaki University Hospital as well as collaborators for conducting the above-mentioned research. I also appreciate the financial support from the Grant-in-Aid for Scientific Research (KAKENHI) of Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology of Japan, Uehara Memorial Foundation, Cosmetology Research Foundation, and the Global COE Program, Nagasaki University, Japan to conduct this research.
The author declares no conflict of interest.
This review of the author’s work was written by the author upon receiving the 2019 Pharmaceutical Society of Japan Award for Divisional Scientific Contribution.