2021 年 44 巻 11 号 p. 1781-1789
2021 年 44 巻 11 号 p. 1781-1789
Dried terrestrial stems of Ephedra sinica are known as ‘Ephedra herb.’ The pharmacological effects are mainly related to two major ingredients, (−)-ephedrine and (+)-pseudoephedrine (total alkaloids which are defined in Japanese Pharmacopoeia, TA). In this study, in order to aid in cultivation and breeding, the stability of TA content and stem dry weight of 46 E. sinica genets was evaluated from the first year of transplantation to the sixth year. TA content and composition ratio of these genets were stable after the second year, and dry weight was stable after the fourth year. These traits showed high inter-genet variability but low annual variability for each genet. Additionally, rank correlation coefficients of each trait among the genets were high. There was no significant correlation between these traits. Furthermore, to assess the reproducibility of these traits in clones, we evaluated TA content and dry weight of three clonal lines with high TA contents. TA content and composition ratio of the clonal lines were also stable after the second year of transplantation, and dry weight of the clonal lines was also stable after the fourth year. Moreover, TA content and composition ratio in each clonal line were comparable with those of each original genet after the second year. These results suggested that ephedrine alkaloids content and dry weight of E. sinica plants are stable, and that these traits are highly reproducible in clones. Therefore, selection breeding of E. sinica using vegetative propagation can be effective for high and stable quality of Ephedra herb.
Ephedra herb, dried terrestrial stems of Ephedra sinica, has been used traditionally for the treatment of common cold, headache, bronchial asthma, and nasal inflammation in traditional Japanese (Kampo) and Chinese medicines. The pharmacological actions of these medicines are thought to be related to ephedrine alkaloids.1) Ephedra herb contains six ephedrine-type alkaloids in the form of three pairs of diastereomeric congeners.2) Among them, the two main congeners, (−)-ephedrine (Eph) and (+)-pseudoephedrine (PEph), commonly represent approx. 90% of these alkaloids.3) Additionally, the efficacy of Eph and PEph in Ephedra herb differs.4) The Japanese Pharmacopoeia states that Ephedra herb intended for medicinal use has to be over 0.7% total alkaloids (TA) (sum of Eph and PEph content) in dry weight (DW).5) Therefore, the content and composition ratio of Eph and PEph in Ephedra herb should be controlled.
Ephedra sinica is a gymnosperm shrub distributed across arid areas of northern China and Mongolia.6) In China, E. sinica has been cultivated commercially since the 1980 s.7) It takes five years until the first harvest of the medicinal parts of E. sinica derived from seedlings in China.7) After harvesting the terrestrial stems of E. sinica, new shoots re-emerge from the plant, and herbal stems are harvested annually.7)
In Japan, the inclusion of Ephedra herb in Kampo medicine relies on imports from China.8) Thus, it would be more convenient to cultivate E. sinica in Japan for a stable supply of Ephedra herb, and this practice started recently in Japan.9) However, the TA content of three quarters of Ephedra herb that were cultivated in Japan did not satisfy the criteria of the Japanese Pharmacopoeia (≥ 0.7% DW).9) Thus, the TA content of E. sinica cultivated in Japan must be higher and more stable.
Previous studies have reported that the content of ephedrine alkaloids in E. sinica varies widely depending on habitat10,11) and is affected by environmental factors,12,13) growth,14) genetic factors,15) and fertilization.16) Additionally, the ephedrine alkaloid composition ratio seemingly depends on genetic factors, but not on environmental factors or growth period.14) Our previous studies demonstrated that the content of ephedrine alkaloids in E. sinica can be improved using selection breeding13) and vegetative propagation.15) On the other hand, few studies have reported the dry weight of its medicinal parts and the relationships between its ephedrine alkaloid contents and dry weight of its medicinal parts. For sustainable and stable supply of Ephedra herb in Japan, it is necessary to understand the stability of both the ephedrine alkaloid content and dry weight of terrestrial stems of E. sinica over the years after transplantation because its medicinal parts are harvested annually. However, this information is lacking.
In the present study, to assess the stability of ephedrine alkaloid content, ratio, and dry weight, we evaluated these traits of terrestrial stems among 46 E. sinica genets derived from seedlings. The evaluations were carried out for six years, from the first year of transplantation to the sixth year of growth. The plants were cultivated in Ibaraki Prefecture, Japan. To assess the reproducibility of these traits in clones, we used the above three clonal lines propagated from genets with high TA content and evaluated the above three traits for five years, from the first year of transplantation to the fifth year of growth.
We used 46 E. sinica genets that have been preserved in the experimental field of Tsumura & Co. in Ibaraki Prefecture, Japan (35°993′N; 140°200′E, approximately 25 m above sea level). The plants used in the study were identified by Hiyama H. Voucher specimens have been deposited in the Herbarium of TSUMURA Botanical Raw Material Research Laboratories, Tsumura & Co., Ibaraki, Japan.
Experimental DesignThe 46 genets were transplanted at a spacing of 1.0 and 0.3 m between and within rows, respectively, on May 11, 2012. Farmyard manure (2000 g·m−2) was used as the basal fertilizer. In April and June from 2013 to 2017, a chemical fertilizer was applied to supply 2, 2, 2, and 0.2 g·m−2 of N, P2O5, K2O, and Mg, respectively.
We partially reutilized our previously published data (ephedrine alkaloid content of 46 genets from 2-year-old to 5-year-old plants, namely, from the first year of transplantation to the fourth year of growth).15) We used our previous data (1) to evaluate the stability of ephedrine alkaloid content and its composition ratio and (2) to evaluate the relationship between TA content and dry weight.
Harvest and ProcessingThe terrestrial stems of E. sinica plants were harvested by cutting them at a height of 5 cm above the ground.
Kajimura et al.17) reported that ephedrine alkaloid content in Ephedra plants was low in the early stages of stem growth, after which it stabilized when terrestrial stem growth stopped. Therefore, samples were collected after stem growth stopped in each year. The terrestrial stems of the 46 genets were harvested on October 11, 2012 (2-year-old plants; first year of transplantation), August 21, 2013 (3-year-old plants; second year of transplantation), September 19, 2014 (4-year-old plants; third year of transplantation), October 27, 2015 (5-year-old plants; fourth year of transplantation), October 11, 2016 (6-year-old plants; fifth year of transplantation), and September 12, 2017 (7-year-old plants; sixth year of transplantation). To avoid mixing with the stems of the previous year, stems were cut every year. Because stem age affects ephedrine alkaloid content, we cut the stems each year but harvested only one-year-old stems. Height of the terrestrial stems of the genets in 2017 was measured at harvest. The number of terrestrial stems of genets in 2017 was determined after harvesting.
Terrestrial stems were dried at 35 °C for three days and then at 50 °C for six h in a food dryer (TB-60-F, Taikisangyo Co., Ltd., Okayama, Japan) to determine their ephedrine alkaloid content and dry weight. The samples were ground to a powder using a vibrating rod mill (TI-100, CMT Co., Ltd., Japan).
Determination of Ephedrine AlkaloidsQuantitative analysis of ephedrine alkaloid content was performed by HPLC on an instrument (LC-10ADvp, Shimadzu Corp., Kyoto, Japan) equipped with an Inertsil ODS-3 column (5 µm, 4.6 × 150 mm; GL Sciences Inc., Tokyo, Japan). (−)-ephedrine (Eph) and (+)-pseudoephedrine (PEph) standards were provided by Tsumura & Co. (Tokyo, Japan). A mixture of water, acetonitrile, and phosphoric acid (650 : 350 : 1, v/v/v) with 0.5% sodium dodecyl sulfate was used as the mobile phase. The flow rate and column temperature were kept constant at 1.1 mL/min and 40 °C, respectively. The powdered samples (0.25 g) were extracted with 50% aqueous methanol (20 mL) using a reciprocal shaker for 30 min, followed by centrifugation at 3000 rpm for 10 min. After supernatant collection, the residue was re-extracted in the same manner. Combined supernatants were prepared by adding 50% aqueous methanol to a total volume of 50 mL, and 10 µL aliquots were injected into the HPLC apparatus. The TA content and TA composition ratio were calculated as follows:
 |
One-way ANOVA was performed to test the effects of the year of transplantation on ephedrine alkaloid content and dry weight, and Tukey–Kramer tests were used for multiple mean comparisons. Spearman’s rank correlation was used to evaluate TA content stability, its composition ratio, and dry weight of genets over the years. To assess the magnitude of the relationship between TA content and dry weight of genets, correlations between TA content and dry weight were analyzed using Pearson’s correlation. To assess the magnitude of the relationship between genet dry weight and number of terrestrial stems or height of terrestrial stems, correlations between dry weight and the number of terrestrial stems or height of terrestrial stems were analyzed using Pearson’s correlation. These statistical analyses were performed using the R software version 3.5.0 (https://www.r-project.org/).
Experiment 2. Assessment of the Reproducibility of Ephedrine Alkaloids and Dry Weight of Clonal LinesPlant MaterialTo assess the reproducibility of the TA content, composition ratio, and dry weight of clonal lines derived from E. sinica genets with high content of TA and different TA composition ratios, we used three clonal lines that have been preserved in the experimental field of Tsumura & Co. in Ibaraki Prefecture. The three clonal lines (#2, #19, and #26) were propagated by stolons from three different genets.15)
Experimental DesignEach of the three clonal lines was transplanted at a spacing of 1.0 and 0.3 m between and within rows on May 11, 2014. Farmyard manure (2000 g·m−2) was used as the basal fertilizer. In April and June from 2015 to 2018, a chemical fertilizer was applied to supply 2, 2, 2, and 0.2 g·m−2 of N, P2O5, K2O, and Mg, respectively.
We partially reutilized our previously published data (ephedrine alkaloid content of three clonal lines from 1-year-old to 2-year-old plants, namely, from the first year of transplantation to the second year).15) We used our previous data to evaluate the reproducibility of the TA content and TA composition ratio in clonal lines.
Harvest and ProcessingHarvest and processing methods were performed as described in Experiment 1.
Terrestrial stems of the three clonal lines were harvested on November 19, 2014 (1-year-old plants; first year of transplantation), August 4, 2015 (2-year-old plants; second year of transplantation), August 3, 2016 (3-year-old plants; third year of transplantation), September 12, 2017 (4-year-old plants; fourth year of transplantation), and July 14, 2018 (5-year-old plants; fifth year of transplantation). The number of terrestrial stems of clones was determined after harvesting in 2017.
Determination of Ephedrine AlkaloidsQuantitative analysis of ephedrine alkaloid content was performed as described in Experiment 1.
Statistical AnalysisTukey–Kramer tests were used for multiple mean comparisons. To assess the magnitude of the relationship between TA content and dry weight of clones, correlations between TA content and dry weight were analyzed using Pearson’s correlation. Additionally, to assess the magnitude of the relationship between clone dry weight and number of terrestrial stems, correlations between dry weight and number of terrestrial stems were analyzed using Pearson’s correlation. These statistical analyses were performed using the R software version 3.5.0 (https://www.r-project.org/).
The mean TA content of genets satisfied the criteria for Ephedra herb defined in the Japanese Pharmacopoeia (≥ 0.7% DW),5) except in the first year of transplantation (0.64 ± 0.28% DW) and in the fourth year of growth (0.69 ± 0.34% DW) (Fig. 1A). There were no significant differences in the mean TA content of E. sinica genets from the first year of transplantation to the sixth year of growth (Fig. 1A). Although the variation in TA content among genets in each year of transplantation was large (i.e., in sixth year, 0.18–1.61% DW) (Fig. 2), range of the TA content in each genet was small after the second year. The TA content of genets with high TA content (i.e., genet #19) was high over the years (Fig. 2), and the TA content of genets with low TA content (i.e., genet #50) was low over the years. Additionally, the rank correlation coefficients of TA content among the 46 genets have been high since the second year (ρ = 0.85–0.94) (Supplementary Table 1).

A: TA content, B: TA composition ratio, C: Dry weight of terrestrial stems of genets. TA, total alkaloids (sum of Eph and PEph); DW, dry weight; Eph, ephedrine; PEph, pseudoephedrine. Different lowercase letters on the bars indicate significant differences (p < 0.05, Tukey–Kramer multiple-comparison test).

TA, total alkaloids (sum of Eph and PEph); DW, dry weight; Eph, ephedrine; PEph, pseudoephedrine. The circles indicate the TA content in each year after transplantation. The genets were arranged by high rank of mean TA content in each genet from second to sixth year of growth. The colors of points shown in the white to black gradient indicate the TA content of genet in second to sixth year of growth, respectively.
Similarly, there were also no differences in the mean TA composition ratio in E. sinica genets from the first year of transplantation to the sixth year of growth (Fig. 1B). Although the variation in TA composition ratio among genets in each year of transplantation was large (i.e., in the sixth year, 0.20–1.00) (Fig. 3), range of the TA composition ratio in each genet was small after the first year. The TA composition ratio of the genets with high TA composition ratio (i.e., genet #2) was high over the years (Fig. 3), and the TA composition ratio of the genets with low TA composition ratio (i.e., genet #23) was low over the years. Additionally, the rank correlation coefficients of TA composition ratio among the 46 genets have been high since the first year (ρ = 0.92–0.99) (Supplementary Table 1).

Eph, ephedrine; PEph, pseudoephedrine. The genets were arranged by high rank of mean ratio in each genet from first year to sixth year of growth. The colors of points shown in the white to black gradient indicate the ratio of genet in first to sixth year of growth, respectively.
However, the result of one-way ANOVA showed that the differences in mean dry weight over years were significant (p < 0.05). In particular, the dry weight of genets in the first year of transplantation was low (Fig. 1C). The mean dry weight of E. sinica genets increased from the first to the fourth year of growth, although there was no significant difference in mean dry weight between the third and fourth year of growth (Fig. 1C). After that, although the mean dry weight in the fifth year decreased temporarily, it increased in the sixth year. The variation in dry weight among genets was large (i.e., in the sixth year, 33.7–150.9 g). The difference in dry weight of each genet among the years was large as well (Fig. 4). Meanwhile, the dry weight of genets with high dry weight (i.e., genet #11) was high after the fourth year of transplantation (Fig. 4), and the dry weight of genets with low dry weight (i.e., genet #37) was low after the fourth year of transplantation. Moreover, the rank correlation coefficients of dry weight among the 46 genets have been high since the fourth year (ρ = 0.66–0.75) (Supplementary Table 1).
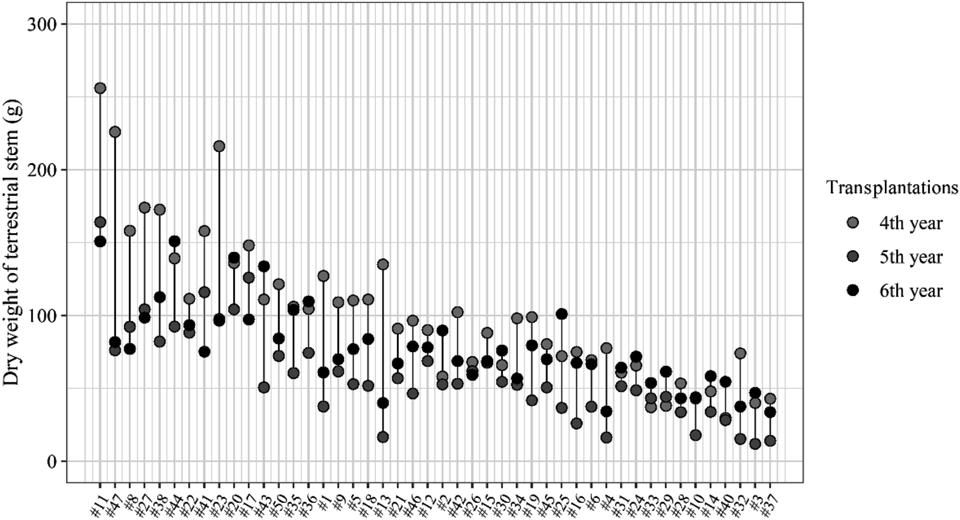
The genets were arranged by high rank of mean dry weight in each genet from fourth year to sixth year of growth. The colors of points shown in the white to black gradient indicate the dry weight of genet in fourth year to sixth year of growth, respectively.
The correlation coefficients between the TA content and dry weight of genets from the first year to the sixth year were 0.04 (first year), −0.18 (second year), −0.28 (third year), −0.31 (fourth year, p < 0.05), −0.23 (fifth year), and −0.08 (sixth year) (Fig. 5). These correlation coefficients were low and not significant, except in the fourth year (p < 0.05). On the other hand, the correlation coefficients between dry weight and the number of terrestrial stems and height of terrestrial stems of genets in the sixth year were 0.89 (p < 0.01) and 0.34 (p < 0.05), respectively (Fig. 6). In particular, the correlation coefficients between dry weight and the number of terrestrial stems were positive and high.

TA, total alkaloids (sum of Eph and PEph); DW, dry weight; Eph, ephedrine; PEph, pseudoephedrine.

A: Relationships between dry weight and number of terrestrial stems, B: Relationships of between dry weight and height of terrestrial stems. *, ** Probability value for the test of significance <0.05 and <0.01, respectively.
In clonal lines #2 and #26, there were no significant differences in the mean TA content from the second year of transplantation onwards (Fig. 7). In contrast, in clonal line #19, the mean TA content between the fourth and fifth year of growth increased significantly (p < 0.05) (Fig. 7). In the second year of growth, the mean TA content was 1.16 ± 0.11% DW in clonal line #2, 1.15 ± 0.22% DW in clonal line #19, and 1.61 ± 0.27% DW in clonal line #26 (Fig. 7). In the second year of growth, the TA content in original genets was 1.09% DW in genet #2, 1.56% DW in genet #19, and 1.40% DW in genet # 26 (Fig. 2). The mean TA content in clonal lines #2 and #26 in the second year was comparable with the TA content in each original genet, whereas the mean TA content in clonal line #19 in the fourth year of growth was comparable with the TA content of the original genet in the second year (Figs. 2, 7). Although the variation in TA content among genets was large (Fig. 2), the variation in TA content among clones in each clonal line was small over the years (Fig. 7). Additionally, the TA content of clones, which were propagated from the selected genets with high TA contents, satisfied the criteria for Ephedra herb defined in Japanese Pharmacopoeia (≥ 0.7% DW)5) since the second year of transplantation (Fig. 7).

TA, total alkaloids (sum of Eph and PEph); DW, dry weight; Eph, ephedrine; PEph, pseudoephedrine. Different lowercase letters on the bars indicate significant differences (p < 0.05, Tukey–Kramer multiple-comparison test). Clonal line #2 (n = 5), clonal line #19 (n = 5), clonal line #26 (n = 4).
Similarly, in clonal lines #2 and #26, there were no significant differences in the mean TA composition ratio after the second year of transplantation (Fig. 8). In contrast, in clonal line #19, there were differences in the mean TA composition ratio among the second (0.91 ± 0.01), third (0.89 ± 0.01), fourth (0.86 ± 0.02) and fifth year of growth (0.88 ± 0.02) (Fig. 8). In the second year, the mean TA composition ratio in the clonal lines was 0.98 ± 0.03 in clonal line #2, 0.91 ± 0.01 in clonal line #19, and 0.69 ± 0.01 in clonal line #26 (Fig. 8). The TA composition ratio in the original genets in the second year was 0.98 in genet #2, 0.87 in genet #19, and 0.69 in genet #26 (Fig. 3). The mean TA composition ratio in each clonal line in the second year was comparable with the TA composition ratio in each original genet (Figs. 3, 8). Although the variation in TA composition ratio among genets was large (Fig. 3), the variation in TA composition ratio among clones in each clonal line was small over the years (Fig. 8).

TA, total alkaloids (sum of Eph and PEph); Eph, ephedrine; PEph, pseudoephedrine. Different lowercase letters on the bars indicate significant differences (p < 0.05, Tukey–Kramer multiple-comparison test). Clonal line #2 (n = 5), clonal line #19 (n = 5), clonal line #26 (n = 4).
In contrast, the mean dry weight of each clonal line increased from the first year of transplantation to the fourth year of growth (Fig. 9). There were no significant differences in the mean dry weight of each clonal line between the fourth and fifth year of growth (Fig. 9). The mean dry weight of the clonal lines in the fourth year was 39.3 ± 17.5 g in clonal line #2, 32.6 ± 12.0 g in clonal line #19, and 50.8 ± 14.3 g in clonal line #26 (Fig. 9). The dry weight of original genets in the fourth year was 58.0 g in genet #2, 98.8 g in genet #19, and 68.0 g in genet #26 (Fig. 4). The mean dry weight of each clonal line in the fourth year was lower than the dry weight of each original genet (Figs. 4, 9).

Different lowercase letters on the bars indicate significant differences (p < 0.05, Tukey–Kramer multiple-comparison test). Clonal line #2 (n = 5), clonal line #19 (n = 5), clonal line #26 (n = 4).
The correlation coefficients between TA content and dry weight of clones in the fourth year of transplantation were low and not significant (r = 0.23) (Fig. 10A). Although the variations in TA content in each clonal line were small, the variations in dry weight of each clonal line were large. On the other hand, the correlation coefficients between dry weight and the number of terrestrial stems of clones in the fourth year were positive and high (r = 0.90, p < 0.01) (Fig. 10B).

A: Relationships between dry weight and TA content. B: Relationships between dry weight and number of terrestrial stems. TA, total alkaloids (sum of Eph and PEph); DW, dry weight; Eph, ephedrine; PEph, pseudoephedrine. ** Probability value for the test of significance <0.01.
In the present study, we assessed the stability and reproducibility of ephedrine alkaloids in E. sinica plants. In the genet cultivation experiment, the range of variation in TA content among genets (error bars in Fig. 1A) is stable over years, and the annual variation in TA content of each genet is small (Fig. 2). Therefore, the TA content among genets has been stable since the second year of transplantation. Similarly, the range of variation in TA composition ratio among genets (error bar in Fig. 1B) is stable over years, and the annual variation in TA composition ratio of each genet is small (Fig. 3). Therefore, the TA composition ratio among genets has been stable since the first year of transplantation. A previous study described that the ephedrine alkaloid composition ratio seemingly depends on genetic factors, which is genetic variance in the total variance, but not on environmental factors, which is environmental variance in the total variance, or growth period,14) which supports our results. These results suggested that ephedrine alkaloids produced in E. sinica were stable over the years and that the potential TA content of genets could be evaluated using data on TA content after the second year of transplantation. However, the mean TA content of the 46 studied genets in the first and fourth year did not meet the criteria of the Japanese Pharmacopoeia (TA ≥ 0.7% DW),5) (Fig. 1A). This may be because the high inter-genet variability (Fig. 2). In contrast, in some genets, the TA content exceeded the standard value of the Japanese Pharmacopoeia over the years. Our previous study13) showed that the selection of ephedrine alkaloid content in E. sinica at various locations in Japan is valid, and E. sinica plants with high TA content can be selected at various locations. Additionally, in the clone cultivation experiment, our results showed that it is possible to cultivate clonal lines with high TA content over the years using vegetative propagation of selected genets with high TA content (Fig. 7). Furthermore, although some previous studies also reported that the content of ephedrine alkaloids in E. sinica varies widely depending on habitat,10,11) our results showed that clonal propagation could be used to reduce the variability and ratio of TA content in E. sinica (Figs. 1, 7, 8). These results suggested that the selection of individuals from E. sinica populations with high variability and subsequent clonal propagation of the selected plants could produce Ephedra herb with not only high and stable TA content, but also a stable TA ratio.
In the present study, we also assessed the stability and reproducibility of E. sinica dry weight. Although the variation in genet dry weight was large over the years (Figs. 1C, 4), it has been stable since the fourth year of growth. In addition, the rank correlation coefficients among genets have been high since the fourth year of growth (Supplementary Table 1). However, the mean dry weight of genets decreased in the fifth year (Fig. 1C). Furthermore, the rank correlation coefficients of dry weight between the year and the previous year were high (Supplementary Table 1). In E. sinica plants, new terrestrial stems are formed from the axillary buds of terrestrial stems. Thus, the way in which plant terrestrial stems are harvested could affect the formation of new terrestrial stems. Although the mean dry weight of the fifth year decreased, the mean dry weight of the sixth year increased and tends to approach the value of the fourth year (Fig. 1C). The decrease in the mean dry weight of genets in the fifth year was considered to have decreased temporarily. The cause of this could not be clarified, but the decrease in dry weight was probably due to the influence of environmental factors. Moreover, we evaluated traits related to dry weight throughout the two cultivation experiments. The correlation coefficients between dry weight and the number of terrestrial stems in genets as well as in clones were positive and high (Figs. 6, 10B). These results suggested that the dry weight of terrestrial stems is strongly influenced by the number of terrestrial stems. In fact, in the present study, the number of terrestrial stems differed among clones that were propagated using stolons in the early stage. Therefore, the variation in the dry weight of clones in each clonal line could have been related to the number of terrestrial stems. Therefore, our results suggested that although there is room for improvement of the selection of genets of higher dry weight, the dry weight of E. sinica is also affected by environmental factors and cultivation methods.
In the present study, we revealed the relationship between TA content and dry weight of E. sinica over the years through two cultivation experiments. The correlation coefficients between the TA content and dry weight of genets were almost negative and low, but there were no significant correlation between the TA content and dry weight, except in the fourth year (Fig. 5). Regarding other plant species, in a medicinal plant Aconitum japonicum, tuberous root size showed a significant negative correlation with aconitine alkaloid content18); in Pinus ponderosa, seedling growth was negatively related to piperidine alkaloid content,19) and in Rauwolfa serpentina, the correlation between reserpine alkaloid content and root length was positive.20) Although Stamp21) reported that plant alkaloid production was negatively correlated with biomass production, the correlation between alkaloid content and biomass differed among different plant species. Kajimura et al.17) reported that ephedrine alkaloid content of Ephedra plants was influenced by their stem growth in a given year, and ephedrine alkaloid content tended to be low or unstable in the early stages of stem growth in each year. Our study also showed that the TA content of genets was unstable during the first year of transplantation, i.e., in the early growth stage. However, in the present study, dry weight did not affect the TA content of E. sinica because of the low correlation coefficients between TA content and dry weight over the years (Fig. 5). This suggested that it is possible to carry out selection breeding of E. sinica genets with not only high TA content but also high dry weight. In particular, the genet #38 could be most suitable for cultivation and breeding because both TA content and dry weight were high and stable over years (in fourth year, TA content was 1.18% DW and dry weight was 172.6 g, in fifth year, TA content was 1.28% DW and dry weight was 82.0 g, in sixth year, TA content was 1.47% DW and dry weight was 112.6 g) (Figs. 2, 4, 5).
Previous studies have described that ephedrine alkaloids content and dry weight of E. sinica terrestrial stems are influenced by environmental factors.11,13) Therefore, it is unclear how ephedrine alkaloids content and dry weight in E. sinica are influenced by the environment and the specific environmental factors that determine these two characteristics. Thus, measuring genotype by environment (G × E) interaction and heritability of ephedrine alkaloids and dry weight is crucial for analyzing this issue. In the future, G × E interactions and heritability should be evaluated by cultivating clonal lines at various locations. In the present study, the number of clones in each clonal line was low. At present, methods for large-scale clonal propagation of E. sinica, such as tissue culture, have not been established. Additionally, it is difficult to perform cross breeding between E. sinica individuals because it is a dioecious species and its flowering is unstable. However, methods of vegetative propagation have been established in this species.22,23) The studies of G × E interactions, heritability and the methods of vegetative propagation could help in the establishment of breeding and cultivation of E. sinica with high and stable content of ephedrine alkaloids and dry weight in Japan.
In conclusion, the present study demonstrated that the ephedrine alkaloids of not only E. sinica genets, but also their clones, are stable since the second year of transplantation, and dry weight is stable since the fourth year. In addition, the present study also demonstrated that the correlation between ephedrine alkaloid content and dry weight of terrestrial stems was low and there was no significant correlation between the ephedrine alkaloid content and the dry weight, over the years. This suggested that selection breeding of E. sinica plays an essential role in the production of Ephedra herb with high quality and quantity. Therefore, Ephedra herb with high TA content and high dry weight can be obtained by selection breeding and can potentially be produced consistently, in Japan.
The authors gratefully acknowledge T. Kurosawa, A. Uetake, and M. Sakai for sampling and sample processing.
This study was funded by Tsumura & Co. (https://www.tsumura.co.jp/english/). The funder provided support in the form of a salary for Hajime Hiyama, Aya Ozawa, and Bunsho Makino. Yosuke Yoshioka and Ryo Ohsawa are supervisors at the University of Tsukuba, to which Hajime Hiyama belongs, and there was no funding from Tsumura & Co.
The online version of this article contains supplementary materials.