2023 年 46 巻 11 号 p. 1498-1505
2023 年 46 巻 11 号 p. 1498-1505
Adiponectin is an abundant adipocytokine secreted by adipocytes. It exists in the plasma in its trimeric, hexameric, high-molecular-weight (HMW), and globular (a proteolytic product) isoforms. Adiponectin’s anti-inflammatory effects on macrophages remain controversial. We have previously reported a simple and effective method for purifying native HMW adiponectin from human plasma. Here, we investigated whether native HMW adiponectin from human plasma has anti-inflammatory effects on macrophages. Pretreatment with human native HMW adiponectin inhibited lipopolysaccharide (LPS)-induced interleukin-1β (IL-1β) gene expression, but not tumor necrosis factor (TNF)-α expression. However, simultaneous treatment with HMW adiponectin and LPS did not inhibit IL-1β expression. Further, HMW adiponectin pretreatment decreases glycogen synthase kinase-3β (GSK-3β) inactivation by abrogating LPS-induced Akt (Ser473) phosphorylation, which subsequently suppresses LPS-induced CCAAT/enhancer binding protein β (C/EBPβ) protein translation and nuclear translocation. However, HMW adiponectin pretreatment did not affect LPS-induced nuclear factor-kappaB (NF-κB) activation. These results suggest that HMW adiponectin mediates potent anti-inflammatory activities in macrophages by inhibiting its Akt–C/EBPβ signaling pathway, thereby suppressing IL-1β gene expression.
Adiponectin is primarily secreted by adipose tissue. The full-length adiponectin produced by mammalian cells is primarily secreted as high-molecular-weight (HMW) multimer including trimer and hexamer. The full-length adiponectin from insect cells is mainly hexamer.1) Conversely, bacteria produce full-length adiponectin that is not secreted and needs to be reconstituted to form trimeric adiponectin. In human plasma, adiponectin circulates as a trimer, hexamer, and HMW multimer. In addition, a bioactive proteolytic globular adiponectin (gAd), produced by macrophage-derived elastase, is also detected in human plasma, albeit at a very low concentration.2) Of note, we have reported a simple and effective method for purifying native HMW adiponectin from human plasma.3) Also, previously, we purified a 420-kDa HMW adiponectin from human plasma using gelatin-Cellulofine and confirmed it to be a novel 28-kDa gelatin-binding protein (GBP28).4) Physiologically, adiponectin isoforms exert different effects.1) Hence, it is important for different studies to specify the recombinant adiponectin isoform used in analyzing its biological activity.
Adiponectin is now recognized to have anti-inflammatory effects on macrophages. All isoforms of adiponectin elicit cellular responses by binding to specific receptors, namely, adiponectin receptor 1 (AdipoR1) and AdipoR2, both of which are expressed on macrophages.5) AdipoR1 is predominantly expressed in macrophages and mediates the anti-inflammatory effects of adiponectin.6,7)
Adiponectin probably acts via regulating cytokine expressions by modulating lipopolysaccharide (LPS) effects on macrophages during preincubation. In fact, to pretreat macrophages with recombinant human gAd (rhgAd, expressed in Escherichia coli) reduces the expression of inflammatory cytokines such as interleukin-1β (IL-1β),8) IL-6 and tumor necrosis factor (TNF)-α.9) In contrast, anti-inflammatory effects of adiponectin are associated with upregulation of anti-inflammatory cytokine, IL-10 and IL-1RA.10–13) However, anti-inflammatory signaling via IL-10 independent pathways have been implicated using the recombinant human adiponectin (rhAd, expressed in HEK293 cells).14) The recombinant mouse gAd (rmgAd, expressed in E. coli) promotes endotoxin tolerance in macrophages by inducing IRAK-M expression post-LPS treatment.15) In addition, the rmgAd influences Toll-like receptor (TLR)-signaling pathways by inhibiting nuclear factor-kappaB (NF-κB) activation via AdipoR.16) Recently, rhAd (from HEK293 cells) has been reported to significantly inhibit LPS-induced TNF-α production in human lung macrophages. In addition, hexameric rhAd (produced in E. coli) inhibits LPS-induced production of cytokines.17) These results indicate that adiponectin importantly regulates inflammatory process.
Despite numerous reports on the anti-inflammatory effects of adiponectin and mechanisms underlying its anti-inflammatory properties, literature on inflammatory role of adiponectin is contradictory. Discrepancies in results can be attributed to the recombinant isoforms used and their sources. Here, using human native HMW adiponectin as the standard we assayed the anti-inflammatory effect of adiponectin on macrophages.
The rhgAd and rmgAd expressed in E. coli were purchased from PeproTech, Inc. (Rocky Hill, NJ, U.S.A.) and BioVision (Mountain View, CA, U.S.A.), respectively. The rhAd and recombinant mouse adiponectin (rmAd) expressed in CHO cells were gifted by the Oriental Yeast Co., Ltd. (Tokyo, Japan). LPS from E. coli serotype 0127:B8 was obtained from Difco Laboratories (Detroit, MI, U.S.A.). Antibodies directed against phospho-Akt (Ser473), Akt, phospho-glycogen synthase kinase-3β (GSK-3β)(Ser9), and inhibitor of kappaBα (IκBα) were purchased from Cell Signaling (Beverly, MA, U.S.A.). The primary antibodies for GSK-3β were purchased from BD Transduction Laboratories (San Diego, CA, U.S.A.), and the antibodies against C/EBPβ were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). The pGL4.32 [luc2P/NF-κB-RE/Hygro] plasmid and pRL-thymidine kinase plasmid were obtained from Promega (Madison, WI, U.S.A.). Mouse IL-1β enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems (Minneapolis, MN, U.S.A.). All other chemicals were purchased form Sigma-Aldrich (St. Louis, MO, U.S.A.).
Cell CultureThe murine RAW264.7 macrophage-like cell line was cultured in Dulbecco’s modified Eagle medium with 10% fetal bovine serum and penicillin–streptomycin at 37 °C and 5% CO2. Cells were pretreated with various adiponectin concentrations and were then stimulated with LPS (1 µg/mL). The human monocytic leukemia cell line, THP-1, was cultured in RPMI 1640 medium with 10% fetal bovine serum and penicillin–streptomycin. Cells were differentiated into macrophages in the presence of 100 ng/mL phorbol 12-myristate-13 acetate (PMA) for 3 d, followed by pretreatment with 10 µg/mL HMW adiponectin for 16 h. They were then stimulated with LPS (1 µg/mL).
Purification of HMW Adiponectin from Human PlasmaHuman plasma was provided by the Japan Red Cross Society (Tokyo, Japan). HMW adiponectin was prepared using reduced formyl-Cellulofine, as described previously.3) A sandwich ELISA specific for HMW adiponectin was performed, as described previously.18) For hexamer adiponectin, pooled human plasma was subjected to 5–12% polyethylene glycol precipitation. The precipitate was dissolved in solution containing 10 mM Tris–HCl (pH 8.0), 0.2 M NaCl, and 5 mM ethylenediaminetetraacetic acid (EDTA) and was applied to a diethylaminoethyl-Sepharose column (3 × 23 cm). After washing the column with 10 mM Tris–HCl (pH 8.0) containing 0.3 M NaCl, remaining bound proteins were serially eluted with 10 mM Tris–HCl (pH 8.0) containing 0.5 M NaCl. The eluted fractions were pooled and dialyzed against 10 mM Tris–HCl (pH 7.4), 0.15 M NaCl, and 5 mM EDTA (Tris-buffered saline); subsequently, they were applied to sulfate-Cellulofine (1 × 10 cm). The flow-through fractions were pooled and applied to a formyl-Cellulofine (1 × 10 cm). The flow-through fractions containing hexamer adiponectin were pooled and concentrated. This study was approved by the Ethical Committee of Showa University (No.184).
Quantitative Real-Time RT-PCRTotal RNA was purified using RNAiso Plus reagent (TaKaRa Bio Inc., Shiga, Japan). The first-strand cDNA was synthesized from 1 µg of total RNA using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, U.S.A.). PCR reactions were performed with TaqMan probes (Applied Biosystems) for IL-1β (Mm01336189_m1, Hs01555410_m1), TNF-α (Mm00443258_m1, Hs00174128_m1), C/EBPβ (Mm00843434_s1), and β-actin (4352933E, Hs99999903_m1) using the StepOnePlus real-time PCR system (Applied Biosystems). Results were normalized to endogenous reference β-actin gene, and the relative quantity of each mRNA was calculated using the comparative Ct (threshold cycle) method.
Western Blot AnalysisRAW264.7 macrophages were lysed in lysis buffer [20 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride, 1× PhosSTOP (Roche Diagnostics, Basel, Switzerland), and 1× Complete protease inhibitor cocktail (Roche Diagnostics)]. The blots were visualized by an enhanced chemiluminescence method using an ECL Advance Western blotting Detection kit (GE Healthcare, Chicago, IL, U.S.A.) with a C-DiGit Blot Scanner (LI-COR Biosciences, Lincoln, NE, U.S.A.).
Immunocytochemical AnalysisImmunocytochemical analysis was performed as previously described, using anti-C/EBPβ antibody and Alexa Fluor 488-labeled goat anti-rabbit immunoglobulin G antibody.19) Immunostained slide was mounted on a drop with PermaFluor Aqueous Mounting Medium (Thermo Fisher Scientific, Waltham, MA, U.S.A.) and visualized with a FLUOVIEW FV10i confocal microscope (Olympus, Tokyo, Japan).
NF-κB Reporter AssayRAW264.7 macrophages were transfected with pGL4.32 [luc2P/NF-κB-RE/Hygro], which contains five copies of an NF-κB response elements (NF-κB-RE) along with the pRL-TK internal control reporter plasmid, by electroporation using Neon transfection system (Invitrogen, Carlsbad, CA, U.S.A.). Luciferase activity of cell lysates was measured, as described previously.20) Promoter activity was expressed as the number of firefly luciferase light units normalized to pRL-TK renilla luciferase light units.
Statistical AnalysisOne-way ANOVA with Tukey’s post-hoc test using JMP Pro 16 software for Windows (SAS Institute Inc., Cary, NC, U.S.A.) were used to compare data. A p-value of <0.05 was statistically significant. All values are expressed as mean ± standard error of mean (mean ± S.E.M.).
To assess the anti-inflammatory properties of native adiponectin, we incubated macrophages with LPS and human native HMW adiponectin. Pretreatment with HMW adiponectin markedly inhibited LPS-induced IL-1β expression by macrophage which peaks at 4 h after LPS treatment (Fig. 1A). Meanwhile, pretreatment with HMW adiponectin did not inhibit LPS-induced TNF-α expression by macrophages which peaks at 2 h after LPS treatment (Fig. 1B). A 16 h exposure of macrophages to hexamer adiponectin attenuated LPS-induced IL-1β expression—albeit, attenuation by hexameric adiponectin was weaker than that by HMW adiponectin—but did not affect TNF-α expression (data not shown). In addition, significant inhibition of IL-1β expression was observed in cells preincubated with 10 or 30 µg/mL HMW adiponectin (Fig. 1C), while TNF-α expression was unaffected (Fig. 1D). Inhibition of LPS-induced IL-1β expression was stronger in macrophages pretreated with HMW adiponectin for longer periods (Fig. 1E). Moreover, co-stimulation with HMW adiponectin and LPS did not suppress LPS-induced IL-1β expression (Fig. 1E). HMW adiponectin did not suppress LPS-induced TNF-α expression, even when macrophages were preincubated with high concentrations of HMW adiponectin (30 µg/mL) (Fig. 1D) or for long durations (up to 48 h) (Fig. 1F). Thus, pretreating with HMW adiponectin suppressed LPS-induced IL-1β expression, but not TNF-α expression.

(A, B) Time course of the expression of the cytokines (A) IL-1β; and (B) TNF-α after LPS stimulation. RAW264.7 macrophages were pretreated with 1 or 10 µg/mL HMW adiponectin for 16 h; then, they were stimulated with 1 µg/mL LPS for the indicated time period (HMW→LPS); (C, D) Effect of HMW adiponectin concentration. RAW264.7 macrophages were pretreated with the indicated concentration of HMW adiponectin for 16 h and were then stimulated with 1 µg/mL LPS for 4 h; (E, F) Effect of adiponectin preincubation time on LPS-induced IL-1β and TNF-α expression. RAW264.7 macrophages were pretreated with 10 µg/mL HMW adiponectin for the indicated time and were then stimulated with 1 µg/mL LPS for 4 h. The mRNA expression levels were determined by real-time PCR [ #, p < 0.05 vs. cells treated with LPS; *, p < 0.001 vs. cells treated with LPS; §, p < 0.0001 vs. cells treated with LPS; n = 3 per group; error bars, standard error (S.E.)].
PI3K/Akt signaling pathway regulates LPS signaling and gene expression in macrophages.21–24) Akt phosphorylation was assessed to determine whether adiponectin affects the PI3K/Akt signaling pathway. LPS caused rapid (within 30 min) phosphorylation of Akt (Ser473). However, Akt phosphorylation was attenuated in macrophages pretreated with HMW adiponectin (Fig. 2A). Akt phosphorylation was predictably paralleled with a progressive increase in the inhibitory phosphorylation of GSK-3β (a downstream Akt substrate). This phosphorylation was also inhibited by the pretreatment of cells with HMW adiponectin (Fig. 2B). To further investigate the involvement of the PI3K/Akt pathway, we examined the effects of a PI3K inhibitor, LY294002 (5 µM), on LPS-stimulated macrophages. PI3K inhibition ameliorated LPS-induced IL-1β expression by approximately 65% (Fig. 2C). Coincubation with HMW adiponectin and LY294002 further ameliorated LPS-induced IL-1β expression. However, PI3K inhibitor did not affect TNF-α expression (Fig. 2D).

(A, B) Effect of HMW adiponectin on LPS-induced phosphorylation of (A) Akt; and (B) GSK-3β. RAW264.7 macrophages were pretreated with 10 µg/mL HMW adiponectin for 16 h and were then stimulated with 1 µg/mL LPS for 30 min. The protein expression and phosphorylation levels were determined by Western blotting; (C, D) Effect of LY294002 on the inhibition of cytokine expression. RAW264.7 macrophages were pretreated with HMW adiponectin in the presence or absence of 5 µM LY294002 for 16 h and were then stimulated with 1 µg/mL LPS for 4 h. The mRNA expression levels were determined by real-time PCR [ n.s., not significant; #, p < 0.05 vs. cells treated with LPS; ##, p < 0.01 vs. cells treated with LPS; §, p < 0.0001 vs. cells treated with LPS; §§, p < 0.0001 vs. cells treated with HMW/LPS; **, p < 0.01 vs. cells treated with LY294002/LPS; n = 3 per group; error bars, S.E.].
NF-κB and C/EBPβ participate in induction of IL-1β genes in response to LPS stimulation.25) To clarify the mechanisms underlying the anti-inflammatory effects of adiponectin, we examined the involvement of the transcription factors NF-κB and C/EBPβ. First, we investigated the effect of HMW adiponectin on the mRNA expression of NF-κB—a major regulator of pro-inflammatory cytokine expression. The LPS-induced enhancement of NF-κB expression was not significantly affected by preincubation with HMW adiponectin (Fig. 3A). LPS treatment markedly induced cytosolic IκBα degradation, indicating increased NF-κB nuclear translocation. Pretreatment of cells with HMW adiponectin did not inhibit IκBα degradation (Fig. 3B). Additionally, we analyzed NF-κB activity using a luciferase reporter gene assay and macrophages transfected with pNF-κB Luc and pRL-TK plasmids. NF-κB reporter activity was significantly increased in LPS-treated cells, and NF-κB reporter activity was not altered by HMW adiponectin pretreatment (Fig. 3C). These results indicate that adiponectin did not suppress NF-κB expression or its activation. Thus, inhibition of NF-κB activation might not be necessary for the adiponectin-induced inhibition of IL-1β expression.
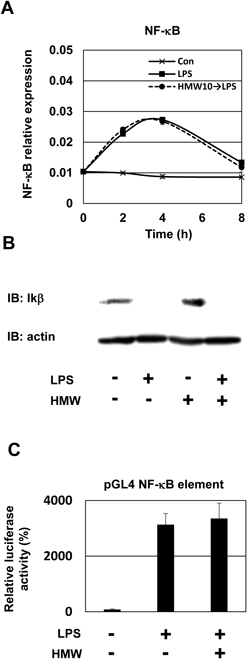
(A) NF-κB expression. RAW264.7 macrophages were pretreated with 10 µg/mL HMW adiponectin for 16 h and were then stimulated with 1 µg/mL LPS for the indicated time. The mRNA expression levels were determined by real-time PCR; (B) IκB expression. RAW264.7 macrophages were pretreated with 10 µg/mL HMW adiponectin for 16 h and were then stimulated with 1 µg/mL LPS for 30 min. The protein expression levels were determined by Western blotting; (C) NF-κB activity. RAW264.7 macrophages transiently transfected with the pGL4.32-NF-κB reporter plasmid were pretreated with 10 µg/mL HMW adiponectin for 16 h and were then stimulated with 1 µg/mL LPS for 4 h [n = 3 per group; error bars, S.E.].
Next, we examined the expression of C/EBPβ in LPS-treated macrophages. As expected, LPS induces C/EBPβ expression. However, contrary to our expectations, HMW adiponectin (10 µg/mL) pretreatment induced a significantly rapid upregulation of C/EBPβ mRNA expression (Fig. 4A). Nonetheless, HMW adiponectin pretreatment significantly downregulated C/EBPβ protein levels in macrophages (Fig. 4B), which was confirmed by fluorescent immunocytochemical staining (Fig. 4C). Further, adiponectin ameliorated LPS-induced acute expression of C/EBPβ protein and tended to reduce its accumulation in the nuclei. These results indicate that reduction in C/EBPβ protein translation and its nuclear translocation may mediate the adiponectin-induced inhibition of LPS-induced IL-1β expression.

(A) C/EBPβ mRNA expression. RAW264.7 macrophages were pretreated with 10 µg/mL HMW adiponectin for 16 h and were then stimulated with 1 µg/mL LPS for the indicated time and subjected to real-time PCR; (B) C/EBPβ protein expression. RAW264.7 macrophages were pretreated with 10 µg/mL HMW adiponectin for 16 h, stimulated with 1 µg/mL LPS for 2 h, and subjected to Western blotting; (C) Subcellular localization of C/EBPβ. RAW264.7 macrophages were treated with 1 µg/mL LPS for 2 h in the presence or absence of 10 µg/mL HMW adiponectin and were subjected to the immunocytochemical analysis employing antibody against C/EBPβ in the presence of 0.2% Triton X-100 [#, p < 0.05 vs. cells treated with LPS; ##, p < 0.01 vs. cells treated with LPS; n = 3 per group; error bars, S.E.].
Adiponectin negatively regulates inflammatory responses.8–17) However, most studies use recombinant adiponectin. Here, we used native HMW adiponectin (purified from human plasma), which is considered to be the most active of the circulating isoforms. We found that all forms of tested adiponectin, including rmAd, rhAd, rmgAd, and rhgAd, as well as HMW multimer and hexamer, ameliorated LPS-induced IL-1β expression (Fig. 1, Supplementary Figs. S1, S2). Moreover, no tested forms of adiponectin ameliorated LPS-induced TNF-α expression in RAW264.7 cells (Fig. 1, Supplementary Figs. S1, S2). Our additional experiment indicated that the anti-inflammatory effects of HMW adiponectin were also observed for the secretion of IL-1β (Supplementary Fig. S3). Notably, our preliminary results in human THP-1-derived macrophages are consistent with these findings. Pretreatment with HMW adiponectin resulted in decreased IL-1β gene expression, but TNF-α expression was not affected (Supplementary Fig. S4).
Although pretreatment with native HMW adiponectin inhibited IL-1β expression, simultaneous treatment with LPS and HMW adiponectin did not inhibit IL-1β expression (Fig. 1C). These results suggest that anti-inflammatory effects of HMW adiponectin are mediated via preconditioning that renders macrophages unresponsive to LPS stimulation. The cell density also influences adiponectin effect on macrophage activity; adiponectin inhibited IL-1β expression at <70% cell densities (but not at >90% cell densities) (data not shown). Recombinant adiponectin induces IL-10 expression, which then inhibits inflammation.11–13) However, here, HMW adiponectin did not induce IL-10 expression even after 24 h (data not shown) of incubation. Future studies are required to investigate the molecular mechanisms underlying the bacterial toxins tolerance mediated by HMW adiponectin. Currently, efforts are underway to profile the gene expression changes induced by native HMW.
The PI3K–Akt pathway is involved in both positive and negative regulation of LPS signaling and cytokine expression.22–25) Our findings suggest that inhibition of LPS-induced Akt phosphorylation (Fig. 3A) may play a critical role in the anti-inflammatory effects of adiponectin. Overexpression of adiponectin in KK/Ta mice, a murine model of metabolic syndrome, causes longevity by attenuating Akt signaling and chronic inflammation.26) Consistently, Akt signaling inhibition by adiponectin attenuates pathological processes, such as osteoclastogenesis,27) and oncogenic action of leptin.28) Putative mechanism involving adiponectin-induced dephosphorylation of Akt has also been reported. Indeed, adiponectin-mediated AMP-activated protein kinase (AMPK) activation reduces metastasis by stimulating dephosphorylation of Akt through increasing protein phosphatase 2A.29) Akt is activated by PI3K, and our results indicate that the PI3K inhibitor LY294002 inhibits LPS-induced IL-1β expression, while slightly decreasing TNF-α expression. Further, coincubation with HMW adiponectin and LY294002 could suppress IL-1β expression, but not TNF-α expression (Figs. 2C, D). Reportedly, although LY294002 blocks LPS-induced IL-1β mRNA production, TNF-α expression was not inhibited in RAW264.7 macrophages.23) Their observations support our hypothesis that the inhibition of Akt activation by adiponectin specifically ameliorates LPS-induced IL-1β expression, without affecting TNF-α expression.
Activated Akt inhibits GSK-3β activity by phosphorylating Ser-9 residue, which is a crucial site for GSK-3β inactivation. HMW adiponectin inhibits LPS-induced phosphorylation of GSK-3β at Ser-9 (Fig. 3B). Consistently, adiponectin pretreatment attenuates serum-induced phosphorylation of Akt (Ser473), and suppresses phosphorylation of GSK-3β (Ser9) in human breast cancer cells.30) Moreover, in a murine model of cardiac hypertrophy, angiotensin II (AngII) intervention in adiponectin knocked-out mice increased the phosphorylation of Akt (Ser473) and GSK-3β (Ser9) as compared to that in wild-type mice.31) Interestingly, these authors observed that adenovirus-mediated adiponectin expression in these mice, significantly reduced AngII-induced phosphorylation of Akt/GSK-3β. Thus, reduced Akt (Ser473) and GSK-3β (Ser9) phosphorylation might play a role in anti-inflammation effects of adiponectin.
Because NF-κB and C/EBPβ are involved in LPS-induced IL-1β expression25) and gAd (from E. coli) inhibits NF-κB activation,16) we examined the effects of native adiponectin on NF-κB. Native HMW adiponectin did not affect LPS-induced IκB degradation (Fig. 3B) and the subsequent NF-κB activation (Fig. 3C). Further, we found that HMW adiponectin reduced LPS-induced C/EBPβ protein translation and nuclear translocation (Figs. 4B, C). The TLR4/PI3K signaling axis controls two divergent pathways—viz., 1) NF-κB activation via Rac1/PAK1, and 2) C/EBPβ activation via Akt/p38 mitogen-activated protein kinase (MAPK)—to induce IL-1β expression.25) Akt kinase signaling regulates IL-1β promoter activity via C/EBPβ, and not via NF-κB.25) C/EBPβ expression is required for IL-1β induction (but not for TNF-α induction). Whereas, NF-κB has a critical role in both TNF-α and IL-1β gene induction.32) Therefore, it is plausible that native HMW adiponectin predominantly inhibits LPS-induced IL-1β expression through the inhibition of Akt-associated C/EBPβ activation, and not via NF-κB activation.
In this study, pretreatment with HMW adiponectin enhanced LPS-induced C/EBPβ mRNA expression while the expression of C/EBPβ protein decreased (Fig. 4A). However, the reason for the contrasting effects of adiponectin pretreatment on the C/EBPβ mRNA and protein levels are unknown. The C/EBPβ gene is transcribed into a single mRNA and translated through the alternative initiation from consecutive in-frame start codons into several N-terminally truncated isoforms. The liver-enriched transcriptional activating protein (LAP) isoform is considered a gene activator, whereas the liver-enriched inhibitory protein (LIP) isoform, which lacks most of the transactivation domain, acts as a dominant-negative repressor.33) LIP forms heterodimers with LAP to inhibit transcription activation.33) A previous study has shown that C/EBPβ is critically involved in the induction of inflammatory genes in activated macrophages, and most are regulated by the 34-kDa LAP.34) These findings indicate that HMW adiponectin might regulate the LAP/LIP ratio to suppress inflammation in RAW264.7 cells. Notably, pretreatment with rmAd also enhanced LPS-induced C/EBPβ mRNA expression, while the protein expression of C/EBPβ decreased (Supplementary Fig. S5). To clarify the molecular mechanism of C/EBPβ expression via adiponectin, it is necessary to analyze the C/EBPβ isoform protein expression, including LIP, after treatment with HMW adiponectin.
In conclusion, human native HMW adiponectin preferentially suppresses LPS-induced IL-1β expression via inhibiting Akt/GSK3β phosphorylation and C/EBPβ protein expression in macrophages. Our results indicate that regulation of Akt/GSK3β/C/EBPβ axis plays an important role in HMW adiponectin-mediated anti-inflammatory action on macrophages. Additionally, (although the responsible mechanisms remain to be elucidated), our data clearly show that preconditioning with HMW adiponectin is essential for manifesting the anti-inflammatory effects of adiponectin on macrophages.
We are grateful to Ms. K. Mori and Ms. M. Mimura, Oriental Yeast Co., Ltd. (Tokyo, Japan), for providing the recombinant human and mouse full-length adiponectin and product information. This work was supported in part by Grant-in-Aid (Nos. 23590474 and 26460494) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The authors declare no conflict of interest.
This article contains supplementary materials.