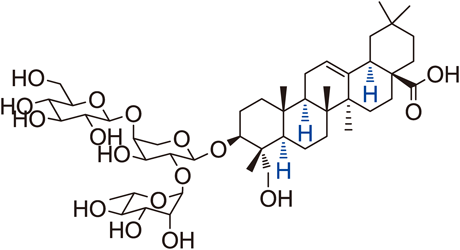
Abstract
Novel saponins that retain a free carboxyl group at the C-17 position and various sugars linked at the C-3 position of hederagenin aglycone were synthesized via stereospecific glycosylation. Since these natural products represented by Pulsatilla saponin D (PSD) were obtained in very small amounts, the total synthesis developed in this paper will resolve this problem of scarcity. The two types of synthesized arabinose- and rhamnose-cored saponins showed potent anticancer activity against a human lung cancer cell line (A549), and most disaccharide moiety saponins possessed more potent anti-lung cancer activity. Among the novel PSD analogues containing disaccharide saponins, compound 10i showed anti-lung cancer activity (6.6 µM) that was four-fold more potent than the clinical agent Iressa® (26.08 µM).