2015 Volume 63 Issue 9 Pages 669-677
2015 Volume 63 Issue 9 Pages 669-677
Novel saponins that retain a free carboxyl group at the C-17 position and various sugars linked at the C-3 position of hederagenin aglycone were synthesized via stereospecific glycosylation. Since these natural products represented by Pulsatilla saponin D (PSD) were obtained in very small amounts, the total synthesis developed in this paper will resolve this problem of scarcity. The two types of synthesized arabinose- and rhamnose-cored saponins showed potent anticancer activity against a human lung cancer cell line (A549), and most disaccharide moiety saponins possessed more potent anti-lung cancer activity. Among the novel PSD analogues containing disaccharide saponins, compound 10i showed anti-lung cancer activity (6.6 µM) that was four-fold more potent than the clinical agent Iressa® (26.08 µM).
Saponins found in many plants are glycosides bearing triterpenes or steroids and have shown various biological and pharmacological activities.1) Saponins with hederagenin such as hederacolchid A {hederagenin 3-O-β-D-glucosyl-(1→4)-α-L-rhamnosyl(1→2)-α-L-arabinoside}, δ-hederin, α-hederin, kalopanaxsaponin A, and sapindoside C have been shown to have potent cytotoxic activities toward various cancer cell lines.2–7) Among hederagenins, Pulsatilla saponin D (PSD) 10 (Fig. 1) not only displayed strong in vitro anticancer activities, but also more potent in vivo antitumor activities in mice bearing Lewis lung carcinoma, exceeding the effects of even taxol and doxorubicin.8) In addition, we reported total synthesis of Pulsatilla saponin D (PSD).9) These natural saponin products were obtained in small amounts, which was a limiting factor. Chemical synthesis may resolve this problem, and may easily provide suitable compounds for study of structure–activity relationships (SAR) and for the other studies.2,10) The result of SAR showed saponins with hederagenin retaining the free carboxyl group at the C-17 position had stronger anticancer activity than related compounds with other substituents. The hederagenin derivatives, that have sugar moieties on the C-3 position, also showed potent anticancer activity.11,12) Most isolated PSD analogs maintained derivative with sugars bound at the 2-OH of the arabinose moiety.8) Therefore, we proposed to study the effect of derivatization at 3-OH on the anticancer activity for SAR study. In this paper, we report total synthesis of new PSD analogs with various sugar moieties linked at C-3 position of hederagenin and at 3-OH of arabinose moiety. We also evaluated the in vitro anticancer activity of these new compounds.

We designed two types of saponin analogues arabinose- and rhamnose- directly glycosylated hederagenins. Stereospecific glycosylation of the glycosyl acceptor, as illustrated by the arabinose moiety 1, with perbenzoylated trichloroacetimidates donors 1a–d13–16) was achieved in the presence of trimethylsilyl trifluoromethanesulfonate (TMSOTf) in dichloromethan (CH2Cl2) at −78°C to give disaccharides 2a–d (Chart 1). Then, the acetyl group was removed using acetyl chloride/CH3OH to give compounds 3a–d.17) Glycosylation of compounds 1a–d with the C-4 free hydroxyl group of compounds 3a–d under the TMSOTf as a promoter at −78°C afforded trisaccharide 4a–g. The hydroxyl groups at the C-3, -4 positions in compound 6 as the glycosyl acceptor were stereospecifically glycosylated with donor 1b and 1d14,16) under the same conditions in 75% and 87% yield, respectively. The benzyl groups at the C-1 positions of 2d, 4a–h were selectively deprotected with palladium carbon (Pd/C) catalyst under H2 to give 5a–i (Chart 1).

Reagents and conditions: Araa=2,3,4-tri-O-benzoyl-α-L-arabinopyranosyl, Rhab=2,3,4-tri-O-benzoyl-α-L-rhamnopyranosyl, Gluc=2,3,4,6-tet-O-benzoyl-β-D-glucopyranosyl, Mand=2,3,4,6-tet-O-benzoyl-α-D-mannopyranosyl.
Treatment of compounds 5a–i using CCl3CN and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) afforded trichloroacetimidate moieties 7a–i as the glycosyl donor. These compounds were further glycosylated with the protected hederagenin 8 to give the fully protected PSD analogs 9a–i (Chart 2). 13C-NMR chemical shifts of each sugar component with the correct configuration for the protected compound 9a–i and the anomeric configuration at C-1 of the arabinopyranoside of structures 9a–i was determined to be trans diaxial on the basis of the 13C–1H coupling constants as previously demonstrated.9)
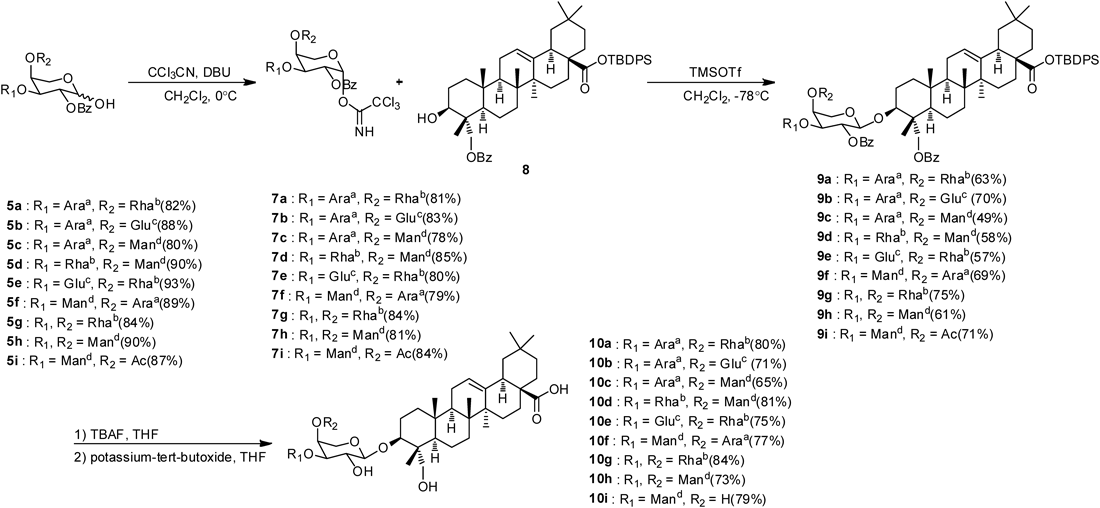
Reagents and conditions: Araa=2,3,4-tri-O-benzoyl-α-L-arabinopyranosyl, Rhab=2,3,4-tri-O-benzoyl-α-L-rhamnopyranosyl, Gluc=2,3,4,5-tet-O-benzoyl-β-D-glucopyranosyl, Mand=2,3,4,5-tet-O-benzoyl-α-D-mannopyranosyl, Arae=α-L-arabinopyranosyl, Rhaf=α-L-rhamnopyranosyl, Glug=β-D-glucopyranosyl, Manh=α-D-mannopyranosyl.
-Butyldiphenylsilyl (TBDPS) groups of hederagenins were deprotected by tetra-n-butylammonium fluoride (TBAF). Total deprotection of the trisaccharide moieties of the saponin analogues was accomplished in situ in one step. Thus, all benzoyl and acetyl groups were removed using potassium t-butoxide to give arabinose-cored saponin analogues 10a–i (Chart 2).
For the synthesis of rhamnose-cored saponins, glycosylation of the acceptor 11 with donors 1a–d13–16) was performed under the standard condition to provide disaccharide moieties 12a–d (Chart 3). Hydrolysis of the isopropylidene group of the compounds 12a–d afforded diols 13a–d in 88%, 84%, 91%, and 90% yield, respectively. Benzoylation of diol moiety of compounds 13a–d followed by selective cleavage of the benzyl group of resulting protected compounds 14a–d gave the disaccharides 15a–d in 82%, 91%, 84%, and 91% yield, respectively. Treatment of compound 13c and 18 with triethyl orthoacetate and p-TsOH following cleavage in aqueous acetic acid (aq. AcOH) afforded the C-2 monoacetylated compounds 16 and 19 with free hydroxyl group at C-3 position as glycosyl acceptor.18,19) Glycosylation of the acceptor 16 and 19 with donors 1a–c13–15) gave trisaccharide moities 17a–c, then benzyl groups were selectively deprotected with Pd/C under the H2 atmosphere to give compounds 20a–c in 85%, 87%, and 82% yield, respectively (Chart 3).
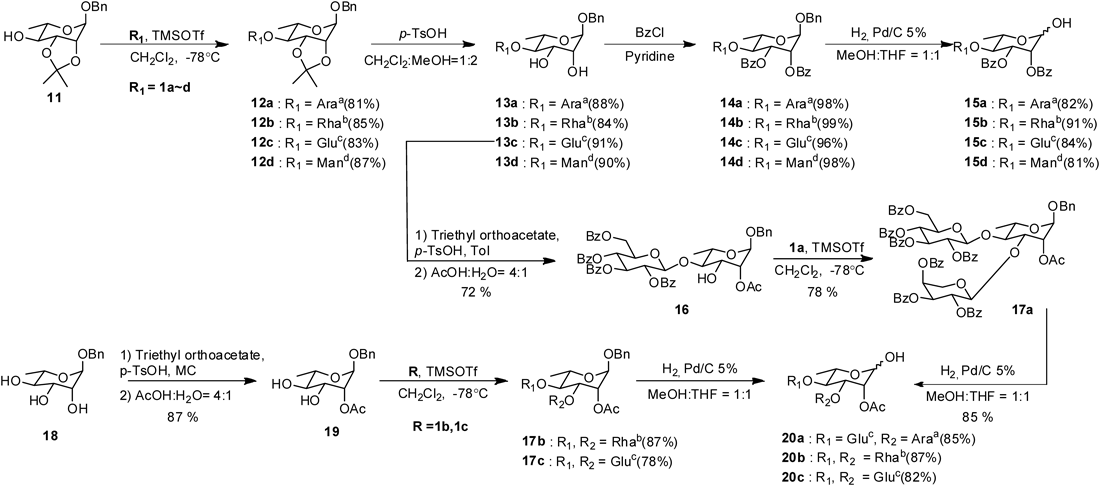
Reagents and conditions: Araa=2,3,4-tri-O-benzoyl-α-L-arabinopyranosyl, Rhab=2,3,4-tri-O-benzoyl-α-L-rhamnopyranosyl, Gluc=2,3,4,6-tet-O-benzoyl-β-D-glucopyranosyl, Mand=2,3,4,6-tet-O-benzoyl-α-D-mannopyranosyl.
For glycosylation, compounds 15a–d and 20a–c were converted to trichloroacetimidate 21a–g using CCl3CN and they were stereospecifically glycosylated with compound 8 to give fully protected trisaccharide 22a–g (Chart 4). The anomeric configuration at C-1 of the a rhamnopyranoside of structures 22a–g was determined as trans diaxial on the basis of the 13C–1H coupling constants, as previously shown.9) Then TBDPS groups of hederagenin were again deprotected by TBAF. Total deprotection of disaccharide moieties of saponin analogs 22a–g was accomplished in situ in one step using potassium t-butoxide to afford the rhamnose-cored saponin analogs 23a–g (Chart 4).

Reagents and conditions: Araa=2,3,4-tri-O-benzoyl-α-L-arabinopyranosyl, Rhab=2,3,4-tri-O-benzoyl-α-L-rhamnopyranosyl, Gluc=2,3,4,6-tet-O-benzoyl-β-D-glucopyranosyl, Mand=2,3,4,6-tet-O-benzoyl-α-D-mannopyranosyl, Arae=α-L-arabinopyranosyl, Rhaf=α-L-rhamnopyranosyl, Glug=β-D-glucopyranosyl, Manh=α-D-mannopyranosyl
These compounds were tested in three cancer cell lines, A-549, MDA-MB-231, and MCF-7. The results clearly indicated that the anticancer activity of new PSD analogues showed potent activity against a lung cancer cell line (A-549) with IC50 values of less 50 µM. However, most of the tested saponin derivatives had no activity on breast cancer cell lines (MDA-MB-231, MCF-7). Position of glycol linkage and structural difference of sugars seem to play an important role in determination of anticancer activity. While rhamnose-cored derivatives directly bound at the C-3 position of hederagenin aglycone showed more anti-breast cancer activity than arabinose-cored derivatives, the two types of the saponins had similar anti-lung cancer activity. Most saponins bearing a disaccharide moiety showed more potent anti-lung cancer activity Particularly, decrease the number of sugars at 2-OH position of trisaccharide moiety of PSD makes easier approach for binding with a target protein, thus enhancing anticancer activity of disaccharides. However, various derivatization at 3-OH position of arabinose of disaccharides generally increase anti-cancer activity.
Most of the derivatives showed lesser activity against breast cancer cell lines than did the PSD trisaccharide. Among the disaccharide saponin, compound 10i possessed four times more potent anti-lung cancer activity (6.6 µM) than did the clinically useful drug Iressa® (26.08 µM). The derivatives which possess sugar moieties on the 3-OH of arabinose moiety generally show more potent anticancer activity against A-549 cell line than corresponding derivatives, in which sugar is bound on 2-OH moiety of PSD.
Although there was little difference in polarity between the saponin derivatives, the absence of a sugar moiety at either 2-OH or 4-OH of the arabinose moiety of trisaccharide could increase the cell membrane permeability,5) thus causing an increase of antitumor activity of disaccharides.
| A-549a) | MDA-MB-231b) | MCF-7c) | |
|---|---|---|---|
| 10a | >50 | >50 | >50 |
| 10b | 41 | >50 | >50 |
| 10c | 37.4 | >50 | >50 |
| 10d | 19.6 | >50 | >50 |
| 10e | 37.2 | >50 | >50 |
| 10f | 17.9 | >50 | >50 |
| 10g | 21.8 | >50 | >50 |
| 10h | 35.3 | >50 | >50 |
| 10i | 6.6 | 21.6 | 19.3 |
| 23a | 17.3 | >50 | 42.1 |
| 23b | 39.4 | >50 | 40.4 |
| 23c | 17.8 | >50 | 41.3 |
| 23d | 18.6 | >50 | 38.7 |
| 23e | >50 | >50 | >50 |
| 23f | 19.7 | >50 | >50 |
| 23g | 42.3 | >50 | >50 |
| PSDd) | 5.9 | 16.8 | 8.1 |
| Iressa® | 26.08 | — | — |
Data are expressed as IC50 values (µM). a) Lung cancer cell line. b) Breast cancer cell line. c) Breast cancer cell line. d) Pulsatilla saponin D.
In conclusion, we synthesized two main types of new PSD analogues mainly including rhamnose and arabinose-attached saponins and tested them in three human cancer cell lines. Practical and stereospecific chemical syntheses of the PSD analogues developed in this paper will resolve the problems of the scarcity of hederagenin-linked saponins represented by natural PSD. Two types of PSD analogues displayed greater potency in anti-lung cancer activity than against the breast cancer cell line. Most of the disaccharide moiety-bearing saponins possessed more potent activity. Among the new saponins containing the disaccharide moiety, compound 10i showed four times more potent anti-lung cancer activities than observed with the clinically useful drug Iressa®. The derivatives which possess sugar moieties on the 3-OH of arabinose moiety generally show more potent anticancer activity against A-549 cell line than corresponding derivatives, in which sugar is bound on 2-OH moiety of PSD. The PSD analogues deserve further evaluation as possible anti-lung cancer drug candidates.
All commercial reagents and solvents were used as received without further purification unless specified. Reaction solvents were distilled from calcium hydride for dichloromethane and from sodium metal and benzophenone for tetrahydrofuran. The reactions were monitored and the Rf values determined using analytical thin layer chromatography (TLC) with Merck silica gel 60 and F-254 precoated plates (0.25-mm thickness). Spots on the TLC plates were visualized using ultraviolet light (254 nm) and a basic potassium permanganate solution or cerium sulfate/ammonium dimolybdate/sulfuric acid solution followed by heating on a hot plate. Flash column chromatography was performed with Merck silica gel 60 (230–400 mesh). 1H-NMR spectra were recorded on Bruker DPX-250, 400 or Varian Unity-Inova 500 Spectrometers. Proton chemical shifts are reported in ppm (δ) relative to internal tetramethylsilane (TMS, δ 0.00) or with the solvent reference relative to TMS employed as the internal standard (IS) (chloroform-d3 (CDCl3), δ 7.26 ppm, d5-pyridine nucleotides (Pyd), δ 7.58 ppm). Data are reported as follows: chemical shift {multiplicity [singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m)], coupling constants [Hz], integration}. 13C-NMR spectra were recorded on Bruker DPX-250 (63 MHz), 400 (100 MHz) or Varian Unity-Inova 500 (125 MHz) spectrometers with complete proton decoupling. Carbon chemical shifts are reported in ppm (δ) relative to TMS with the respective solvent resonance as the internal standard (CDCl3, δ 77.0 ppm; d5-Pyd δ 135.9 ppm). Infrared (IR) spectra were recorded on a Nicolet Model Impact FT-IR 400 spectrometer. Data are reported in wave numbers (cm−1). Matrix assisted laser desorption/ionization-time of flight (MALDI-TOF) masses were recorded on an Applied Biosystems 4700 proteomics analyzer spectrometer.
General Procedure for the Synthesis of Compounds 5a–iA suspension of compounds 2d, 4a–h (0.23 mmol) and 10% Pd/C (50 mg) in methanol–tetrahydrofuran (MeOH–THF) (1 : 1, 15 mL) was stirred under a H2 atmosphere for 4 h. The reaction mixture was filtered with celite and concentrated under reduced pressure. The residue was purified by a silica gel column chromatography (n-hexane–ethyl acetate (EtOAc), 2 : 1, v/v) to give mixture compounds 5a–i (80–93%).
2,3,4-Tri-O-benzoyl-α-L-O-arabinopyranosyl-(1→3)-[2,3,4-tri-O-benzoyl-α-L-O-rhamnopyranosyl-(1→4)]-2-O-benzoyl-α-L-arabinopyranoside (5a)
Yield 82%, white solids, (α : β=5 : 1); 1H-NMR (250 MHz, CDCl3) δ: 8.02–8.11 (m, 6H) 7.96–8.02 (m, 3H) 5.82–5.90 (m, 1H) 5.73–5.81 (m, 3H) 5.65 (dd, J=8.07, 3.30 Hz, 1H) 5.57 (br s, 1H) 5.41–5.48 (m, 1H) 5.36 (dd, J=8.44, 2.20 Hz, 1H) 5.19 (d, J=5.50 Hz, 1H) 4.49–4.57 (m, 2H) 4.38–4.48 (m, 1H) 4.27–4.38 (m, 1H) 4.16–4.23 (m, 1H) 4.01–4.09 (m, 1H) 3.97 (dd, J=12.47, 4.40 Hz, 1H) 3.46 (d, J=5.14 Hz, 1H) 1.39 (d, J=6.24 Hz 3H); 13C-NMR (63 MHz, CDCl3) δ: 165.98 165.85 165.61 165.53 165.15 165.04 133.38 133.33 133.22 133.17 133.09 133.01 132.89 129.97 129.92 129.84 129.73 129.53 129.40 129.32 129.22 129.12 128.44 128.37 128.31 128.16 101.23 98.69 91.15 74.49 72.03 70.97 70.85 70.26 70.08 69.94 69.90 67.91 67.25 62.21 62.01.
2,3,4,6-Tetra-O-benzoyl-α-D-O-mannopyranosyl-(1→3)-[2,3,4,6-tetra-O-benzoyl-α-D-O-mannopyranosyl-(1→4)]-2-O-benzoyl-α-L-arabinopyranoside (5h)
Yield 90%, white solids, (α : β=5 : 1); 1H-NMR (250 MHz, CDCl3) δ: 6.82–8.16 (m, 45H) 6.30 (t, J=9.35 Hz, 1H) 6.15–6.22 (m, 1H) 6.11 (t, J=8.99 Hz, 1H) 6.02 (br s, 1H) 5.88 (br s, 2H) 5.78–5.86 (m, 3H) 5.51 (d, J=13.57 Hz, 3H) 4.81–4.96 (m, 3H) 4.78 (d, J=9.17 Hz, 1H) 4.59–4.71 (m, 2H) 4.48 (br s, 1H) 4.14–4.25 (m, 2H); 13C-NMR (63 MHz, CDCl3) δ: 166.27 166.20 165.79 165.75 165.37 165.35 165.24 165.11 133.35 133.20 133.08 132.81 130.16 129.85 129.73 129.62 129.38 129.05 129.00 128.69 128.49 128.41 128.32 128.22 128.13 128.06 128.01 95.53 94.68 91.38 71.72 70.96 70.85 70.69 70.36 70.05 68.97 66.41 65.91 65.83 62.74 62.53 57.36.
2,3,4,6-Tetra-O-benzoyl-α-D-O-mannopyranosyl-(1→3)-4-O-acetyl-2-O-benzoyl-α-L-arabinopyranoside (5i)
Yield 87%, white solids, (α : β=5 : 1); 1H-NMR (250 MHz, CDCl3) δ: 87.16–8.22 (m, 25H) 5.99–6.09 (m, 1H) 5.71 (d, J=3.32 Hz, 1H) 5.56–5.68 (m, 3H) 5.52 (d, J=2.69 Hz, 1H) 5.28–5.37 (m, 1H) 4.67–4.78 (m, 1H) 4.64 (d, J=1.90 Hz, 1H) 4.54 (br s, 1H) 4.16–4.37 (m, 2H) 3.74–3.87 (m, 1H) 2.30 (s, 3H); 13C-NMR (63 MHz, CDCl3) δ: 166.08 165.52 165.30 165.06 137.27 133.46 133.38 133.20 133.10 129.93 129.74 129.55 128.98 128.77 128.55 128.47 128.37 128.31 127.85 98.61 96.99 77.15 70.66 70.33 70.19 69.79 69.22 66.23 64.78 63.52 62.44.
General Procedure for the Synthesis of Compounds 9a–iA solution of compounds 5a–i (0.24 mmol), CCl3CN (1.2 mmol) and DBU (0.12 mmol) in CH2Cl2 (10 mL) was stirred for 1 h at 0°C, then the reaction mixture was filtered with silica gel. The filtrate was concentrated under reduced pressure. The trichloro acetimidate residue compounds 7a–i and protected hederagenin 8 were treated with TMSOTf (0.04 mmol) at −78°C under a N2 atmosphere. After stirring for 1 h at this temperature, the mixture was neutralized with triethylamine (Et3N), filtered and concentrated under reduced pressure. The residue was purified by a silica gel column chromatography (n-hexane–EtOAc, 2 : 1, v/v) to give compounds 9a–i (49–75%).
28-O-t-Butyldiphenylsilyl-23-O-benzoyl-hederagenin-3-O-2,3,4-tri-O-benzoyl-α-L-O-arabinopyranosyl-(1→3)-[2,3,4-tri-O-benzoyl-α-L-O-rhamnopyranosyl-(1→4)]-2-O-benzoyl-α-L-arabinopyranoside (9a)
Yield 63%, white solids, Rf=0.50 (n-hexane–EtOAc, 2 : 1, v/v); [α]D +106° (c 0.05, CHCl3); 1H-NMR (400 MHz, CDCl3) δ: 7.07–8.17 (m, 50H) 5.59–5.93 (m, 7H) 5.35–5.45 (m, 2H) 5.25 (br s, 1H) 5.12–5.21 (m, 1H) 4.66–4.73 (m, 1H) 4.36–4.44 (m, 1H) 4.24–4.32 (m, 2H) 4.17–4.24 (m, 2H) 4.10–4.17 (m, 1H) 3.96–4.10 (m, 2H) 3.55 (d, J=8.44 Hz, 1H) 3.42–3.50 (m, 1H) 2.78–2.94 (m, 1H) 1.39 (d, J=6.24 Hz, 3H) 1.16–1.96 (m, 22H) 1.12 (s, 9H) 0.97 (s, 3H) 0.93 (s, 3H) 0.89 (s, 3H) 0.84 (s, 3H) 0.74 (s, 3H) 0.35 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ: 166.05 165.77 165.67 165.54 165.50 165.45 165.14 164.70 133.44 133.27 130.00 129.85 129.71 129.60 129.40 129.24 129.17 129.06 128.93 128.74 128.6 128.47 128.41 128.38 128.33 128.28 128.14 101.93 99.56 90.98 74.34 73.43 73.18 72.29 72.23 70.99 70.74 70.19 69.82 68.92 63.17 62.71 48.54 47.56 47.18 43.76 42.61 42.48 40.16 38.95 37.31 34.92 33.25 31.44 30.44 28.80 26.59 25.92 24.50 24.29 24.17 20.46 19.12 18.63 17.97 16.46 14.18 14.04; IR vmax cm−1 (KBr) 2933.51 2864.11 1728.71 1604.57 1453.54 1384.32 1265.76 1092.14; MALDI-TOF-MS m/z 1975.7391 [M+Na]+ (Calcd for C118H124O24SiNa. Found: 1975.8150).
28-O-t-Butyldiphenylsilyl-23-O-benzoyl-hederagenin-3-O-2,3,4,6-tetra-O-benzoyl-α-D-O-mannopyranosyl-(1→3)-4-O-acetyl-2-O-benzoyl-α-L-arabinopyranoside (9i)
Yield 71%, white solids, Rf=0.54 (n-hexane–EtOAc, 2 : 1, v/v); [α]D +46° (c 0.05, CHCl3); 1H-NMR (400 MHz, CDCl3) δ: 7.23–8.22 (m, 40H) 6.01–6.10 (m, 1H) 5.53–5.69 (m, 3H) 5.38–5.43 (m, 1H) 5.28 (br s, 1H) 5.25 (s, 1H) 4.65–4.69 (m, 1H) 4.51–4.57 (m, 1H) 4.26 (br s, 2H) 4.09–4.16 (m, 2H) 3.92 (s, 2H) 3.57–3.64 (m, 2H) 2.89 (dd, J=13.75, 3.48 Hz, 1H) 1.28 (s, 3H) 1.21–1.99 (m, 22H) 1.13 (s, 9H) 1.01 (s, 3H) 0.96 (s, 3H) 0.93 (s, 3H) 0.92 (s, 3H) 0.74 (s, 3H) 0.37 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ: 166.08 165.52 165.30 165.06 137.27 133.46 133.38 133.20 133.10 129.93 129.74 129.55 128.98 128.77 128.55 128.47 128.37 128.31 127.85 98.61 96.99 77.15 70.66 70.33 70.19 69.79 69.22 66.23 64.78 63.52 62.44 48.54 47.56 47.18 43.76 42.61 42.48 40.16 38.95 37.31 34.92 33.25 31.44 30.44 28.80 26.59 25.92 24.50 24.29 24.17 20.46 19.12 18.63 17.97 16.46 14.18 14.04; IR vmax cm−1 (KBr) 2935.22 2865.38 1724.14 1602.34 1452.15 1381.45 1266.61 1092.84; MALDI-TOF-MS m/z 1694.0912 [M+Na]+ (Calcd for C101H110O20SiNa. Found: 1693.7257).
General Procedure for the Synthesis of 10a–iTo a solution of compounds 9a–i (0.027 mmol) in THF (3 mL) was added TBAF (0.054 mmol) at room temperature. When the starting material was completely consumed on TLC, potassium t-butoxide (0.4 mmol) was added and stirred overnight. The reaction mixtures was acidified with amberlite IR-120H H+ resin to pH 7 and filtered, the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (CHCl3–MeOH–H2O, 40 : 10 : 1, v/v/v) to give compounds 10a–i (65–84%).
Hederagenin-3-O-α-L-arabinopyranosyl-(1→3)-[α-L-rhamopyranosyl-(1→4)]-α-L-arabinopyranoside (10a)
Yield 80%, white solids, Rf=0.25 (CHCl3–MeOH–H2O, 40 : 10 : 1, v/v/v); [α]D +12° (c 0.05, MeOH); 1H-NMR (400 MHz, Pyr) δ: 5.91 (s, 1H) 5.47 (br s, 1H) 5.07 (d, J=6.97 Hz, 2H) 4.97 (d, J=7.34 Hz, 2H) 4.66 (d, J=1.83 Hz, 1H) 4.50–4.56 (m, 1H) 4.42–4.50 (m, 3H) 4.25–4.36 (m, 4H) 4.17–4.22 (m, 1H) 4.03–4.12 (m, 1H) 3.72 (br s, 2H) 3.42–3.51 (m, 2H) 3.24–3.37 (m, 1H) 1.62 (d, J=6.24 Hz, 3H) 1.26–2.23 (m, 22H) 1.01 (s, 3H) 0.94 (s, 3H) 0.93 (s, 3H) 0.92 (s, 3H) 0.91 (s, 3H) 0.90 (s, 3H); 13C-NMR (100 MHz, Pyr) δ: 180.32 144.63 106.17 105.17 105.05 81.62 80.71 78.15 77.99 76.60 75.40 73.79 72.52 71.87 71.24 68.48 65.94 65.85 63.93 62.39 47.92 47.28 46.43 43.25 41.91 41.74 39.52 38.51 36.68 33.98 33.01 32.63 30.71 28.10 25.89 23.62 23.54 23.44 17.88 17.24 15.86 13.3; IR vmax cm−1 (KBr) 3435.61 2933.11 1702.54 1632.56 1455.68 1387.31 1266.32 1071.24; MALDI-TOF-MS m/z 905.5343 [M+Na]+ (Calcd for C46H74O16Na. Found: 905.4875).
Hederagenin-3-O-α-L-arabinopyranosyl-(1→3)-[β-D-glucopyranosyl-(1→4)]-α-L-arabinopyranoside (10b)
Yield 71%, white solid, Rf=0.30 (CHCl3–MeOH–H2O, 40 : 10 : 1, v/v/v); [α]D +104° (c 0.05, MeOH); 1H-NMR (400 MHz, Pyr) δ: 5.45 (br s, 1H) 5.36 (d, J=6.97 Hz, 2H) 4.91 (d, J=6.97 Hz, 1H) 4.50–4.60 (m, 3H) 4.40–4.49 (m, 2H) 4.28–4.40 (m, 4H) 4.15–4.26 (m, 5H) 3.94 (t, J=8.25 Hz, 1H) 3.89 (br s, 1H) 3.82 (d, J=11.00 Hz, 1H) 3.67 (d, J=10.64 Hz, 1H) 3.62 (d, J=12.10 Hz, 1H) 3.22–3.31 (m, 1H) 1.23–2.18 (m, 22H) 1.21 (s, 3H) 0.99 (s, 3H) 0.98 (s, 3H) 0.91 (s, 6H) 0.86 (s, 3H); 13C-NMR (100 MHz, Pyr) δ: 180.75 144.63 105.05 81.62 80.71 78.15 77.99 76.60 75.40 73.79 72.52 71.87 71.24 68.48 65.96 65.85 63.93 63.18 62.39 47.92 47.28 46.43 43.25 41.91 41.74 39.52 38.51 36.68 33.98 33.01 32.63 30.71 28.10 25.89 23.62 23.54 23.44 17.88 17.24 15.86 13.37; IR vmax cm−1 (KBr) 3433.21 2935.32 1705.72 1631.66 1451.21 1383.15 1262.73 1072.63; MALDI-TOF-MS m/z 921.4430 [M+Na]+ (Calcd for C46H74O17Na. Found: 921.4824).
Hederagenin-3-O-α-L-arabinopyranosyl-(1→3)-[α-D-mannopyranosyl-(1→4)]-α-L-arabino-pyranoside (10c)
Yield 65%, white solids, Rf=0.25 (CHCl3–MeOH–H2O, 40 : 10 : 1, v/v/v); [α]D +38° (c 0.05, MeOH); 1H-NMR (400 MHz, Pyr) δ: 5.57 (s, 1H) 5.45 (br s, 1H) 5.04 (d, J=6.60 Hz, 2H) 4.89–4.96 (m, 3H) 4.55–4.61 (m, 2H) 4.45–4.54 (m, 3H) 4.35–4.43 (m, 2H) 4.17–4.32 (m, 4H) 4.13 (dd, J=8.62, 2.75 Hz, 1H) 4.02 (dd, J=9.54, 2.93 Hz, 1H) 3.75 (d, J=11.37 Hz, 1H) 3.68 (d, J=10.64 Hz, 1H) 3.47 (d, J=12.84 Hz, 1H) 3.23–3.31 (m, 1H) 1.26–2.19 (m, 22H) 1.22 (s, 3H) 0.98 (br s, 6H) 0.91 (s, 3H) 0.88 (s, 3H) 0.86 (s, 3H); 13C-NMR (100 MHz, Pyr) δ: 180.75 144.61 122.29 106.96 106.42 98.10 81.87 74.09 73.55 72.62 72.43 72.27 71.77 71.60 70.36 69.04 66.52 63.86 62.35 47.90 47.26 46.43 46.20 43.26 41.91 41.74 39.52 38.49 36.66 33.97 33.02 32.64 30.71 28.10 25.91 23.61 23.54 23.43 17.89 17.23 15.81 14.32 13.35; IR vmax cm−1 3434.23 2932.67 1702.71 1634.65 1453.55 1382.51 1264.36 1073.41; MALDI-TOF-MS m/z 921.4034 [M+Na]+ (Calcd for C46H74O17Na. Found: 921.4824).
Hederagenin-3-O-α-L-rhamnopyranosyl-(1→3)-[α-D-mannopyranosyl-(1→4)]-α-L-arabinopyranoside (10d)
Yield 81%, white solids, Rf=0.63 (CHCl3–MeOH–H2O, 40 : 10 : 1, v/v/v); [α]D +44° (c 0.05, MeOH); 1H-NMR (400 MHz, Pyr) δ: 6.20 (s, 1H) 5.67 (s, 1H) 5.48 (br s, 1H) 4.78–4.90 (m, 3H) 4.47–4.72 (m, 7H) 4.38 (dd, J=11.74, 6.24 Hz, 2H) 4.20–4.34 (m, 5H) 3.69 (d, J=10.64 Hz, 1H) 3.40 (d, J=12.84 Hz, 1H) 3.26–3.34 (m, 1H) 1.68 (d, J=6.24 Hz, 3H) 1.27–2.21 (m, 22H) 1.25 (s, 3H) 1.02 (s, 3H) 1.01 (br s, 3H) 0.94 (s, 3H) 0.91 (s, 3H) 0.86 (s, 3H); 13C-NMR (100 MHz, Pyr) δ: 180.75 145.31 135.46 130.78 128.81 124.46 123.43 117.10 107.45 103.48 98.11 82.51 77.01 75.34 75.24 73.14 72.99 72.88 72.63 72.56 72.43 70.82 69.91 64.58 62.51 48.60 47.94 47.11 46.89 43.92 42.62 42.44 40.22 40.16 39.21 37.36 34.67 33.71 33.35 31.41 30.44 28.80 26.60 24.32 24.24 24.15 19.18 18.57 17.93 16.52 14.04; IR vmax cm−1 (KBr) 3431.23 2932.62 1702.41 1633.41 1453.76 1384.13 1263.78 1073.28; MALDI-TOF-MS m/z 935.5480 [M+Na]+ (Calcd for C47H76O17Na. Found: 935.4980).
Hederagenin-3-O-β-D-glucopyranosyl-(1→3)-[α-L-rhamnopyranosyl-(1→4)]-α-L-arabinopyranoside (10e)
Yield 75%, white solids, Rf=0.33 (CHCl3–MeOH–H2O, 40 : 10 : 1, v/v/v); [α]D +26° (c 0.05, MeOH); 1H-NMR (400 MHz, Pyr) δ: 5.97 (s, 1H) 5.48 (br s, 1H) 5.15 (d, J=7.70 Hz, 1H) 4.98 (br s, 6H) 4.70 (br s, 1H) 4.38–4.57 (m, 4H) 4.20–4.34 (m, 4H) 4.05–4.14 (m, 1H) 3.92–4.01 (m, 1H) 3.73 (d, J=10.64 Hz, 1H) 3.66 (d, J=11.74 Hz, 1H) 3.30 (dd, J=13.39, 3.48 Hz, 1H) 1.62 (d, J=5.87 Hz, 3H) 1.27–2.22 (m, 22H) 1.25 (s, 3H) 1.02 (br s, 3H) 1.01 (br s, 3H) 0.94 (s, 6H) 0.91 (s, 3H); 13C-NMR (100 MHz, Pyr) δ: 181.30 145.28 123.02 106.91 106.89 103.28 83.99 82.48 79.09 78.60 76.16 75.36 72.92 72.51 72.24 71.94 70.76 66.34 64.62 63.27 48.61 47.99 47.11 43.99 42.61 42.43 40.22 39.24 37.38 35.59 34.66 33.75 33.35 31.41 28.81 26.60 24.30 24.22 24.14 19.02 18.59 17.93 16.52 14.1; IR vmax cm−1 (KBr) 3433.76 2935.27 1704.22 1632.12 1452.61 1382.35 1264.72 1074.54; MALDI-TOF-MS m/z 935.7244 [M+Na]+ (Calcd for C47H76O17Na. Found: 935.4980).
Hederagenin-3-O-α-D-mannopyranosyl-(1→3)-[α-L-arabinopyranosyl-(1→4)]-α-L-arabinopyranoside (10f)
Yield 77%, white solids, Rf=0.27 (CHCl3–MeOH–H2O, 40 : 10 : 1, v/v/v); [α]D +50° (c 0.05, MeOH); 1H-NMR (400 MHz, Pyr) δ: 5.87 (br s, 1H) 5.48 (br s, 1H) 4.92 (d, J=5.87 Hz, 2H) 4.88 (br s, 1H) 4.75–4.82 (m, 2H) 4.66 (d, J=8.44 Hz, 1H) 4.48–4.56 (m, 2H) 4.47 (br s, 1H) 4.39 (d, J=6.60 Hz, 2H) 4.32–4.37 (m, 2H) 4.26 (d, J=11.74 Hz, 1H) 4.20 (br s, 3H) 4.00 (d, J=6.97 Hz, 1H) 3.64 (dd, J=15.77, 12.10 Hz, 2H) 3.55 (d, J=12.10 Hz, 1H) 3.30 (d, J=11.74 Hz, 1H) 1.29–2.23 (m, 22H) 1.25 (br s, 3H) 1.02 (s, 3H) 1.01 (s, 3H) 0.94 (br s, 9H); 13C-NMR (100 MHz, Pyr) δ: 180.71 145.29 123.02 107.44 106.96 99.30 82.55 77.72 75.26 74.81 73.29 72.85 72.53 71.59 69.99 69.63 67.56 66.37 64.87 63.54 48.60 48.03 47.11 46.87 43.89 42.62 42.43 40.22 39.16 37.38 34.66 33.71 33.35 31.40 30.44 28.79 26.61 26.55 24.32 24.24 24.14 18.60 17.94 16.54 14.17; IR vmax cm−1 (KBr) 3434.82 2932.64 1703.67 1635.87 1455.68 1385.81 1263.33 1072.31; MALDI-TOF-MS m/z 921.5866 [M+Na]+ (Calcd for C46H74O17Na. Found: 921.4824).
Hederagenin-3-O-α-L-rhamnopyranosyl-(1→3)-[α-L-rhamnopyranosyl-(1→4)]-α-L-arabinopyranoside (10g)
Yield 84%, white solids, Rf=0.28 (CHCl3–MeOH–H2O, 40 : 10 : 1, v/v/v); [α]D +16° (c 0.05, MeOH); 1H-NMR (400 MHz, Pyr) δ: 5.94 (br s, 1H) 5.80 (br s, 1H) 5.44 (br s, 1H) 4.92 (d, J=6.24 Hz, 1H) 4.68 (d, J=12.84 Hz, 2H) 4.58 (d, J=7.34 Hz, 1H) 4.10–4.52 (m, 9H) 3.60–3.71 (m, 2H) 3.41–3.52 (m, 2H) 3.26 (d, J=11.74 Hz, 1H) 1.64 (d, J=5.87 Hz, 3H) 1.58 (d, J=5.87 Hz, 3H) 1.25–2.21 (m, 22H) 1.22 (s, 3H) 0.98 (s, 3H) 0.91 (s, 3H) 0.90 (s, 3H) 0.87 (br s, 6H); 13C-NMR (100 MHz, Pyr) δ: 180.75 144.64 122.27 106.48 103.19 103.09 81.87 78.94 75.56 73.87 73.79 72.48 72.35 71.96 70.19 69.97 63.90 58.61 47.90 47.23 46.43 46.23 43.25 41.91 41.75 39.52 38.49 36.66 34.00 33.03 32.96 32.64 30.71 28.10 25.91 23.88 23.62 23.56 23.46 19.78 18.49 18.30 17.89 17.25 15.82 13.54 13.42; IR vmax cm−1 (KBr) 3436.83 2934.34 1703.21 1633.56 1453.61 1383.98 1264.77 1074.51; MALDI-TOF-MS m/z 919.6133 [M+Na]+ (Calcd for C47H76O16Na. Found: 919.5031).
Hederagenin-3-O-α-L-mannopyranosyl-(1→3)-[α-L-mannopyranosyl-(1→4)]-α-L-arabinopyranoside (10h)
Yield 73%, white solids, Rf=0.30 (CHCl3–MeOH–H2O, 40 : 10 : 1, v/v/v); [α]D +140° (c 0.05, MeOH); 1H-NMR (400 MHz, Pyr) δ: 5.98 (br s, 1H) 5.57 (br s, 1H) 5.44 (br s, 1H) 4.86 (br s, 3H) 4.68–4.75 (m, 2H) 4.58–4.68 (m, 4H) 4.45–4.58 (m, 4H) 4.34–4.43 (m, 3H) 4.17–4.27 (m, 2H) 4.14 (m, 1H) 3.62 (d, J=10.27 Hz, 1H) 3.27 (br s, 2H) 1.26–2.18 (m, 22H) 1.22 (br s, 3H) 0.98 (br s, 6H) 0.91 (s, 3H) 0.88 (br s, 6H); 13C-NMR (100 MHz, Pyr) δ: 180.75 144.61 122.29 106.44 98.57 97.34 82.24 75.43 75.10 74.54 72.50 72.18 71.97 70.59 69.08 68.78 68.59 64.33 63.40 62.83 62.04 47.89 47.41 46.41 46.19 43.13 41.91 41.73 39.51 38.45 36.68 33.96 33.02 32.96 32.67 30.70 28.09 25.90 25.71 23.62 23.54 23.43 17.91 17.22 15.81 13.39; IR vmax cm−1 (KBr) 3433.56 2937.16 1703.63 1634.71 1454.89 1385.78 1266.21 1072.46; MALDI-TOF-MS m/z 951.7219 [M+Na]+ (Calcd for C47H76O18Na. Found: 951.4929.5031).
Hederagenin-3-O-α-D-mannopyranosyl-(1→3)-α-L-arabinopyranoside (10i)
Yield 79%, white solids, Rf=0.28 (CHCl3–MeOH–H2O, 40 : 10 : 1, v/v/v); [α]D +146° (c 0.05, MeOH); 1H-NMR (400 MHz, Pyr) δ: 5.83 (s, 1H) 5.45 (br s, 1H) 4.91–4.95 (m, 3H) 4.74 (s, 1H) 4.62–4.69 (m, 2H) 4.51–4.58 (m, 1H) 4.45 (s, 2H) 4.25–4.37 (m, 3H) 4.21 (d, J=12.47 Hz, 2H) 3.63–3.70 (m, 1H) 3.48–3.56 (m, 1H) 3.21–3.32 (m, 1H) 1.29–2.25 (m, 22H) 1.22 (s, 3H) 0.99 (s, 3H) 0.98 (s, 3H) 0.93 (s, 3H) 0.91 (s, 6H); 13C-NMR (100 MHz, Pyr) δ: 180.75 144.67 106.44 98.09 81.79 77.65 74.64 72.71 72.09 70.73 68.97 66.64 65.22 64.20 62.93 47.90 47.33 46.42 43.22 41.91 41.73 41.39 39.51 38.48 36.68 34.00 33.64 32.65 30.69 28.13 25.88 23.54 23.43 17.91 17.24 15.82 13.47; IR vmax cm−1 (KBr) 3432.36 2934.32 1702.78 1636.31 1453.66 1382.54 1263.79 1073.45; MALDI-TOF-MS m/z 789.4209 [M+Na]+ (Calcd for C41H66O13Na. Found: 789.4401).
General Procedure for the Synthesis of Compounds 15a–dA suspension of compounds 14a–d (0.33 mmol) and 10% Pd/C (80 mg) in MeOH–THF (1 : 1, 15 mL) was stirred under a H2 atmosphere for 4 h. The reaction mixture was filtered with celite and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (n-hexane–EtOAc, 2 : 1, v/v) to give a mixture compounds 15a–d (81–91%).
2,3,4-Tri-O-benzoyl-α-L-arabinopyranosyl-(1→4)-2,3-di-O-benzoyl-α-L-rhamnopyranoside (15a)
Yield 82%, white solids, (α : β=5 : 1); 1H-NMR (400 MHz, CDCl3) δ: 7.14–8.07 (m, 25H) 5.57–5.68 (m, 4H) 5.39 (dd, J=9.17, 3.30 Hz, 1H) 5.30 (d, J=3.67 Hz, 1H) 5.05 (d, J=5.87 Hz, 1H) 4.34 (dd, J=12.84, 3.30 Hz, 1H) 4.23–4.29 (m, 1H) 4.11 (t, J=9.17 Hz, 1H) 3.92 (d, J=12.47 Hz, 1H) 3.51 (d, J=3.67 Hz, 1H) 1.50 (d, J=6.60 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ: 165.85 165.64 165.56 165.27 165.04 133.67 133.55 133.47 133.43 133.32 133.20 133.13 130.07 129.96 129.94 129.82 129.67 129.61 129.57 129.50 129.35 129.24 128.99 128.93 128.85 128.65 128.58 128.53 128.43 128.28 101.11 92.30 76.71 72.55 71.12 70.86 70.07 68.53 67.21 63.09 18.37.
General Procedure for the Synthesis of Compounds 20a–cA suspension of compounds 17a–c (0.3 mmol) and 10% Pd/C (80 mg) in MeOH–THF (1 : 1, 15 mL) was stirred under the H2 atmosphere for 4 h. The reaction mixture was filtered with celite and concentrated under the reduced pressure. The residue was purified by a silica gel column chromatography (n-hexane–EtOAc, 2 : 1, v/v) to give α, β mixtures compounds 20a–c (82–87%).
2,3,4-Tri-O-benzoyl-α-L-arabinopyranosyl-(1→3)-[2,3,4,6-tetra-O-benzoyl-β-D-glucopyranosyl-(1→4)]-2-O-acetyl-α-L-rhamnopyranoside (20a)
Yield 85%, white solids, (α : β=5 : 1); 1H-NMR (400 MHz, CDCl3) δ: 7.23–8.33 (m, 35H) 5.59–5.70 (m, 2H) 5.43–5.51 (m, 2H) 5.38 (dd, J=10.27, 3.67 Hz, 1H) 5.30 (br s, 1H) 5.27 (br s, 1H) 5.06 (br s, 1H) 4.64 (d, J=8.07 Hz, 1H) 4.47 (d, J=7.34 Hz, 1H) 4.42 (dd, J=12.10, 3.30 Hz, 1H) 4.19 (br s, 2H) 3.92 (dd, J=9.54, 5.87 Hz, 1H) 3.84 (d, J=11.74 Hz, 1H) 3.60 (t, J=9.54 Hz, 1H) 3.31 (d, J=3.67 Hz, 1H) 2.51–2.58 (m, 1H) 2.39 (d, J=13.20 Hz, 1H) 2.22 (s, 3H) 1.22 (d, J=6.60 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ: 170.25 165.99 165.92 165.75 165.23 165.06 164.87 134.20 134.10 133.64 133.48 133.43 133.40 133.28 130.35 130.24 130.22 130.04 129.88 129.82 129.78 129.72 129.62 129.58 129.47 129.39 129.06 128.83. 128.62 128.57 128.52 128.48 128.47 100.66 100.30 92.36 77.44 73.68 72.87 72.57 71.18 71.08 70.42 69.36 68.49 66.76 63.82 62.34 21.09 17.86.
2,3,4-Tri-O-benzoyl-α-L-rhamnopyranosyl-(1→3)-[2,3,4-tri-O-benzoyl-α-L-rhamnopyranosyl-(1→4)]-2-O-acetyl-α-L-rhamnopyranoside (20b)
Yield 87%, white solids, (α : β=20 : 1); 1H-NMR (400 MHz, CDCl3) δ: 7.08–8.02 (m, 30H) 6.04 (br s, 1H) 5.94–6.02 (m, 2H) 5.88 (br s, 1H) 5.79 (t, J=9.90 Hz, 1H) 5.65 (t, J=9.90 Hz, 1H) 5.59 (s, 1H) 5.41 (s, 1H) 5.34 (br s, 1H) 5.27 (br s, 1H) 4.34–4.45 (m, 2H) 4.10–4.23 (m, 2H) 3.94 (t, J=9.54 Hz, 1H) 3.87 (d, J=3.67 Hz, 1H) 2.26 (s, 3H) 1.49 (d, J=5.87 Hz, 3H) 1.41 (d, J=6.60 Hz, 3H) 1.32 (d, J=5.87 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ: 170.80 165.99 165.89 165.60 165.59 165.27 165.16 133.38 133.29 133.17 132.99 132.91 132.76 130.00 129.87 129.81 129.77 129.71 129.65 129.49 129.46 129.44 129.39 128.50 128.45 128.42 128.31 128.19 128.08 100.13 99.76 91.36 81.02 79.02 73.13 72.32 72.05 71.97 71.43 69.58 69.38 67.78 67.72 67.20 21.32 18.69 17.66.
General Procedure for the Synthesis of Compounds 22a–gA solution of compounds 15a–d, 20a–c (0.25 mmol), CCl3CN (1.25 mmol) and DBU (0.125 mmol) in CH2Cl2 (10 mL) was stirred for 1 h at 0°C, then the reaction mixture was filtered with silica gel. The filtrate was concentrated under reduced pressure. The trichloro acetimidate residue compounds 21a–g and protected hederagenin 8 was treated with TMSOTf (0.05 mmol) at −78°C under a N2 atmosphere. After stirring for 1 h at this temperature, the mixture was neutralized with triethylamine (Et3N), filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (n-hexane–EtOAc, 2 : 1, v/v) to give compounds 22a–g (73–82%).
28-O-t-Butyldiphenylsilyl-23-O-benzoyl-hederagenin-3-O-2,3,4-tri-O-benzoyl-α-L-arabinopyranosyl-(1→4)-2,3-di-O-benzoyl-α-L-rhamnopyranoside (22a)
Yield 77%, white solids, Rf=0.43 (n-hexane–EtOAc, 2 : 1, v/v); [α]D +45° (c 0.1, CHCl3); 1H-NMR (400 MHz, CDCl3) δ: 7.93–7.99 (m, 4H) 7.78–7.86 (m, 4H) 7.12–7.70 (m, 32H) 5.64 (br s, 2H) 5.58 (br s, 1H) 5.52 (d, J=8.80 Hz, 1H) 5.39 (d, J=8.80 Hz, 1H) 5.26 (br s, 1H) 5.04 (d, J=5.14 Hz, 1H) 4.98 (br s, 1H) 4.24–4.37 (m, 2H) 4.04–4.17 (m, 3H) 3.91 (d, J=13.20 Hz, 1H) 3.62 (t, J=7.34 Hz, 1H) 2.87 (d, J=12.47 Hz, 1H) 1.13 (s, 9H) 0.97 (br s, 3H) 0.95 (br s, 3H) 0.94 (br s, 3H) 0.90 (br s, 2H) 0.87 (br s, 3H) 0.85–2.03 (m, 22H) 0.39 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ: 176.66 165.93 165.80 165.60 165.29 165.20 165.02 143.78 135.89 133.55 133.41 133.28 133.22 133.14 132.85 132.09 130.62 130.04 129.98 129.95 129.87 129.71 129.66 129.63 129.09 129.00 128.65 128.50 128.43 128.30 127.67 127.63 122.39 101.05 99.89 82.96 76.89 72.87 70.99 70.93 70.04 68.52 67.42 65.69 62.99 48.11 48.07 46.40 42.58 41.98 41.84 39.37 38.53 36.61 34.14 33.20 32.63 32.49 30.85 27.55 27.23 25.33 25.08 23.65 23.54 19.46 18.27 18.22 17.18 15.88 13.05; IR vmax cm−1 (KBr) 2935.24 2864.23 1725.52 1602.56 1455.72 1384.78 1262.35 1094.15; MALDI-TOF-MS m/z 1635.4953 [M+Na]+ (Calcd for C99H108O18SiNa. Found: 1635.7203).
28-O-t-Butyldiphenylsilyl-23-O-benzoyl-hederagenin-3-O-2,3,4,6-tetra-O-benzoyl-β-D-glucopyranosyl-(1→3)-[2,3,4,6-tetra-O-benzoyl-β-D-glucopyranosyl-(1→4)]-2-O-acetyl-α-L-rhamnopyranoside (22g)
Yield 80%, white solids, Rf=0.3 (n-hexane–EtOAc, 2 : 1, v/v); [α]D +66.7° (c 0.1, CHCl3); 1H-NMR (400 MHz, CDCl3) δ: 8.30 (d, J=6.60 Hz, 2H) 8.23 (d, J=7.34 Hz, 2H) 7.23–8.01 (m, 51H) 5.73 (t, J=9.54 Hz, 1H) 5.64 (t, J=9.54 Hz, 1H) 5.38–5.53 (m, 4H) 5.18–5.26 (m, 2H) 4.69 (br s, 1H) 4.65 (d, J=7.34 Hz, 1H) 4.60 (d, J=6.60 Hz, 1H) 4.43 (d, J=11.74 Hz, 1H) 4.25–4.30 (m, 1H) 4.13–4.21 (m, 2H) 4.02–4.12 (m, 1H) 3.85 (d, J=11.74 Hz, 1H) 3.76–3.83 (m, 1H) 3.60 (t, J=9.17 Hz, 1H) 3.48 (t, J=7.34 Hz, 1H) 2.86 (d, J=12.47 Hz, 1H) 2.58 (d, J=5.87 Hz, 1H) 2.49 (d, J=7.34 Hz, 1H) 2.12 (s, 3H) 1.21 (br s, 3H) 1.12 (br s, 9H) 0.93 (s, 6H) 0.89 (s, 3H) 0.78 (s, 3H) 0.75–1.95 (m, 22H) 0.57 (s, 3H) 0.35 (s, 3H); 13C-NMR (100 MHz, CDCl3) 176.65 169.92 166.07 165.93 165.84 165.63 165.23 165.03 164.97 164.45 143.74 135.87 133.83 133.68 133.61 133.52 133.41 133.28 133.20 132.81 130.57 130.19 130.07 130.00 129.95 129.83 129.71 129.60 128.58 128.41 127.65 127.61 122.38 100.31 100.20 100.05 82.34 77.32 74.04 72.93 72.85 72.71 72.40 72.28 72.09 71.28 69.42 69.39 67.01 65.41 62.46 62.39 48.05 48.01 47.92 46.36 42.36 41.92 41.80 39.29 38.43 36.46 34.14 33.18 32.53 32.49 30.82 27.51 27.21 25.25 25.10 23.62 23.48 21.02 19.44 18.13 17.55 17.13 15.68 12.57; IR vmax cm−1 (KBr) 2931.56 2864.78 1724.57 1604.71 1453.47 1384.48 1262.36 1092.79; MALDI-TOF-MS m/z 2181.9798 [M+Na]+ (Calcd for C129H134O28SiNa. Found: 2181.8729).
General Procedure for the Synthesis of Compounds 23a–gTo a solution of compounds 22a–g (0.04 mmol) in THF (3 mL) was added TBAF (0.08 mmol) at room temperature. When the starting material was completely consumed on TLC, potassium t-butoxide (0.6 mmol) was added and stirred overnight. The reaction mixtures was acidified with amberlite IR-120H H+ resin to pH 7 and filtered, the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (CHCl3–MeOH–H2O, 40 : 10 : 1, v/v/v) to give compounds 23a–g (78–84%).
Hederagenin-3-O-α-L-arabinopyranosyl-(1→4)-α-L-rhamnopyranoside (23a)
Yield 81%, white solids, Rf=0.58 (CHCl3–MeOH–H2O, 20 : 10 : 1, v/v/v); [α]D +31.5° (c 0.05, MeOH); 1H-NMR (400 MHz, Pyr) δ: 5.63 (br s, 1H) 5.48 (br s, 1H) 5.08 (d, J=4.40 Hz, 1H) 4.62 (br s, 1H) 4.55 (br s, 2H) 4.28–4.37 (m, 2H) 4.22–4.28 (m, 2H) 4.08–4.19 (m, 2H) 3.72 (d, J=9.54 Hz, 1H) 3.61 (d, J=11.74 Hz, 2H) 3.29 (d, J=13.20 Hz, 1H) 0.90–2.21 (m, 22H) 1.45 (d, J=4.40 Hz, 3H) 1.22 (s, 3H) 1.04 (s, 3H) 1.01 (s, 3H) 0.95 (br s, 6H) 0.74 (s, 3H); 13C-NMR (100 MHz, Pyr) δ: 180.64 145.29 123.01 107.66 104.14 85.34 81.55 75.62 74.47 73.51 72.52 70.18 68.35 68.08 64.57 48.52 74.53 47.08 46.86 43.77 42.58 42.41 40.19 39.01 37.30 34.66 33.72 33.66 33.24 31.40 28.79 26.62 26.01 24.65 24.30 24.24 24.12 20.52 18.85 18.61 17.89 16.48 13.99; IR vmax cm−1 (KBr) 3435.51 2934.33 1702.51 1635.81 1454.56 1383.78 1263.99 1073.21; MALDI-TOF-MS m/z 773.5143 [M+Na]+ (Calcd for C41H66O12Na. Found: 773.4452).
Hederagenin-3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranoside (23b)
Yield 84%, white solids, Rf=0.6 (CHCl3–MeOH–H2O, 20 : 10 : 1, v/v/v); [α]D −14.6° (c 0.05, MeOH); 1H-NMR (400 MHz, Pyr) δ: 6.30 (s, 1H) 5.62 (s, 1H) 5.49 (br s, 1H) 4.91 (br s, 1H) 4.50–4.59 (m, 3H) 4.26–4.45 (m, 4H) 4.17 (dd, J=11.74, 4.40 Hz, 1H) 3.71 (d, J=11.00 Hz, 1H) 3.62 (d, J=11.00 Hz, 1H) 3.30 (d, J=11.00 Hz, 1H) 1.63 (d, J=5.87 Hz, 3H) 1.61 (d, J=6.60 Hz, 3H) 1.04 (s, 3H) 1.01 (s, 3H) 0.96 (s, 3H) 0.94 (br s, 6H) 0.88–2.21 (m, 22H) 0.76 (s, 3H); 13C-NMR(100 MHz, Pyr) δ: 180.71 145.32 123.00 104.21 103.62 81.65 80.71 74.46 74.03 73.41 73.16 70.82 68.31 64.61 48.54 47.55 47.10 46.88 43.79 42.59 42.43 40.19 39.06 37.32 34.68 33.72 33.68 33.24 31.41 28.80 26.63 26.05 24.64 24.32 24.24 24.13 20.51 19.36 18.98 18.62 17.90 16.49 14.27 13.98; IR vmax cm−1 (KBr) 3433.23 2931.98 1701.69 1637.93 1459.54 1384.12 1264.63 1075.18; MALDI-TOF-MS m/z 787.5653 [M+Na]+ (Calcd for C42H68O12 Na. Found: 787.4608).
Hederagenin-3-O-β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranoside (23c)
Yield 83%, white solid, Rf=0.48 (CHCl3–MeOH–H2O, 20 : 10 : 1, v/v/v); [α]D −7° (c 0.1, MeOH); 1H-NMR (400 MHz, Pyr) δ: 5.57 (s, 1H) 5.48 (s, 1H) 5.23 (d, J=5.13 Hz, 1H) 4.58 (br s, 2H) 4.09–4.47 (m, 8H) 3.79 (br s, 1H) 3.59–3.71 (m, 2H) 3.29 (d, J=12.47 Hz, 1H) 1.28 (d, J=12.47 Hz, 3H) 1.21 (br s, 3H) 1.04 (s, 3H) 1.01 (s, 3H) 0.96 (s, 3H) 0.94 (s, 3H) 0.90–2.22 (m, 22H) 0.76 (s, 3H); 13C-NMR (100 MHz, Pyr) δ: 180.68 145.29 123.00 107.20 103.95 85.77 81.44 79.02 78.92 76.94 73.28 72.45 71.91 68.49 64.63 63.07 48.52 47.54 47.09 46.85 43.75 42.58 42.41 40.18 37.29 34.67 33.72 33.66 33.23 31.41 28.79 26.62 25.98 24.57 24.30 24.24 24.11 20.49 18.80 18.62 17.89 16.47 14.01; IR vmax cm−1 (KBr) 3432.35 2931.89 1702.32 1636.79 1458.11 1385.31 1263.95 1074.81; MALDI-TOF-MS m/z 803.1849 [M+Na]+ (Calcd for C42H68O13 Na. Found: 803.4558).
Hederagenin-3-O-α-D-mannopyranosyl-(1→4)-α-L-rhamnopyranoside (23d)
Yield 82%, white solids, Rf=0.44 (CHCl3–MeOH–H2O, 20 : 10 : 1, v/v/v); [α]D +35° (c 0.05, MeOH); 1H-NMR (400 MHz, Pyr) δ: 5.58 (s, 1H) 5.54 (s, 1H) 5.48 (br s, 1H) 4.92 (t, J=6.97 Hz, 1H) 4.58–4.71 (m, 4H) 4.52 (br s, 1H) 4.33–4.45 (m, 2H) 4.19–4.28 (m, 2H) 4.12–4.18 (m, 1H) 3.58–3.68 (m, 2H) 3.29 (d, J=10.27 Hz, 1H) 1.42 (d, J=5.14 Hz, 3H) 1.21 (s, 3H) 1.03 (s, 3H) 1.00 (s, 3H) 0.95 (s, 3H) 0.93 (s, 3H) 1.54 (m, 22H) 0.74 (s, 3H); 13C-NMR (100 MHz, Pyr) δ: 180.71 145.30 123.00 104.28 103.88 84.90 81.65 76.26 73.50 73.95 72.78 71.79 69.74 68.39 64.64 63.48 48.52 47.54 47.10 46.86 43.74 42.58 42.42 40.18 39.01 37.30 34.67 33.72 33.66 33.22 31.41 28.79 26.62 26.01 24.31 24.24 24.12 18.64 18.61 17.89 16.47 13.97; IR vmax cm−1 (KBr) 3434.31 2932.75 1702.33 1636.79 1458.17 1383.89 1265.54 1074.37; MALDI-TOF-MS m/z 803.1768 [M+Na]+ (Calcd for C42H68O13Na. Found: 803.4558).
Hederagenin-3-O-α-L-arabinopyranosyl-(1→3)-[β-D-glucopyranosyl-(1→4)]-α-L-rhamnopyranoside (23e)
Yield 78%, white solids, Rf=0.24 (CHCl3–MeOH–H2O, 20 : 10 : 1, v/v/v); [α]D −7.5° (c 0.1, MeOH); 1H-NMR (400 MHz, Pyr) δ: 5.57 (s, 1H) 5.53 (d, J=7.34 Hz, 1H) 5.49 (br s, 1H) 5.25 (d, J=8.07 Hz, 1H) 4.81 (br s, 1H) 4.62–4.67 (m, 1H) 4.59 (t, J=9.17 Hz, 1H) 4.51 (t, J=8.07 Hz, 1H) 4.15–4.42 (m, 7H) 4.11 (d, J=8.80 Hz, 1H) 4.04 (t, J=8.07 Hz, 1H) 3.72 (d, J=11.00 Hz, 1H) 3.60–3.68 (m, 2H) 3.46–3.56 (m, 2H) 3.30 (d, J=10.27 Hz, 1H) 1.73 (d, J=6.60 Hz, 3H) 1.22 (s, 3H) 1.09–2.22 (m, 22H) 1.05 (s, 3H) 1.01 (s, 3H) 0.97 (s, 3H) 0.93 (s, 3H) 0.81 (s, 3H); 13C-NMR (100 MHz, Pyr) δ: 180.71 145.29 123.02 106.78 105.01 103.84 83.01 81.33 79.80 79.65 18.22 46.46 75.17 73.37 72.46 72.33 70.27 68.80 67.84 64.66 63.21 48.52 47.55 47.10 46.85 43.77 42.59 42.43 40.19 38.98 37.30 34.67 33.70 33.66 33.25 31.40 30.44 28.79 26.60 25.98 24.55 24.30 24.23 24.13 20.48 19.15 18.61 17.89 16.47 14.20 14.05; IR vmax cm−1 (KBr) 3435.32 2931.68 1701.41 1636.89 1459.14 1384.98 1264.45 1074.32; MALDI-TOF-MS m/z 935.5243 [M+Na]+ (Calcd for C47H76O17Na. Found: 935.4980).
Hederagenin-3-O-α-L-rhamnopyranosyl-(1→3)-[α-L-rhamnopyranosyl-(1→4)]-α-L-rhamnopyranoside (23f)
Yield 79%, white solids, Rf=0.32 (CHCl3–MeOH–H2O, 20 : 10 : 1, v/v/v); [α]D −5.7° (c 0.05, MeOH); 1H-NMR (400 MHz, Pyr) δ: 5.95 (s, 1H) 5.74 (s, 1H) 5.55 (s, 1H) 5.49 (br s, 1H) 4.93 (br s, 1H) 4.77 (br s, 1H) 4.71 (br s, 1H) 4.65 (dd, J=9.17, 6.24 Hz, 1H) 4.45–4.60 (m, 4H) 4.25–4.40 (m, 4H) 4.21 (dd, J=12.10, 4.03 Hz, 1H) 3.69–3.80 (m, 2H) 3.30 (d, J=10.27 Hz, 1H) 1.58–1.62 (m, 9H) 1.21 (s, 3H) 1.04 (s, 3H) 1.01 (s, 3H) 0.97 (s, 3H) 0.93 (s, 3H) 0.85 (s, 3H) 0.80–2.21 (m, 22H); 13C-NMR (100 MHz, Pyr) δ: 180.79 145.32 122.99 105.14 104.28 104.04 82.36 81.82 79.76 74.30 74.06 73.24 73.15 72.99 72.61 71.01 70.59 68.91 64.73 48.54 47.55 47.11 46.88 43.84 42.59 42.44 40.19 39.01 37.34 34.68 33.71 33.67 33.23 31.41 30.44 28.80 26.61 26.10 24.48 24.32 24.24 24.13 20.46 19.25 18.96 18.79 18.63 17.89 16.46 14.00; IR vmax cm−1 (KBr) 3433.13 2930.89 1702.53 1636.41 1459.13 1385.23 1263.77 1074.92; MALDI-TOF-MS m/z 933.6139 [M+Na]+ (Calcd for C48H78O16Na. Found: 933.5188).
Hederagenin-3-O-β-D-glucopyranosyl-(1→3)-[β-D-glucopyranosyl-(1→4)]-α-L-rhamnopyranoside (23g)
Yield 82%, white solids, Rf=0.16 (CHCl3–MeOH–H2O, 20 : 10 : 1, v/v/v); 1H-NMR (400 MHz, Pyr) δ: 5.45–5.56 (m, 3H) 5.39 (d, J=8.07 Hz, 1H) 4.86 (s, 1H) 4.63–4.69 (m, 1H) 4.57 (t, J=9.17 Hz, 1H) 4.40–4.46 (m, 1H) 4.29–4.39 (m, 4H) 4.10–4.27 (m, 5H) 4.00–4.08 (m, 2H) 3.81 (br s, 1H) 3.72 (d, J=10.27 Hz, 1H) 3.54–3.66 (m, 2H) 3.28 (d, J=11.00 Hz, 1H) 1.70 (d, J=5.87 Hz, 3H) 1.20 (br s, 3H) 1.02 (s, 3H) 1.00 (s, 3H) 0.96 (s, 3H) 0.93 (s, 3H) 0.81 (s, 3H) 0.76–2.22 (m, 22H); 13C-NMR (100 MHz, Pyr) δ: 180.77 145.29 123.00 105.88 105.08 103.60 83.11 81.38 79.79 79.54 78.98 78.48 78.30 76.40 75.89 72.35 72.29 72.07 68.79 64.75 63.21 63.00 48.50 47.53 47.10 46.87 43.74 42.58 42.41 40.18 38.93 37.29 34.67 33.73 33.66 33.22 31.41 30.43 28.79 26.61 25.91 24.30 24.25 24.12 19.11 18.61 17.89 16.44 14.03; IR vmax cm−1 (KBr) 3433.56 2931.35 1701.69 1637.53 1459.83 1384.80 1264.31 1075.22; MALDI-TOF-MS m/z 965.7416 [M+Na]+ (Calcd for C48H78O18Na. Found: 965.5086).
Dulbecco’s modified Eagle’s medium (DMEM), DMEM/F12, fetal bovine serum (FBS), RPMI1640 were purchased from Gibco BRL (Rockville, MD, U.S.A.). 3-(4,5-Dimethylthiazol-2-yl)-2,4-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), cholera toxin, hydrocortisone, insulin, transferrin, triiodothyronine (T3), and cisplatin were obtained from Sigma-Aldrich Chemical (St. Louis, MO, U.S.A.).
Cell Viability AssayThe viability of cancer cells was determined via a MTT assay. MCF-7 and MDA-MB-231 breast cancer cells were cultured in DMEM supplemented with 10% FBS in a humidified atmosphere of 5% CO2 at 37°C, and A549 lung cancer cells were maintained in 10% FBS-RPMI1640 medium. Cancer cells were cultured in DMEM/F12 (3 : 1) supplemented with 10% FBS, 1×10−10 M cholera toxin, 0.4 mg/mL hydrocortisone, 5 µg/mL insulin, 5 µg/mL transferrin or 2×10−11 M T3. The compounds were dissolved in DMSO and diluted with culture media. Cancer cells (1.0×104 cells/mL) were seeded onto each well of a 96-well plate with the respective media and incubated to adhere overnight. The cells were then treated with various concentrations of each newly synthesized compound in serum-free medium for 24 h. MTT solution (20 µL, 5 mg/mL) was added to each well, and the cells were incubated for 4 h at 37°C. The medium was then removed, and 200 µL of DMSO was added to each well. The absorbance was determined at 570 nm using a microplate reader (Bio-Rad Laboratories, Hercules, CA, U.S.A.).
This study was supported by a Grant of the Korean Health Technology R & D Project from the Ministry of Health and Welfare, the Republic of Korea (No. A121443).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials. The supplementary materials data file (1H- and 13C-NMR, IR, Mass) of the synthesized Pusatilla saponin D analogs.