2016 Volume 64 Issue 10 Pages 1509-1513
2016 Volume 64 Issue 10 Pages 1509-1513
The hybrid compounds 1–5 comprised of five nitroxides with ibuprofen were synthesized and their reduction rate for ascorbic acid (AsA) and methyl radicals were measured in comparison with 3-hydroxy-tetramethylpyrrolidine-1-oxyl (PROXYL) 6. The rate constants in reduction reaction with 200-fold excess of AsA were determined in following order: 1 (0.42±0.06), 3 (0.17±0.06), 2 (0.10±0.05), and 6 (0.09±0.02 M−1s−1). The remaining two sterically shielded nitroxides 4 and 5 scarcely reacted with AsA. In the reaction with the more reactive methyl radicals, produced by 200-fold excess of Fenton’s reagent, the reduction rates of 2, 4, and 5 were in the following decreasing order: 2 (1.1±0.2), 4 (0.76±0.09), and 5 (0.31±0.03 M−1s−1).
The representative nitroxide radicals, a six-membered-ring nitroxide, tetramethylpiperidine-1-oxyl (TEMPO), and a five-membered-ring nitroxide, tetramethylpyrrolidine-1-oxyl (PROXYL), have been used as in-vivo probes. Recently, various nitroxides in which the tetramethyl groups around the nitroxyl group were replaced by bulkier groups have been synthesized. They had different reduction rates for reductants such as ascorbic acid (AsA), and these were applied as an in-vivo probe.1–17) The rate constant of reduction of a nitroxide probe in vitro is expected to be a guideline of its half-life in vivo.9–11) The rate constants of them are greatly different and could not measure under the same conditions.9,10,17) For the nitroxides resistant to AsA, we planned to examine the measurement of their rate constants using methyl radicals formed by Fenton reaction. The balance between their sensitivity for reductants and half-life is important for use of nitroxide as an in-vivo probe. TEMPO radical is highly sensitive for reductants and a good detection reagent for AsA,5,11) but, its half-life as an in-vivo probe was too short and unusable.14)
It has been shown that ibuprofen-PROXYL passed through the blood–brain barrier (BBB) in mice and was indicated the anti-inflammatory action in inflamed mice brains. Real-time changes in the redox status of mice brains were successfully visualized by magnetic resonance imaging (MRI) and electron paramagnetic resonance (EPR) imaging. Both shows a functionality as a theranostic compound.18) However, for exploring the redox status of brain, nitroxides with different reduction rate for reductants such as reactive oxygen species (ROS) or vitamin C or E are required. Five nitroxides with different reduction activities and their condensates with ibuprofen were synthesized (Fig. 1). The reduction reactivity of the five ibuprofen–nitroxides were measured by addition of AsA, and for the reduction-resistant nitroxides by the addition of the methyl radicals produced by the Fenton reaction. The reduction rate constants of the five ibuprofen–nitroxides for AsA or methyl radicals were determined and compared with the suitable time-dependent reduction–decay curve.

Six nitroxides (6–11) were synthesized by the synthetic methods shown in Chart S1. Sterically shielded nitroxides 7, 10 and 11 were synthesized according to the method established by Yamada’s group4–7) and Rajca’s group.9–11) Since there were some low-yield reaction steps in the synthesis of the sterically shielded nitroxides (7, 10, 11), the reaction conditions were partially improved and synthesis was achieved.
Nitroxides with tetramethyl groups 6, 8 and 9 were synthesized by conventional methods.19–21)
The ibuprofen–nitroxides 1–5 were synthesized by a condensation reaction using dicyclohexylcarbodiimide (DCC) and N,N-dimethyl-4-aminopyridine (DMAP) in quantitative yields.
The purity of the ibuprofen–nitroxides 1–5 was determined by 1H-NMR and EPR spectroscopies (Figs. S1–S11). In 1H-NMR spectroscopy the assignment was achieved as a hydroxylamine by adding excess of hydrazobenzene. EPR spectra of ibuprofen–nitroxides 3–5 in dimethyl sulfoxide (DMSO) show triplet patterns due to 14N hyperfine splitting, aN=1.43–1.53 mT, and g-values of about 2.006, similar to those for tetramethyl-linked 1 and 2 (Figs. S6–S11). EPR line-widths of nitroxides with tetramethyl groups 1, 2 and 6 were 0.086–0.096 mT and those of nitroxides with the more sterically hindered groups 3, 4 and 5 were broad, 0.103–0.198 mT18) (Table 1). DMSO was used as solvent for the measurement of the reduction kinetics.
| Compound | g | aN (mT) | Line width (mT) |
|---|---|---|---|
| 1 | 2.0066 | 1.56 | 0.096 |
| 2 | 2.0069 | 1.47 | 0.086 |
| 3 | 2.0060 | 1.53 | 0.198 |
| 4 | 2.0063 | 1.46 | 0.141 |
| 5 | 2.0068 | 1.43 | 0.103 |
| 6 | 2.0069 | 1.47 | 0.088 |
Partition coefficients (log Po/w) of nitroxides 1–6 between n-octanol and phosphate buffer solution (PBS) (0.1 M, pH 7.4) were measured by EPR spectroscopy and compared.7–9) Since ibuprofen–nitroxides were insoluble in PBS due to their lipophilicity, each nitroxide was added in a mixture of 1 mL of n-octanol and 1 mL of PBS (0.1 M, pH 7.4), and the resultant mixture was mixed vigorously for 1 h, and then the mixture was centrifuged at 3000 rpm for 5 min. Both n-octanol and PBS were subjected to the EPR measurement. Due to the lipophilicity of the ibuprophen–nitroxides, nitroxide-radicals in compounds 2–5 were no detectable in PBS. Log Po/w of 1 was also 3.49, very lipophilic.
Reduction Rate Constants, k (M−1s−1) for 200-Folds Excess AsAThe rates of reduction for nitroxides 1–5 were studied under pseudo-first-order conditions using a 200-fold excess of AsA in DMSO. Second-order rate constants, k were obtained by monitoring the decay of the low-field EPR peak height of nitroxides/peak height of the Mn marker from 2 min to 10 min after reaction at 295 K (Fig. 2, Table 2).

Each point is the mean of three experiments (±S.D.).
| Compound | k (M−1s−1) | R2 |
|---|---|---|
| 1 | 0.42±0.07 | 0.9998 |
| 2 | 0.10±0.05 | 0.9815 |
| 3 | 0.17±0.06 | 0.9753 |
| 6 | 0.09±0.02 | 0.9787 |
The reduction reaction of five ibuprofen–nitroxides and 3-hydroxymethyl-2,2,5,5-tetramethylpyrrolidin-1-oxyl (6) with 200-fold excess of AsA, showed that the sterically shielded nitroxides 4 and 5, linked with tetraethyl groups, were scarcely subject to the reduction. The hybrid compounds comprised of ibuprofen and the more reactive six-membered ring compounds (1, 3) or the tetramethyl group-linked five-membered ring compounds (2, 6) were subjected to the reduction, and had the order 1>3>2>6. Rate constant was calculated about 1, 2, 3, and 6 which showed the appropriate decay curve. Tetramethylpiperidine nitroxide 1 was 2.47 times faster than dicyclohexylpiperidine nitroxide 3, which was 1.70 times faster than tetramethylpyroline nitroxide 2, which in turn was 1.11 times faster than tetramethylpyrrolidine nitroxide 6. It was assumed that the six-membered ring nitroxides 1 and 3 are ready to react with AsA compared to the five-membered ring nitroxide because they take the same chair-form. The decay by the reduction of 3 was variable and R2 was low because of the EPR spectrum was unstable and signal width of the splitting pattern was broad.
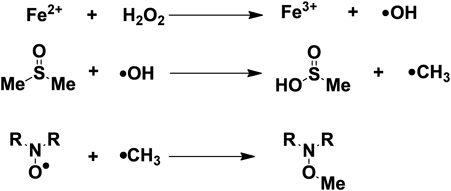
Reduction Rate Constants, k (M−1s−1) for 200-Fold Excess Methyl Radicals Produced by Fenton’s ReagentAs the reaction of AsA with the sterically shielded nitroxides 4 and 5 was limited, a more reactive radical was necessary to obtain more exact rate constants. One such readily preparable radical is the methyl radical, which is produced by the Fenton reaction22–25) (Chart 1).
For the nitroxides resistant to AsA, the methyl radicals produced by the Fenton reaction were used. Rate constant, k was determined in the same way to that of AsA. To determine the concentration and conditions of the Fenton’s reagent to be used in the reaction with the nitroxides, a pre-examination using 20-, 100-, and 200-fold excess of Fenton’s reagent with 20 µM nitroxide 5 was carried out. It was found that 200-fold of reagent gave the best decay curve. The structure of the methyl adduct product was confirmed (see Experimental, compound 12). The reaction of nitroxide with methyl radicals is irreversible, unlike the conversion of nitroxide by AsA into hydroxylamine. The reactions of the five ibuprofen–nitroxides with 200-fold excess of Fenton’s reagent were conducted (Fig. 3). Since the appropriate decay curves were showed, the rate constants were calculated for 2, 4, and 5. Nitroxides 1 and 3 reacted immediately. The remaining sterically shielded nitroxides (2, 4, 5), which had hardly reacted with AsA, reacted in the order 2, 4, and 5. There was not a large difference between the rate constants. Nitroxides 2 and 4 reacted within 30 min, however, in the most sterically shielded nitroxide 5, 25% had not been reduced after 30 min. It was surprising that 5 was resistant not only to AsA but also to the labile methyl radicals.

Each point is the mean of three experiments (±S.D.).
Five ester-linked hybrid compounds between ibuprofen and five nitroxides with a variety of nitroxyl-protecting groups were synthesized and the reactivities to AsA and methyl radicals were measured. For three hybrid compounds and 6, moderate decay curves for 200-fold excess AsA were determined, with the reduction rate constants in following order: 1 (0.42±0.06), 3 (0.17±0.06), 2 (0.10±0.05), and 6 (0.09±0.02 M−1s−1). For the AsA-resistant compounds, moderate decay curves with 200-fold excess methyl radicals were determined, with the reduction rate constants in the following decreasing order: 2 (1.1±0.2), 4 (0.76±0.09), and 5 (0.31±0.03 M−1s−1) (Table 3). Since the rate constant of 2 was determined for AsA and methyl radicals as 0.10±0.05, and 1.1±0.2, respectively, it is assumed that reduction ability of 200-fold excess Fenton’s reagent for ibuprofen-nitroxides is 10 times higher than that of 200-fold excess AsA. Although each reduction rate constant was here determined using DMSO as a solvent due to the lipophilicity of the ibuprofen–nitroxides, since that of 6 for AsA in PBS was 0.12±0.003 M−1 s−1and analogous to 0.09±0.02 M−1 s−1 in DMSO, it was demonstrated that this method using both AsA and methyl radical as a reductant is also available in PBS for the measurement of the reduction rate constants of the nitroxide derivatives with various reactivity.
| Compound | k (M−1 s−1) | R2 |
|---|---|---|
| 2 | 1.1±0.2 | 0.9997 |
| 4 | 0.76±0.09 | 0.9998 |
| 5 | 0.31±0.03 | 0.9933 |
All starting materials and reagents were purchased from Wako Pure Chemical Industries, Ltd., Osaka, Japan and Sigma-Aldrich, St. Louis, MO, U.S.A. Reactions were monitored by TLC on 0.25-mm silica gel F254 plates (E. Merck, Japan). UV radiation and a 7% ethanolic solution of phosphomolybdic acid with heating, were used for coloration. Flash column chromatography was performed on silica gel (silica-gel 60, 40–50 µm, Kanto Chemical Co., Inc., Tokyo, Japan) to separate and purify the reaction products. Melting points were determined using an ASONE micro-melting point apparatus and uncorrected values reported. NMR spectra were recorded on a JEOL ECX-500 spectrometer using Me4Si as the internal standard. The 1H-NMR spectra of the hybrid compounds were measured and assigned for the corresponding hydroxylamines, which were prepared by the reduction of nitroxide radicals under the presence of excess of hydrazobenzene (Wako Pure Chemical Industries, Ltd.). Mass spectra and high-resolution mass spectra (HR-MS) were obtained under electron spray ionization (ESI) conditions on a JEOL JMS-T100LP.
General ProceduresSynthesis of Nitroxides 6–11The nitroxide radicals used in the present study were synthesized by established methods, as shown in Supplementary materials (Chart S1). Nitroxides 6, 7, 8, 9, and 10 were synthesized via 6, 4, 1, 4, and 3 steps from tetramethyl-4-piperidone in overall yield of 52, 17, 85, 62, and 0.8%, respectively. Nitroxide 11 was synthesized via 4 steps from tetraethyl-4-hydroxypiperidine with an overall yield of 6.05%. The synthetic yield of 10 was very low, 0.8%, because of the change of gem-methyl group to a gem-cyclohexyl group and the subsequent oxidation to nitroxide radical had a low unimprovable yield of 16 and 5.5%, respectively.
Synthesis of (2,2,6,6-Tetramethylpiperidin-1-oxyl)-4-yl (±)-2-(p-Isobutylphenyl)propionate (1)To a solution of ibuprofen (216 mg, 1.05 equiv.) and 3-hydroxymethyl-2,2,5,5-tetramethylpyperidin-1-oxyl (172 mg) in dry CH2Cl2 (6 mL), DCC (229 mg, 1.11 equiv.) and DMAP (13.5 mg, 0.111 equiv.) were added and the mixture was stirred at room temperature under an Ar atmosphere for 18 h. To the reaction mixture ethyl ether (20 mL) was added and the resulting precipitates were filtered through a celite pad. The filtrate was evaporated in vacuo and then purified by silica-gel column chromatography (n-hexane/ethyl acetate=3 : 1) to give 1 (360 mg, 100%) as red crystals.
The other ibuprofen nitroxides 2–5 were synthesized in a similar way to that of 1 in quantitative yields.
(2,2,6,6-Tetramethylpiperidin-1-oxyl)-4-yl (±)-2-(p-Isobutylphenyl)propionate (1)Red crystals, mp=48°C. ESI-MS (m/z) 362 (M+2H)+. 1H-NMR (CDCl3) δ (ibuprofen moiety) 0.89 (6H, d, J=6.8 Hz, CH3×2), 1.47 (3H, d, J=7.5 Hz, 2-CH3), 1.84 (1H, m, H8), 2.44 and 2.45 (each 1H, s, CH2), 3.64 (1H, q, J=7.5 Hz, H2), 7.08 and 7.18 (each 2H, d, J=8.2 Hz, ArH ×4), (piperidine moiety) 1.13, 1.15, 1.17, 1.18 (each 3H, s, –CH3×4), 1.45 and 1.54 (each 1H, t, J=11.6 Hz, CH2), 1.78 and 1.88 (each 1H, dt, J=0.4, 11.6 Hz, CH2), 5.02 (1H, m, >CH–). HR-MS (ESI+) m/z: [M + 2H]+, calcd for C22H36NO3 362.26952, found 362.26998.
(2,2,5,5-Tetramethyl-3-pyrroline-1-oxyl)-3-yl-methyl (±)-2-(p-Isobutylphenyl)propionate (2)Pale-yellow crystals, mp=45°C. ESI-MS (m/z) 381 (M+Na)+, 358 (M)+. 1H-NMR (CDCl3) δ (ibuprofen moiety) 0.89 (6H, d, J=6.7 Hz, CH3×2), 1.51 (3H, d, J=7.5 Hz, 2-CH3), 1.83 (1H, m, H8), 2.43 and 2.45 (each 1H, s, CH2), 3.72 (1H, q, J=7.5 Hz, H2), 7.09 and 7.21 (each 2H, d, J=8.3 Hz, ArH ×4), (piperidine moiety) 1.11, 1.12, 1.14, 1.15 (each 3H, s, –CH3×4), 4.52 and 4.61 (each 1H, d, J=14.1 Hz, CH2), 5.33 (1H, s, olefin H). HR-MS (ESI+) calcd for C22H32NO3 358.23822, found 358.23819.
(7-Aza-dispiro[5.1.5.3]hexadec-7-yl-oxyl)-15-yl (±)-2-(p-Isobutylphenyl)propionate (3)Pale-red crystals, mp=102°C. ESI-MS (m/z) 463 (M+Na)+, 442 (M+2H)+. 1H-NMR (CDCl3) δ (ibuprofen moiety) 0.88 (6H, d, J=6.8 Hz, CH3×2), 1.49 (3H, d, J=6.8 Hz, CH3), 1.84 (1H, m, H8), 2.43 and 2.45 (each 1H, s, CH2), 3.65 (1H, q, J=6.8 Hz, H2), 7.09 and 7.19 (each 2H, d, J=8.6 Hz, ArH ×4), (piperidine moiety) 1.00–1.45 (11H, m, cyclohexyl H), 1.51–1.68 (9H, m, cyclohexyl H), 2.24 and 2.35 (each 2H, br dt, J=12.1 Hz, CH2×2), 4.19 (1H, m, >CH–). HR-MS (ESI+) calcd for C28H42NNaO3 463.30624, found 463.30686.
(2,2,6,6-Tetraethylpiperidin-1-oxyl)-4-yl (±)-2-(p-Isobutylphenyl)propionate (4)Red powder. ESI-MS (m/z) 439 (M+Na)+. 1H-NMR (CDCl3) δ (ibuprofen moiety) 0.88 (6H, d, J=6.8 Hz, CH3×2), 1.47 (3H, d, J=6.8 Hz, 2-CH3), 1.28 (1H, m, H8), 2.42 and 2.44 (each 1H, s, CH2), 3.64 (1H, q, J=6.8 Hz, H2), 6.81 and 7.18 (each 2H, d, J=8.4 Hz, ArH ×4), (piperidine moiety) 0.78, 0.81, 0.82, 0.85 (each 3H, t, J=7.5 Hz, –CH2CH3×4), 1.24–1.30 (2H, –CH2CH3×1), 1.60–1.70 (4H, m, –CH2CH3×2), 1.60–1.70 (4H, m, –CH2CH3×2), 1.86 (2H, –CH2CH3×1), 1.43 and 1.97 (each 2H, t, J=9.1 Hz, CH2×2), 4.97 (1H, m, >CH–). HR-MS (ESI+) calcd for C26H42NNaO3 439.30624, found 439.30413.
(2,2,5,5-Tetraethyl-3-pyrroline-1-oxyl)-3-ylmethyl (±)-2-(p-Isobutylphenyl)propionate (5)Yellow powder. ESI-MS (m/z) 437 (M+Na)+. 1H-NMR (CDCl3) δ (ibuprofen moiety) 0.76 and 0.82 (each 3H, d, J=6.8 Hz, 8-CH3×2), 1.50 (3H, d, J=6.8 Hz, 2-CH3), 1.82 (1H, m, H8), 2.41 and 2.43 (each 1H, s, CH2), 3.72 (1H, q, J=6.8 Hz, H2), 6.81 and 7.19 (each 2H, d, J=6.8 Hz, ArH×4), (piperidine moiety) 0.87 and 0.89 (each 6H, br s, –CH2CH3×4), 1.40–1.62 (8H, m, –CH2CH3×4), 4.43 and 4.58 (each 1H, d, J=14.4 Hz, CH2), 5.24 (1H, s, olefin H). HR-MS (ESI+) calcd for C26H40NNaO3 437.29059, found 437.28977.
(1-Methoxy-2,2,5,5-tetramethyl-3-pyrrolin)-3-ylmethyl (±)-2-(p-Isobutylphenyl)propionate (12)To a solution of 2 (20 mg, 0.056 mmol) in DMSO (5 mL), 30% H2O2 aqueous solution (265 µL, 2.8 mmol) was added, with stirring. FeSO4·7H2O (770 mg, 2.8 mmol) was added by portions. After stirring for 1h, 50 mL of water was added to the reaction mixture and extracted with ethyl ether three times. The combined organic layer was washed with brine and dried over anhydrous Na2SO4. After removing the organic layer in vacuo, the residue was purified by silica-gel column chromatography (n-hexane/ether=10 : 1) to give 12 (17 mg, 82%) as a colorless powder.
Data of 12Colorless powder. ESI-MS (m/z) 396 (M+Na)+, 374 (M+H)+. 1H-NMR (CDCl3) δ (ibuprofen moiety) 0.89 (6H, d, J=6.8 Hz, CH3 ×2), 1.50 (3H, d, J=7.6 Hz, CH3), 1.84 (1H, m, H8), 2.43 and 2.45 (each 1H, s, CH2), 3.71 (1H, q, J=7.6 Hz, H2), 7.09 and 7.19 (each 2H, d, J=8.4 Hz, ArH ×4), (pyrroline moiety) 1.131 and 1.137 (each br s, CH3×2), 1.162 (6H, br s, CH3×2), 4.48 and 4.58 (each 1H, dd, J=1.5, 13.6 Hz, CH2), 5.25 (3H, s, OMe). 13C-NMR (CDCl3) δ (ibuprofen moiety) 18.39 (C9), 22.47 (C10), 30.31 (C8), 45.1 (C7), 45.3 (C2), 127.3 (C5), 129.4 (C4), 137.6 (3), 140.7 (C6), 174.4 (C1), (pyrroline moiety) 60.7 (CH2), 67.5, 69.7, 132.2, 139.1. HR-MS (ESI+) calcd for C23H36NNaO3 396.25146, found 396.25284.
AnalysisApparatusEPR spectra were obtained on a JEOL JES-FR30 EPR spectrometer. Samples were drawn into quartz capillaries, the bottoms of the capillaries were sealed and the capillaries were placed in standard 2-mm-i.d. quartz EPR tubes. The EPR spectrometer settings were as follows: microwave power, 4.0 mW; frequency, 9.5 GHz; and modulation amplitude, 1.25 G. Quick mixing of sample was conducted by VOLTEX®.
ReagentsThe reagents used in this study were commercial products: L-ascorbic acid (Wako, Japan), and dimethyl sulfoxide (DMSO; Wako, Japan), 30% H2O2 aqueous solution (Wako, Japan), and FeSO4·7H2O (Wako, Japan). DMSO was deoxygenated by microwave under reduced pressure and used.
EPR Intensity Measurement of Time-Dependent Reduction of Six Nitroxides with 200-Fold AsA in DMSOEach sample 1–6 and ascorbic acid were dissolved in DMSO to prepare 2 mM stock solutions. Twenty microliters of 2 mM sample and 1.98 mL of ascorbic acid in DMSO were mixed by VOLTEX® and an EPR measurement taken after 2 min. Subsequent measurements were taken at an interval of 1 min up to 10 min, and then the interval was changed to 2 min until 30 min had passed.
The conditions of the EPR measurement were: field, 336.5 mT; power, 4.0 mW; gain, 10.0; sweep width, 5.0 mT; modulation width, 0.1 mT; sweep time, 0.5 min; time constant, 0.1 s; date points, 0; accumulation, 1; accumulation method, no; and frequency, 9.2 GHz.
EPR intensity was calculated by following equation:
 |
Twenty microliters of 2 mM nitroxide was diluted with 1.98 mL of DMSO to prepare 20 µM sample solution. EPR intensity of 20 µM sample solution was measured and the relative value of the EPR intensity was determined to be 1.0.
EPR Intensity Measurement of Time-Dependent Reduction of Five Nitroxides with Methyl Radicals in DMSOMethyl radical was prepared by the Fenton reaction. Thirty percent H2O2 aqueous solution was diluted with DMSO to prepare 4.04 mM H2O2 DMSO solution. Twenty microliters of each 2 mM sample DMSO solution and 1.98 mL of 4.04 mM H2O2 DMSO solution were mixed by VOLTEX® and the resulting mixture was used in the EPR measurement at 0 min. To this mixture, 2.2 mg of FeSO4·7H2O (8 µmol) was added and the mixture was mixed by VOLTEX® for 10 s. An EPR measurement was taken on the resulting mixture.
The EPR measurement and the determination of EPR intensity were conducted in the similar way to the above described.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.
1. Chart S1. Synthesis of six nitroxides.
2. Figures S1–S5. 1H-NMR spectra of the nitroxides 1–5 in CDCl3.
3. Figures S6–S11. EPR spectra of the nitroxides 1–6 in DMSO.
4. Table S1. Partition coefficients of nitroxides between n-octanol and PBS.