2018 Volume 66 Issue 3 Pages 235-238
2018 Volume 66 Issue 3 Pages 235-238
WFQ-101 with a unique N-1 substituent, 5-amino-4-fluoro-2-(hydroxymethyl)phenyl group, was selected as a lead compound through combination screening based on antimicrobial activity and the efflux index against quinolone-resistant (QR) Pseudomonas aeruginosa (P. aeruginosa). Through structural optimization, we identified WFQ-228 as a novel fluoroquinolone antibiotic candidate. WFQ-228 had potent and superior activity in comparison to levofloxacin (LVX) and ciprofloxacin (CIP) against clinical isolates of P. aeruginosa, Escherichia coli and Acinetobacter baumannii, including QR strains. Furthermore, WFQ-228 demonstrated the potential to overcome major mechanisms of drug resistance; its antimicrobial activity was less affected by both pump-mediated efflux and mutations of the quinolone resistance-determining region in P. aeruginosa compared with LVX and CIP. These results suggest that WFQ-228 is a promising candidate for further evaluation in the treatment of infections caused by QR Gram-negative pathogens.
The spread of multiple drug-resistant (MDR) bacteria has become a serious global concern. WHO has warned against a ‘post-antibiotic’ era,1) and published a global priority list of antibiotic-resistant bacteria.2) In this list, MDR Gram-negative bacteria, particularly MDR Pseudomonas aeruginosa (P. aeruginosa), Enterobacteriaceae and Acinetobacter baumannii (A. baumannii), are defined as high priority pathogens. In fact, an increase in mortality and hospital length of stay in patients with MDR bacteria has been reported.3,4) Such circumstances demonstrate the urgent need to develop novel antibiotics in order to combat these threatening bacteria.
Fluoroquinolones (FQs) are one of the most prescribed classes of antibiotics. FQs exhibit bactericidal activity by inhibiting DNA gyrase and topoisomerase IV, which play the essential roles of DNA replication and transcription in bacteria.5–7) This class of antibiotics, which includes levofloxacin (LVX) and ciprofloxacin (CIP) with broad and potent antimicrobial activities, has been successfully used to treat various bacterial infections including urinary tract, genitourinary, and respiratory tract infections.8–10) However, their continual usage has also resulted in the spread of FQ-resistance in bacteria.11)
It is well known that bacterial resistance to FQs occurs via one of the following mechanisms or a combination of these: i) target-site mutation,12,13) ii) overexpression of efflux pumps,14–16) iii) plasmid-mediated resistance,17) iv) changes in membrane structure,18) and v) enzymatic modification of FQs.19) In Gram-negative pathogens such as P. aeruginosa and Escherichia coli (E. coli), the mutations in the quinolone resistance-determining regions (QRDRs) of the subunit of the targeted enzymes, known as gyrA and parC, and the overexpression of efflux pumps are the most common resistance mechanisms.13,15,16,20)
We previously reported that novel FQ derivatives having a unique group of 5-amino-2,4-difluorophenyl or 6-amino-3,5-difluoropyridin-2-yl at the N-1 position displayed potent antimicrobial activity against various clinical isolates of pathogens with QRDR mutations.21,22) Despite this noteworthy potent activity, however, those FQ derivatives are less active against several Gram-negative resistant pathogens such as P. aeruginosa and E. coli. Reported herein is the result of our study to create novel FQs to overcome MDR Gram-negative pathogens.
Among the global priority pathogens listed by WHO, we placed the most importance on MDR P. aeruginosa because of the high mortality rate from this infection and the paucity of effective therapeutic options.23) In order to find lead compounds for MDR Gram-negative pathogens, we conducted library screening by measuring activity against P. aeruginosa in parallel with evaluating pump-mediated efflux property. Anti-P. aeruginosa activity was assessed as the mean value of minimum inhibitory concentration (MIC) against three clinical isolates of quinolone-resistant (QR) strains. The pump-mediated efflux property was represented as a MIC ratio and estimated by comparing the mean MIC determined in the presence and absence of phenylalanine-arginine β-naphthylamide (PAβN),20,24) a broad-spectrum efflux pump inhibitor; a small MIC ratio (PAβN(−)/PAβN(+)) indicates low pump-mediated efflux of the compound. We therefore aimed to identify compounds with lower MICs and smaller MIC ratios as the targeted lead compounds.
The MICs (PAβN(−)) and MIC ratios (PAβN(−)/PAβN(+)) of three representative compounds (A–C) against clinical isolates of P. aeruginosa are plotted in Fig. 1. Compound B (WFQ-101) showed preferable results, with lower MIC and the smallest MIC ratio. The activity of WFQ-101 (mean MIC of 3.2 µg/mL) was superior or comparable to LVX (20 µg/mL) and CIP (3.2 µg/mL) in the absence of PAβN. The MIC ratio of WFQ-101 was 10, which was 17- and 2.1-fold smaller than those of LVX and CIP, respectively (MIC ratios of 166 and 21, respectively). In addition, WFQ-101 was also attractive in terms of its unique chemical structure, having a 5-amino-4-fluoro-2-(hydroxymethyl)phenyl group at the N-1 position. Compound A was more potent (mean MIC of 1.0 µg/mL) than WFQ-101; however, its MIC ratio was 101, which was much larger than that of WFQ-101, and it lacked structural novelty. Compound C had a comparable MIC ratio to WFQ-101; however, its antimicrobial activity (mean MIC of 32 µg/mL) was 10-fold weaker. We thus selected WFQ-101 as a lead compound and conducted a structural optimization study.

a) The mean MIC was obtained from MIC data for three strains of QR P. aeruginosa. b) The MIC ratio was calculated from the comparison of MIC (PAβN(−)/PAβN(+)). The concentration of PAβN used in this assay was 50 µg/mL.
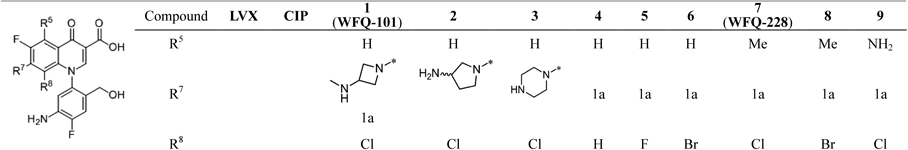
Structure–activity relationships (SAR) of WFQ-101 at the 5, 7, and 8 positions against several clinically important Gram-negative bacteria are shown in Table 1. At the 7-position of WFQs with unique aryl substituents (compounds 1–3) at the N-1 position, smaller substituents tended to be preferable for potent antimicrobial activity; the mean MICs of WFQs having 3-methylaminoazetidinyl (WFQ-101), 3-aminopyrrolidinyl (compound 2) and a piperazinyl group (compound 3) were 0.051, 1.0, and 0.49 µg/mL, respectively, for quinolone-susceptible (QS) Gram-negative strains.
 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Strain | ||||||||||||
| QS | E. coli | NIHJ-JC2 | 0.03 | 0.015 | 0.06 | 1 | 0.5 | 0.25 | 0.25 | 0.12 | 0.03 | 0.06 | 0.12 |
| E. coli | NIHJ | 0.015 | 0.008 | 0.008 | 0.12 | 0.03 | 0.03 | 0.03 | 0.015 | 0.008 | 0.015 | 0.015 | |
| P. aeruginosa | IFO3445 | 1 | 0.25 | 0.12 | 4 | 2 | 0.25 | 0.5 | 0.25 | 0.12 | 0.06 | 0.5 | |
| P. aeruginosa | 15846 | 4 | 1 | 0.12 | 2 | 2 | 1 | 0.5 | 0.12 | 0.12 | 0.12 | 0.12 | |
| Geometric mean | 0.21 | 0.074 | 0.051 | 1.0 | 0.49 | 0.21 | 0.21 | 0.086 | 0.043 | 0.050 | 0.10 | ||
| QR | A. baumannii | 09-04 | 8 | 32 | 32 | 4 | 16 | 32 | |||||
| A. baumannii | 09-12 | 4 | 16 | 0.5 | 0.12 | 0.06 | 1 | ||||||
| E. coli | 2010-05 | 32 | 64 | 32 | 4 | 16 | 32 | ||||||
| E. coli | 2010-29 | 32 | 64 | 4 | 1 | 1 | 8 | ||||||
| P. aeruginosa | 2014-01 | 64 | 32 | 8 | 2 | 4 | 8 | ||||||
| P. aeruginosa | 2014-02 | 64 | 64 | 8 | 2 | 2 | 8 | ||||||
| P. aeruginosa | 2014-17 | 64 | 16 | 8 | 4 | 4 | 8 | ||||||
| Geometric mean | 26 | 35 | 7.2 | 1.6 | 2.4 | 9 | |||||||
Among 8-position analogues of WFQ-101 (compounds 4–6), WFQ-101 with a chlorine atom and its analogue 6 with a bromine atom had similar activities against the QS pathogens, with mean MICs of 0.051 and 0.086 µg/mL, respectively, while WFQs with a smaller atom (compound 4 with a hydrogen atom and 5 with a fluorine atom) showed decreased activities (both with mean MICs of 0.21 µg/mL) compared with WFQ-101. These results suggest that a relatively large atom at the 8-position is required for potent antimicrobial activity.
In the SAR of substituents at the 5-position, the introduction of a methyl group to WFQ-101 and 6 (compounds 7, 8) resulted in enhanced antimicrobial activities, especially against the QR pathogens. Compound 7 (WFQ-228) was more potent than 8 against both QS (mean MICs of 0.043 and 0.050 µg/mL, respectively) and QR bacteria (1.6 and 2.4 µg/mL, respectively). On the other hand, the introduction of an amino group to WFQ-101 (compound 9) decreased the activity by 2- and 1.2-fold, respectively, against QS and QR pathogens. As a result, WFQ-228 exhibited the most potent antimicrobial activity against both QS and QR pathogens, with mean MIC of 0.043 and 1.6 µg/mL, respectively.
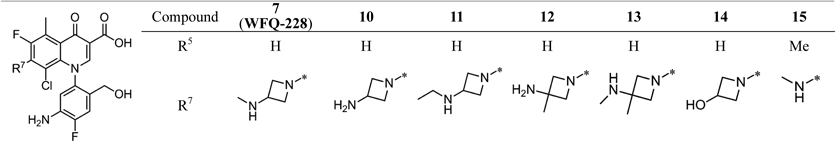
We subsequently conducted structural modification at the 3-position of the 7-azetidinyl group of WFQ-228 (compounds 10–14, Table 2). All analogues showed potent activity against QS pathogens, with mean MIC values of ≤0.12 µg/mL. Against QR pathogens, the antibacterial activity of WFQ-228 was decreased 5.5-fold by the replacement of 3-methylaminoazetidine (WFQ-228, mean MIC of 1.6 µg/mL) with 3-aminoazetidine (compound 10, 8.8 µg/mL), while the replacement with 3-ethylaminoazetidine (compound 11, 2.0 µg/mL) did not largely affect the activity. The 3,3-disubstituted analogues (compounds 12, 13) had similar activities, with mean MIC values of 2.0 and 2.4 µg/mL, respectively. Compound 14, with 3-hydroxyazetidine, exhibited slightly decreased activity from WFQ-228, but was still potent against both QS and QR Gram-negatives (with mean MICs of 0.12 and 3.0 µg/mL, respectively) in spite of the loss of basicity. We therefore introduced a methylamino group, a smaller substituent than the azetidinyl group, at the 7-position (compound 15). Compound 15 had the most potent activity against QS Gram-negative pathogens, with mean MIC of 0.036 µg/mL, while its activity against QR pathogens was decreased 2.1-fold from WFQ-228.
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Strain | ||||||||
| QS | E. coli | NIHJ-JC2 | 0.03 | 0.06 | 0.06 | 0.06 | 0.06 | 0.25 | 0.06 |
| E. coli | NIHJ | 0.008 | 0.008 | 0.008 | 0.015 | 0.015 | 0.03 | 0.008 | |
| P. aeruginosa | IFO3445 | 0.12 | 0.25 | 0.25 | 0.12 | 0.25 | 0.25 | 0.06 | |
| P. aeruginosa | 15846 | 0.12 | 0.12 | 0.25 | 0.12 | 0.25 | 0.12 | 0.06 | |
| Geometric mean | 0.043 | 0.062 | 0.074 | 0.060 | 0.087 | 0.12 | 0.036 | ||
| QR | A. baumannii | 09-04 | 4 | 2 | 4 | 4 | 4 | 4 | 8 |
| A. baumannii | 09-12 | 0.12 | 2 | 0.06 | 0.25 | 0.12 | 0.25 | 1 | |
| E. coli | 2010-05 | 4 | 16 | 4 | 8 | 8 | 16 | 8 | |
| E. coli | 2010-29 | 1 | 4 | 1 | 2 | 1 | 2 | 2 | |
| P. aeruginosa | 2014-01 | 2 | 32 | 4 | 2 | 4 | 4 | 2 | |
| P. aeruginosa | 2014-02 | 2 | 32 | 4 | 2 | 4 | 4 | 2 | |
| P. aeruginosa | 2014-17 | 4 | 16 | 8 | 2 | 8 | 4 | 8 | |
| Geometric mean | 1.6 | 8.8 | 2.0 | 2.0 | 2.4 | 3.0 | 3.3 | ||
We conducted further evaluation of WFQ-228. MICs and MIC ratios of WFQ-228, LVX, and CIP against clinical isolates of QR P. aeruginosa (three strains) are compared in Table 3. WFQ-228 was more potent than LVX and CIP in both the absence and presence of PAβN, with mean MICs of 0.79 and 0.048 µg/mL for WFQ-228, 20 and 0.12 µg/mL for LEV, and 3.2 and 0.15 µg/mL for CIP, respectively. Furthermore, WFQ-228 inherited the properties of WFQ-101, less affected by pump-mediated efflux, with an MIC ratio of 17. WFQ-228 also showed the highest antimicrobial activity against clinical isolates of P. aeruginosa with various QRDR mutations (Table 4). Compared with the wild type (Entry 1), although several types of mutations in QRDR (Entries 2–7) decreased the activities of WFQ-228 by 2- to 8-fold, WFQ-228 still exhibited potent activity, with MICs of ≤2 µg/mL even against strains with multiple mutations in both gyrA and par C, such as 2014-18, 2014-20, and PA532 (Entries 5–7). On the other hand, the activities of LVX and CIP were decreased by 16- to >32-fold and 16- to >128-fold, respectively. These results demonstrated that the antibacterial activity of WFQ-228 was less affected by QRDR mutations of the target enzymes, and therefore suggest that its binding mode with DNA gyrase and/or topoisomerase IV might differ from those of LVX and CIP.
| Compounds | Geometric mean MIC (µg/mL)a) | MIC ratio | |
|---|---|---|---|
| PAβNb) | PAβN −/+ | ||
| − | + | ||
| WFQ-228 | 0.79 | 0.048 | 17 |
| LVX | 20 | 0.12 | 166 |
| CIP | 3.2 | 0.15 | 21 |
a) The geometric mean MIC was obtained from MIC data for three strains of P. aeruginosa. b) The concentration of PAβN used in this assay was 50 µg/mL.
| Entry | Strain | Mutation(s) | MIC (µg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| gyrA | parC | |||||||
| Thr83 | Asp87 | Gly85 | Ser87 | WFQ-228 | LVX | CIP | ||
| 1 | PA528 | — | — | — | — | 0.25 | 4 | 1 |
| 2 | 2014-17 | Ile | — | — | — | 2 | 64 | 16 |
| 3 | PA405 | — | Tyr | — | — | 2 | 64 | 16 |
| 4 | 2014-02 | Ile | — | — | Leu | 2 | 64 | 64 |
| 5 | 2014-18 | Ile | Asn | — | Leu | 2 | >128 | >128 |
| 6 | 2014-20 | Ile | Gly | — | Leu | 2 | 128 | 64 |
| 7 | PA532 | Ile | Asn | Asp | — | 0.5 | >128 | 64 |
Additionally, we evaluated the activity of WFQ-228 against clinical isolates of P. aeruginosa, E. coli and A. baumanni (Table 5). WFQ-228 was 8- to 32-fold more potent than LVX and CIP, with MIC90 values of 2, 2, and 1 µg/mL for those three pathogens, respectively. WFQ-228 has the potential to successfully treat infections caused by these pathogens, judging from the MIC interpretive criteria of CIP by the Clinical and Laboratory Standards Institute.25)
| Species | n | MIC90 (µg/mL) | ||
|---|---|---|---|---|
| WFQ-228 | LVX | CIP | ||
| P. aeruginosa | 130 | 2 | 64 | 32 |
| E. coli | 111 | 2 | 32 | 64 |
| A. baumannii | 23 | 1 | 8 | 32 |
As a result, we have now identified WFQ-228 as a novel candidate to combat QR pathogens, for which there have previously been few or no effective clinical options.
WFQ-101, with a unique N-1 substituent, was selected as a lead compound based on its potent antimicrobial activity and low pump-mediated efflux property against QR P. aeruginosa. Through structural optimization, we identified WFQ-228 as a novel candidate for the development of treatments for drug-resistant bacteria. WFQ-228 showed potent and superior activity in comparison to LVX and CIP against QR Gram-negative bacteria. Furthermore, WFQ-228 showed the potential to overcome major drug resistance; its antimicrobial activity was less affected by both pump-mediated efflux and QRDR mutations in P. aeruginosa compared with LVX and CIP. These results suggest that WFQ-228 is a promising candidate for further evaluation to treat infections caused by QR Gram-negative pathogens.
This work was supported by International Health Management Associates, Inc. (U.S.A.), who supplied bacterial isolates for our research.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.